Abstract
Dendritic cells (DCs) are a heterogeneous population. Murine DCs consist of conventional DCs (cDCs) and plasmacytoid DCs (pDCs). In human, the analogous populations are myeloid DCs (mDCs) and pDCs. Though distinct in phenotypes and functions, studies have shown that these DC subsets may interact or ‘crosstalk’ during immune responses. For example, cDCs may facilitate pDC maturation, while pDCs may enhance antigen presentation of cDCs in certain pathogenic conditions or even take on a cDC phenotype themselves. The role of DCs in non-infectious uveitis has been studied primarily in the experimental autoimmune uveitis mouse model and to a more limited extent in patients. Recent evidence shows that the number, phenotype and function of DC subsets are altered in this disease. We provide an overview of selected recent developments of pDCs and cDCs/mDCs, with special attention to their interaction and the dual roles of DC subsets in non-infectious uveitis.
Keywords: Conventional dendritic cells, myeloid dendritic cells, plasmacytoid dendritic cells, non-infectious uveitis, experimental autoimmune uveitis
1. Introduction of Dendritic Cells and Non-infectious Uveitis
Dendritic cells (DCs) were discovered in the late 1970s by Ralph Steinman of Rockefeller University58, leading to his Nobel Prize in Physiology or Medicine in 201142. DCs are professional antigen-presenting cells (APCs) that connect the innate and adaptive immune responses with critical roles in immune tolerance and defense against pathogens59. Although DCs all share the ability to activate naïve T cells, they are a heterogeneous population in terms of their further phenotypic and functional characteristics57. Categorizations include: conventional (also known as classical or myeloid) DCs vs. plasmacytoid DCs; ‘steady-state’ DCs (present at all times) vs. ‘inflammatory’ DCs (develop in response to inflammation); anatomical location (e.g. lymphoid tissue ‘resident’ DCs vs. non-lymphoid tissue peripheral ‘migratory’ DCs).
The study of DC biology has elucidated these subsets in both mouse and human, identifying some degree of inter-species correspondence between subsets, but also important differences. Murine and human DCs are both comprised of two major subsets, the key distinction being between conventional (also known as classical or myeloid DCs) and plasmacytoid DCs (pDCs). There is some variation in the terms used in the literature, but for the purposes of this review we will use the terms conventional DC (cDCs) to describe the non-plasmacytoid DCs in mice and myeloid DC (mDCs) to describe the equivalent group in humans8; 18; 37. In both species pDCs are the more homogeneous group, being distinguished by a non-dendritic plasma cell-like morphology in their resting state and an ability to rapidly secrete type I interferons (IFNs) in response to viral infection. The cDC/mDC grouping contains a number of different subsets with a range of functions directed towards directing T cell responses31. Classification of DCs and their discrimination from other mononuclear phagocytes (monocytes and macrophages) is made more challenging by plasticity especially under inflammatory conditions, which means that surface phenotype is not always a reliable guide to ontogenic relationship.
Human non-infectious uveitis is a potentially blinding condition characterized by intraocular inflammation. There is considerable evidence that most non-infectious uveitis is autoimmune (or at least auto-inflammatory) in origin32. The immune dysregulation observed in patients can be modeled and interrogated in animal models of uveitis, notably with the immunization of uveitogenic antigens supplemented with complete Freund adjuvant (CFA) in experimental autoimmune uveitis (EAU)62. Such models provide evidence of loss of tolerance to important intra-ocular antigens, such as S-antigen and interphotoreceptor retinoid-binding proteins (IRBP), and enable elucidation of the immune processes leading to the generation of autoreactive CD4+ T cells and their pathological function within the eye11. Critically, EAU has also been successfully induced by intravenous injection of mature DC pulsed with uveitogenic antigens61.
Data in humans is less complete, but still compelling. There is histological evidence of T cell infiltrates at sites of inflammation in eyes with uveitis3, which is supported by flow cytometric studies of intraocular fluid samples from patients with active inflammation10; 44. With regard to the role of auto-antigens in human disease, De Smet noted lymphocyte stimulation responses to peptide determinants of retinal S antigen in a range of uveitis conditions, being most frequent in uveitis associated with Behcet disease or sarcoidosis12; 13. Our recent work has been directed towards determining the role of DCs in the pathogenesis of non-infectious uveitis [Ping, et al., Abstract in 2014 ARVO]. In this review, we discuss recent advances in the understanding of DC subsets in both mice and human and explore the implications of these recent findings to non-infectious uveitis.
