Abstract
Enzymes dissociated from corn (hybrid B73 × Mo17) seedling cell walls by solutions of high ionic strength possess the capacity to degrade Avena caryopsis glucan. Inhibitor studies disclosed that both endo- and exoenzyme activities were involved and that the reaction sequence paralleled the autolytic solubilization of β-d-glucan in isolated cell walls.
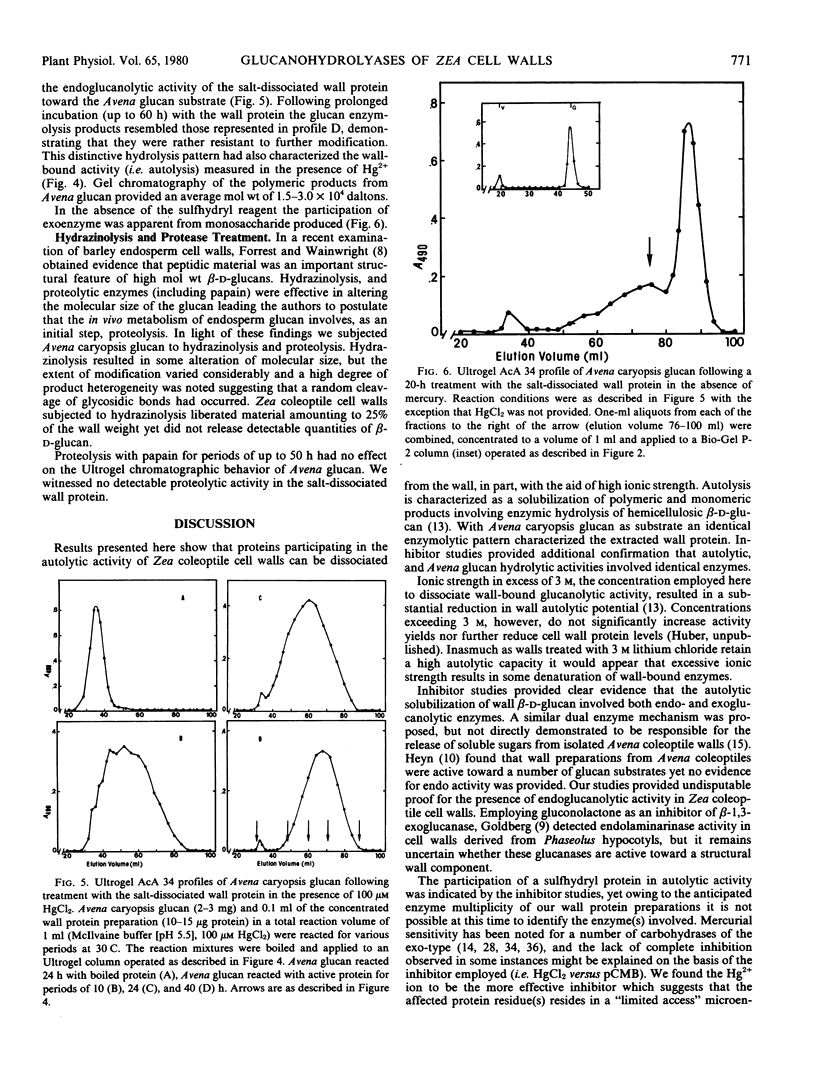
The salt-dissociated exoenzyme activity was strongly inhibited by HgCl2 and to a lesser extent by parachloromercuribenzoate at a concentration of 100 micromolar. In the absence of these inhibitors, Avena caryopsis glucan was converted to monosaccharide, whereas in the presence of the mercurials, only endoenzyme activity was apparent and the glucan substrate was hydrolyzed yielding products with an average molecular size of 1.5 to 3.0 × 104 daltons. Endoenzyme hydrolysis of the caryopsis glucan could not be attributed to the participation of an enzyme specific for mixed-linkage substrates.