2. Characteristics of Murine Dendritic Cell Subsets
As introduced earlier, murine DC subsets consist of two main populations: cDCs and pDCs. In terms of surface phenotype, cDCs are CD11c+ and pDCs are PDCA1+ 66 (Table I). Conventional DCs are found in lymphoid tissues including spleen, lymph nodes and bone marrow, but are also widely distributed amongst non-lymphoid tissues. The major cDC subsets are defined according to the presence of CD8α and CD11b. The CD8+ cDC subset is well-characterized lymphoid tissue based subset with important roles in cross-presentation of exogenous antigens to CD8+ T cells14, and IL-12 secretion. Interestingly transcriptome profiling identified that there is an equivalent subset in non-lymphoid tissues which is CD8− and is defined by the integrin CD103+ 43; the lymphoid CD8+ subset and the non-lymphoid CD103+ subset share a number of features including responsiveness to TLR3 stimulation and expression of the chemokine receptor XCR1.
Table 1.
Comparison of Human and Murine DC Subsets
| Subsets | Phenotypes | Function | References | |
|---|---|---|---|---|
| Human | pDCs | BDCA2 (CD303) BDCA4 (CD304) CD123 TLR7, 9 |
Type I interferon production in response to viral or self nucleic acids (via TLR7, 9) | 5; 6; 7; 48 |
| mDC1 | BDCA1 (CD1c) CD11c TLR1–8 |
Stimulate CD4+ T cells; Wide-range of TLR and lectins; Secrete IL-12, IL-8, IL-10, IL-23 and TNFα |
5; 6 | |
| mDC2 | BDCA3 (CD141) XCR1 |
Antigen cross presentation to CD8+ T cells | 5; 6 | |
| Mouse | pDCs | PDCA1 | Type I interferon production in response to viral or self nucleic acids (via TLR7, 9) | 15 |
| CD8+/CD103+ cDCs |
CD11c CD8α (lymphoid) or CD103 (tissue) XCR1 |
Antigen cross presentation to CD8+ T cells | 15 | |
| CD8− cDCs | CD11c CD11b |
Stimulate CD4+ T cells; Wide-range of TLR and lectins; Secrete IL-12, IL-8, IL-10, IL-23 and TNFα |
CD8+ cDCs in the lymphoid tissues and CD103+ in non-lymphoid tissues share XCR1, and the ability to cross-present antigen to CD8+ T cells. They are therefore grouped as the same subset here.
The CD11b+ (CD8−) subset is less well characterized. They are the most abundant cDC in lymphoid tissue and are also found in non-lymphoid tissues. This is a highly heterogeneous group. Further segregation has been attempted on the basis of surface markers such as CD4, but this has not been supported by transcriptome profiling26. CD11b+ DC differ functionally from CD8+ DC being more effective in inducing CD4+ T cell responses, and capable of producing IL-6 and IL-23, whilst being poor at crosspresentation and the production of IL-12.
Plasmacytoid DCs are found in the blood and periphery. The term ‘plasmacytoid’ refers to their appearance when resting of a non-dendritic plasma cell-like morphology. Their key function is the detection of virus by toll-like receptor (TLR) 7 or TLR9, with production of high levels of type I IFNs. They are characterized by being PDCA-1+, but also express DEC-205 and B220.
DCs may also arise from monocytes, both in vivo and in vitro. In vivo such monocyte-derived ‘inflammatory’ DCs (infDCs) arise secondary to infection or inflammation. In vitro they may be generated from bone marrow cells (bone marrow-derived DCs; BMDCs) under the stimulation of recombinant granulocyte macrophage-colony stimulating factor (GM-CSF)25; 36. A key function of infDCs is to produce large amounts of TNF-α and iNOS (so-called TNF-iNOS producing DCs or ‘Tip DCs’). They have a critical role in pathogen clearance, with an important influence in the appropriate polarization of a T cell response.
A challenge to the study of DC biology in the eye is the limitation that DC numbers in vivo are too low to isolate enough for performing the functional and mechanical studies. For this reason most functional studies in mouse and human have depended on the use of in vitro cultures of bone marrow/monocyte-derived DCs. Although we, among others, have found these model systems useful, the extent to which these in vitro BMDCs reflect cDCs and/or infDCs is not yet fully established. Gene expression profiles have been shown to differ significantly between cDCs (in which development is Flt3-ligand dependent) and BMDCs (in which development is GM-CSF dependent)67. Conversely, cDCs and BMDCs do share expression of the transcription factor Zbtb4652.
3. Characteristics of Human Dendritic Cell Subsets
As outlined earlier, there are shared features, but also important differences, between murine and human DC systems. Inter-species comparison based purely on surface phenotype of DC subsets is generally unhelpful, whereas more recent studies based on gene expression have been more rewarding. The key distinction of conventional DCs (hereafter referred to as myeloid DCs; mDCs) vs. plasmacytoid DCs is maintained with clear separation in both phenotype and function. Due mainly to the availability of tissue and other practical limitations, the study of DC subsets in humans has primarily been focused on peripheral blood. Indeed it was in human blood that pDCs were first identified.
As observed in the mouse, human DCs are relatively rare in the peripheral blood compared to other immune cells18. In blood there are two main populations of DCs: an mDC population, which is CD1c/BDCA-1+CD11chiCD123− (described as mDC1) and a pDC population, which is CD11c−CD123+BDCA-2/CD303+ 18; 57. There is also a second population of mDCs (mDC2), which are CD141/BDCA-3+CD11clo. All three subsets are negative for lineage 1 markers (Lin1−) and express HLA-DR (i.e. Lin1−HLADR+)18; 37; 70 (Table I). In humans CD11c is not restricted to DCs, with 90% of human monocytes expressing CD11c49. Gene expression studies and the study of rare genetic mutations affecting DC function in humans supported by the detailed functional characterization across DC subsets in both species, has helped establish the equivalence of DC subsets in mouse and human. Thus CD1c/BDCA-1+CD11chiCD123− mDC1 in the human are equivalent to CD11b+CD8− cDCs in the mouse; CD141/BDCA-3+CD11clo mDC2 are equivalent to CD8+ cDCs, with the chemokine receptor XCR1 being expressed by this subgroup in both species; and CD11c−CD123+BDCA-2/CD303+ pDCs being equivalent to the murine PDCA-1+ pDCs65.
In terms of function, the human subsets appear to behave similarly to their murine equivalents. pDCs secrete high levels of type I IFNs in response to viruses and other suitable stimuli; mDC1 and mDC2 are effective at presenting antigen and inducing CD4+ and CD8+ T cell responses, with mDC2 being particularly effective at cross-presentation of exogenous antigens to CD8+ T cells. These shared features support the idea that the study of murine DCs can support our understanding of human DC biology and related autoimmunity.
Even more than in the mouse, the concept of human ‘inflammatory’ DCs is controversial. In vitro it has long been established that human DCs can be derived from monocytes (MoDCs). These have been widely studied to inform human DC biology and have even been used as a tool for vaccine generation and cancer therapy6. Typically CD14+ monocytes from peripheral blood are cultured with recombinant GM-CSF and IL-4 in vitro for 5–7 days33; 55. Further ‘maturation’ may be induced through stimulation with appropriate TLR ligands and/or pro-inflammatory cytokines29. Although a number of ‘inflammatory’ DC phenotypes in humans have been identified in vivo, it is unclear whether these truly represent monocyte-derived DCs or whether they are a form of activated monocyte. Monocytes may become activated during infection or inflammation with enhanced expression of CD16 and reduced level of CD14. CD16+ monocytes are significant producers of inflammatory cytokines (such as TNF-α) and display both DC and macrophage-like characteristics including antigen processing and presentation. When noting these parallels between the generation of MoDCs in vitro and the activation of monocytes in vivo, it is tempting to extrapolate the development of these monocytes and to assume that they become inflammatory DCs in vivo. In human studies, however, ‘inflammatory DC’ subsets, such as the 6-sulfo LacNAc (slan)+ DC subset, have generally been indistinguishable from activated monocytes9. This is clearly an area requiring further study. Table 1 summarizes several studies of the phenotypic and functional analysis in DC subsets in mice and humans.