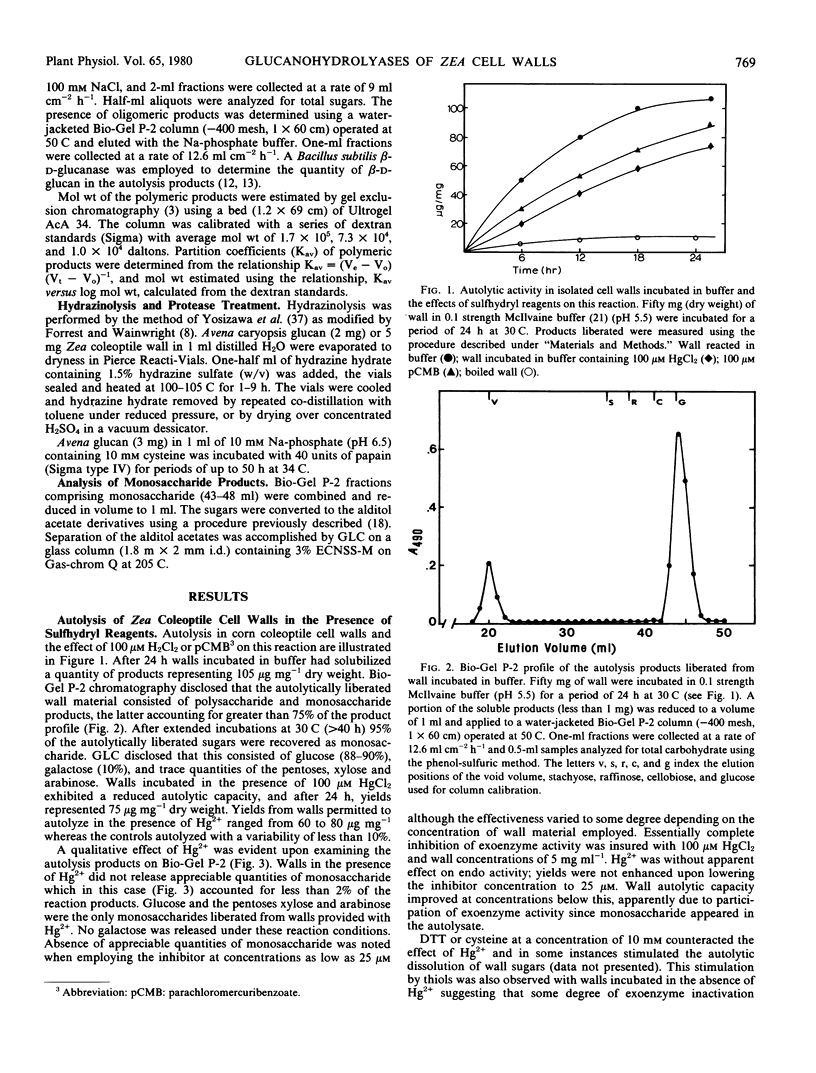
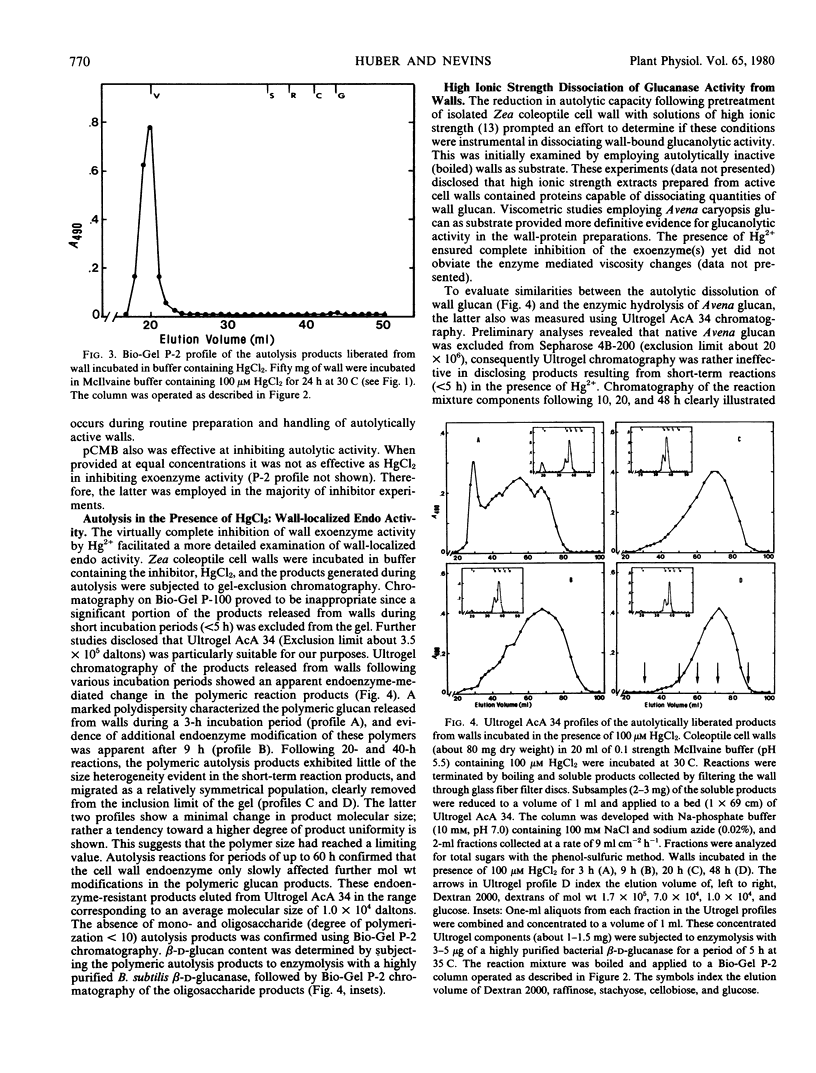
The autolytic capacity of isolated cell walls was similarly affected by inhibitors. In the presence of 100 micromolar HgCl2, cell walls released from 60 to 80 micrograms per milligram dry weight as polymeric glucan during a 24-hour period. Monosaccharide accounted for less than 2% of the autolytically solubilized products. Analysis of the polymeric glucan product revealed a similarity in molecular size to the products obtained following treatment of Avena caryopsis glucan with salt-dissociated wall protein. The results suggest that among the salt-dissociated proteins are those responsible for the autolytic capacity of isolated cell walls.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans M. L. Evidence Against the Involvement of Galactosidase or Glucosidase in Auxin- or Acid-stimulated Growth. Plant Physiol. 1974 Aug;54(2):213–215. doi: 10.1104/pp.54.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn A. N. Glucanase activity in coleoptiles of Avena. Arch Biochem Biophys. 1969 Jul;132(2):442–449. doi: 10.1016/0003-9861(69)90387-7. [DOI] [PubMed] [Google Scholar]
- Huber D. J., Nevins D. J. Preparation and Properties of a beta-d-Glucanase for the Specific Hydrolysis of beta-d-Glucans. Plant Physiol. 1977 Aug;60(2):300–304. doi: 10.1104/pp.60.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Daniels D., Dowler M. J., Rayle D. L. Activation of Avena coleoptile cell wall glycosidases by hydrogen ions and auxin. Plant Physiol. 1974 Feb;53(2):224–228. doi: 10.1104/pp.53.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz M., Ordin L. A cell wall polysaccharide-hydrolyzing enzyme system in Avena sativa L. coleoptiles. Biochim Biophys Acta. 1967 Jun 13;141(1):126–134. doi: 10.1016/0304-4165(67)90251-6. [DOI] [PubMed] [Google Scholar]