4. Imbalanced Dendritic Cell Subset Proportions in the Immune System
Conventional DCs and pDCs are fms-like tyrosine kinase 3 (Flt3) ligand-dependent. They are derived from a common DC precursor (CDP) that is a Lin− c-Kitlo CD115+ CX3CR1+ Flt3+ progenitor cell, which in turn is derived from a bipotent macrophage/DC precursor (MDP; Lin− c-Kithi CD115+ CX3CR1+ Flt3+)68,1,21. Studies show that Flt3 ligand is essential for the regulation of homeostatic DCs (cDCs and pDCs) development in mice spleen, where it maintains normal DC numbers by regulating cell division in the periphery66. Another study shows that repeated injections of Flt3 ligand result in an increase in the number of functional cDCs38. Although GM-CSF is commonly added to in vitro cultures, it does not appear to be essential for the development of any DC subsets in vivo, although it does have important effects on DC function, notably in cross-presentation51. Cytokines such as Flt-3 ligand not only affect the generation of DC subsets, but also influence the balance between DC subset proportions, and so may in turn impact on the pathogenesis of autoimmune diseases.
Another factor that may influence the balance of proportions between DC subsets is the conversion from one DC subset to another. Notably pDCs have been observed to convert into cDCs in response to certain stimuli. For example during viral infection, pDCs may convert to mDCs with enhanced antigen-presenting capacity and TLR upregulation71. Similarly pDCs also convert into cDCs when mice are injected with double-stranded RNA (poly I:C) and type I interferon63. Furthermore, a subpopulation of pDCs, CCR9− pDCs, differentiates into cDCs after they are adoptively transferred into peripheral lymphoid organs, lung, and intestine54; 56. Similarly, CCR9− pDCs have been shown to convert into CD11b+CD8α+ MHC class IIhi cDC-like cells under the stimulation of GM-CSF or soluble factors produced by intestinal epithelial cells. This conversion is supported by a switch from typical pDCs to CD8+ cDCs regulators, notably a downregulation of the E2-2 transcription factor and upregulation of ID2, PU.1 and Batf353. Therefore, the plasticity of pDCs in switching to fully functional cDCs can lead to alteration of the ratio between DC subsets in vivo.
5. Interaction of Dendritic Cell Subsets during Immune Responses
As explained, both murine and human DCs include two major subsets, and those subsets exhibit distinct functions. Interestingly, it appears that there is significant interrelationship between these subsets. Kuwajima et al. have shown that murine cDCs fail to produce IL-12 in the absence of pDCs in vivo, suggesting that normal function of cDCs is dependent on the presence of pDCs. Moreover, it appeared that cell-to-cell contact (CD40–CD40L) between cDCs and pDCs was required30. Similarly, in human immunodeficiency virus (HIV) infection, pDCs promote mDC function in a bystander fashion, while they produce more type I interferon in response to HIV19. This discovery has prompted further investigation into when and how cDCs/mDCs interact or crosstalk with pDCs. Furthermore Wang et al.35 found that antigen-specific CD8+ T cells are increased and antitumor responses are enhanced after mice are immunized with a mixture of activated pDCs and mDCs, compared to mice immunized with either mDCs or pDCs. The increased anti-tumor responses arise because of enhanced antigen presentation in mDCs, which is facilitated by pDCs. Consistent with in vivo studies, co-culturing of pDCs and mDCs in vitro leads to pDC maturation and enhanced mDC antigen presentation (but not cytokine production) in response to bacterial infections47. In summary, given these interactions, it is possible that the imbalance of DC subset proportions may significantly contribute to the immune responses seen in autoimmune diseases, such as uveitis.
6. Role of Dendritic Cell Subsets in Experimental Autoimmune Uveitis (EAU)
The critical role of DCs in non-infectious uveitis has been investigated in the murine model of uveitis (EAU). The immunological characteristics observed in EAU show distinct similarities to human disease states in which activation of CD4+ T cells is central17; 40. The central role of DCs in uveitis may be argued from their key role taking up and presenting antigens to CD4+ T cells, thereby activating them and inducing immune responses. In autoimmune non-infectious uveitis, uveitogenic antigen-specific CD4+ T cells are crucial effector cells that drive inflammation and tissue damage after they are activated by DCs; however, these rare antigen-specific CD4+ T cells must need to encounter their uveitogenic antigens presented by APCs to successfully initiate the uveitis. As specialized APCs, DCs exhibit abnormal function in human non-infectious uveitis and EAU model, although the pathogenic mechanisms of DCs in uveitis are not fully understood (see Figure).
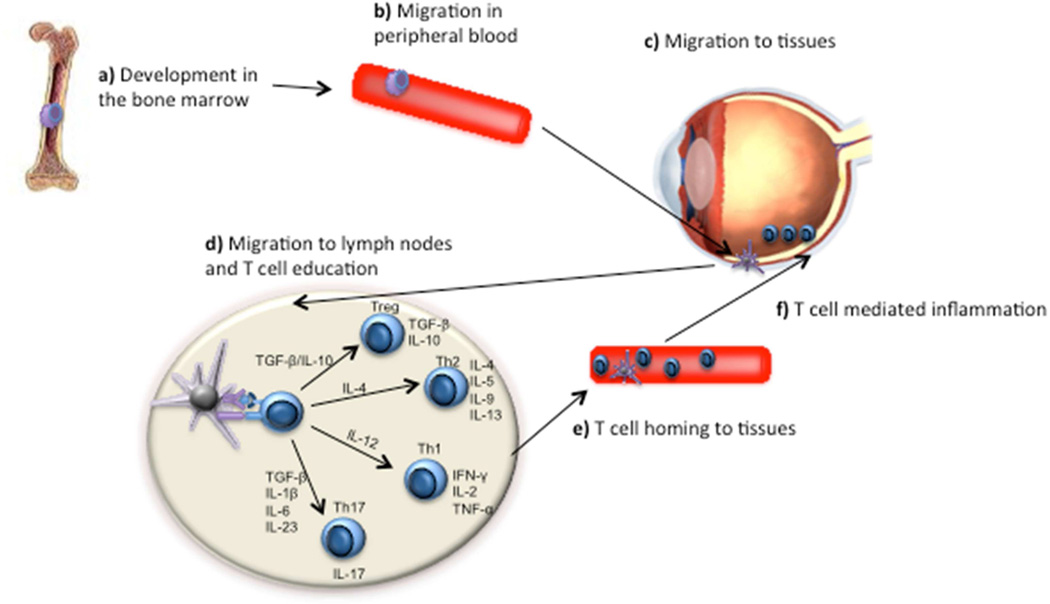
Figure 1. Role of DCs in Non-infectious Uveitis.
a) Precursors of DCs from bone marrow migrate into peripheral blood and become immature DCs in response to microenvironment challenge. b) Immature DCs circulate in the peripheral blood and eye under the attraction of chemokine or cytokines. c) Immature DCs sense and take up the antigens in the eye. d) Immature DCs become mature after antigen uptake, which express high MHC and co-stimulatory molecules. Mature DCs migrate into draining lymph nodes where they educate naïve T cells to be Th1, Th2, T regulatory cells and Th17 cells depending on signals. e) Educated T cells migrate into eyes to secrete cytokines, which cause non-infectious uveitis.
There is clear evidence that mDCs are present in the ocular tissues under both resting and inflammatory conditions. Choudhury et al. found functional mDCs in the choroid and thus may play a significant role in the inflammatory process during posterior uveitis7. McMenamin et al. have shown that antigen-presenting cells residing in the iris and ciliary body in normal rat eyes have access to ocular antigens on both sides of the blood-ocular barrier and thus are capable of activating circulating antigen-specific T cells41. Furthermore, Butler et al. suggested that DCs may act as local APCs in the induction of uveitis2. More specifically, Gregerson and his co-workers confirmed that cDCs are distinct from microglia. They are present in quiescent retinas, and they effectively respond to injured neurons. Interestingly cDCs from quiescent retinas promote generation of Foxp3+ T cells and inhibit T cell activation, whereas cDCs from injured retinas inhibit Foxp3+ T cell generation and stimulate T cell activation. Local delivery of exogenous BMDCs increases the incidence and severity of EAU, which is induced by the adoptive transfer of activated CD4+ or CD8+ T cells. Therefore, local conditions in the retina determine cDC function and affect the pathogenesis of EAU by both CD4+ and CD8+ T cells22. A key part of this mechanism may be a direct chemoattractant effect by IRBP and S-Ag. Howard et al. showed that these autoanitgens attract immature DCs and T cells expressing CXCR3 and CXCR5 to initiate innate and adaptive immune responses24.