- Kivilaan A., Bandurski R. S., Schulze A. A partial characterization of an autolytically solubilized cell wall glucan. Plant Physiol. 1971 Oct;48(4):389–393. doi: 10.1104/pp.48.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Kivilaan A., Bandurski R. S. In vitro autolysis of plant cell walls. Plant Physiol. 1967 Jul;42(7):968–972. doi: 10.1104/pp.42.7.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loescher W., Nevins D. J. Auxin-induced Changes in Avena Coleoptile Cell Wall Composition. Plant Physiol. 1972 Nov;50(5):556–563. doi: 10.1104/pp.50.5.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners D. J., Wilson G. Purification of malted-barley endo-beta-D-glucanases by ion-exchange chromatography: some properties of an endo-barley-beta-D-glucanase. Carbohydr Res. 1976 Jun;48(2):255–264. doi: 10.1016/s0008-6215(00)83221-8. [DOI] [PubMed] [Google Scholar]
- Masuda Y., Yamamoto R. Effect of auxin on beta-1, 3-glucanase activity in Avena coleoptile. Dev Growth Differ. 1970 Mar;11(4):287–296. doi: 10.1111/j.1440-169x.1970.00287.x. [DOI] [PubMed] [Google Scholar]
- Nevins D. J., Huber D. J., Yamamoto R., Loescher W. H. beta-d-Glucan of Avena Coleoptile Cell Walls. Plant Physiol. 1977 Oct;60(4):617–621. doi: 10.1104/pp.60.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Sugar composition of oat-coleoptile cell walls. Biochem J. 1963 Oct;89(1):144–150. doi: 10.1042/bj0890144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosizawa Z., Sato T., Schmid K. Hydrazinolysis of alpha-1-acid glycoprotein. Biochim Biophys Acta. 1966 Jun 29;121(2):417–420. doi: 10.1016/0304-4165(66)90134-6. [DOI] [PubMed] [Google Scholar]