In addition, the types of immune responses largely depend on the maturation status of DCs. Pretreatment with fixed immature DCs, but not fixed mature DCs, ameliorate EAU progression by inhibiting uveitogenic CD4+ T cell activation and differentiation45. Suzuki et al. suggested that impaired cDC maturation may be the underlying mechanism for the observed anti-inflammatory effect of aminoimidazole carboxamide ribonucleotide (AICAR)60. Impaired murine cDC maturation prevents the generation of Ag-specific Th1 and Th17 cells to attenuated EAU by the treatment of AICAR60. Further indirect evidence of the importance of DC maturation in the pathogenesis of uveitis may be inferred from the role of pertussis toxin assisting the induction of uveitis in mice. Pertussis toxin exerts an adjuvant role on cDC to promote cDC maturation and the production of proinflammatory cytokines, thereby eliciting a Th1 response in EAU23.
As discussed elsewhere ‘maturation’ is more complex and more plastic than a simple unidirectional linear progression, and thus there are a number of other DC states that should be considered50. For example, IL-10-conditioning BMDCs induce an ‘immature’ phenotype which can suppress EAU development and significantly reduce IRBP-specific T-cell proliferation and IFN-γ production, but increase IL-10 production64.
Liang et al. has also shown that a specific CD25+ splenic DC subset contributes to the development of EAU, with ablation of this DC subset resulting in decreased activation and expansion of γδ T cells, with decreased activation of IL-17+ IRBP-specific T cells and attenuated inflammation in the EAU34.
Taken together, data from the EAU model suggest that DCs have an important role in the induction or inhibition of inflammation. Now, a major challenge lies in extrapolating these data to human non-infectious uveitis.
7. Abnormalities of Dendritic Cell Subsets in Non-infectious Uveitis Patients
An important question that remains is whether perturbations in the number, frequency, or ratio of DC subsets can lead to the development of autoimmunity. Studying DCs in non-infectious uveitis patients is a challenge owing to the low number of DCs in the peripheral blood and eye tissues. Despite this, DC subsets have been successfully quantified in patients with non-infectious uveitis secondary to Sarcoidosis, Behcet diseases, Vogt-Koyanagi-Harada (VKH) syndrome, or birdshot chorioretinopathy. Through multicolor flow cytometry analysis, we have determined that non-infectious uveitis patients have an increased frequency and absolute number of CD1c+ mDC1 when compared to the levels in the peripheral blood of healthy controls (Ping et al., manuscript under review).
In addition to the alteration in DC numbers, DCs exhibit a more ‘mature’ phenotype in non-infectious uveitis patients. Studies show that the deficiencies of mDCs correlate with the clinical severity observed in sarcoidosis, but mDCs still exhibit the upregulated co-stimulatory and maturation markers (e.g., CD86, HLA-DR)39. Another study revealed that increased CD86 and HLA-DR expression on mDCs in uveitis patients are strongly correlated with inflammatory activity. Interestingly, the expression of these surface molecules is not completely downregulated to the baseline levels measured in healthy controls, even during uveitis remission28. We have observed that only HLA-DR, not co-stimulatory molecules such as CD80, on CD1c+ mDC1 is increased in the patient peripheral blood when compared with healthy controls (Ping et al., manuscript under review).
Looking within the eye, Chang et al. used cadaveric tissue to identify DC, based on noting dendritiform stromal cells, which were HLA-DR positive and CD68 negative (a macrophage marker). This study was of interest because it showed that these cells expressed TLR4 and the associated LPS receptor complex and were localized to the uvea, but not to other ocular tissues4. This may be particularly relevant to those forms of uveitis that have a proposed association with gram negative bacteria such as HLA-B27 associated anterior uveitis5. Denniston et al. reported that mDCs isolated from human uveitic aqueous humor (AqH) were characterized by elevated MHC I and II, but reduced CD86 compared with matched peripheral blood mDCs16.
Unlike mDCs, there exists no consistent trend among different studies when comparing pDC frequency and function in non-infectious uveitis. Pay et al. reported a higher frequency of IFNα+ pDCs in Behcet disease patients with higher sensitivity of these cells to CpG D ODN stimulus, which they inferred contributed to high serum IFN-α levels found in these patients promoting a Th1 type immune response46. Plskova et al. studied a series of patients with severe, refractory sight-threatening uveitis pre- and post-treatment with IFN-α. They noted no significant difference between patients and control subjects in the number of pDCs, but there was a significant decrease in the capability of patients' pDCs to produce IFN-α in response to CpG and an alteration in the T cell phenotype with lower activation markers and increased intracellular T-cell IL-10 levels48. Data from our laboratory show that pDC frequency is significantly decreased in all types of non-infectious uveitis patients regardless of disease status when comparing to that in healthy controls and that this decreased pDC frequency is highly correlated with decreased regulatory T (Treg) cells (unpublished data). This observation supports the suggestion of Plskova that pDC may help the generation of Treg cells48. This hypothesis is also supported by observations in rheumatoid arthritis, where Kavousanaki et al. noted that pDC from inactive RA (but not active RA) expressed high levels of indoleamine 2,3-dioxygenase and promoted the differentiation of allogeneic naive CD4+CD25− T cells into IL-10-secreting Treg cells that showed poor proliferation in vitro27. While for active disease they noted reduced pDC levels (similar to our observations in uveitis), in treatment-induced remission they found elevated pDC levels (in contrast to our observations). The role of pDCs in human autoimmune disease requires further elucidation.
From practical limitations relating to the numbers of mDCs and pDCs available, the limited numbers of functional or mechanistic studies conducted in human uveitis have depended on in vitro generation of MoDCs. Frassanito et al. observed that when MoDCs are generated from the peripheral blood of patients with non-infectious uveitis, these cells display an enhanced ability to stimulate cell proliferation of allogeneic T cells in mixed lymphocyte reactions (MLR) and higher levels of IL-12 production compared to healthy controls or compared to uveitis patients who had received systemic treatment20. More recently, Yang et al. demonstrated the immunosuppressive effects of the plant extract Berberine on MoDCs from patients with active VKH69. Similarly, Denniston et al. used human MoDCs as a model to test the effects of non-inflammatory and inflammatory AqH supernatant, noting that exposure of MoDCs from healthy controls to uveitic AqH from patients with active inflammation induced upregulation of MHC I and II and reduced CD86. Interestingly these changes were characteristic of the mDCs that had been directly isolated from these uveitis patients, providing evidence for the potential role of the ocular microenvironment in DC regulation, maturation and function15; 16.
In light of our increased understanding of DC biology, and our own observations of alterations in DC subsets in human uveitis (notably an increase in mDCs and a reduction in pDCs), we hypothesize that these DC alterations are at least partially responsible for the biased CD4+ T cell immune responses observed in human non-infectious uveitis (increased Th1 and decreased Treg cells).
8. Conclusions
The investigation of DCs is complicated by the fact that they account for a relatively small proportion in the immune system. An even bigger challenge persists is the translation and application of the findings from animal models such as EAU into more complex human non-infectious uveitis. There is, however, accumulating evidence that indicates that DCs may have either a protective or pathogenic role in the uveitis, depending on their maturation status and local microenvironment.
We hypothesize that, while most studies of DCs in uveitis emphasize the impact of abnormalities in an individual DC subset, important pathogenic effects may arise from imbalances in DC subset proportions and their mutual interaction. Even as DC-based therapies for cancer and vaccines continue to progress, we need to address these more fundamental questions regarding the role of DCs in the pathogenesis of uveitis. Therefore, we argue that an equivalent depth of understanding on the function of individual DC subsets as well as their interaction in autoimmunity will be a necessary and potentially powerful tool for preventing, controlling, and effectively treating human non-infectious uveitis.
Method of Literature Search
The literature for this review was searched from PubMed, using the key words of uveitis, dendritic cells, plasmacytoid dendritic cells, classical dendritic cells and myeloid dendritic cells. The date range was from 1947 and 2014. Non-English manuscripts were included where a screen of the abstract identified it as being relevant.
Acknowledgements
The research was supported by the Intramural Research Program of the National Institute of Eye, NIH. We thank Drs. Igal Gery, William Tucker, and Carlos Blades for critical reading the manuscript.
Abbreviations
- APCs
Antigen presenting cells
- AqH
Aqueous humor
- BDCA
Blood Dendritic Cell Antigen
- BMDCs
Bone marrow-derived dendritic cells
- CFA
Complete Freund adjuvant
- cDCs
Conventional dendritic cells
- DCs
Dendritic cells
- EAU
Experimental Autoimmune Uveitis
- GM-CSF
Granulocyte macrophage-colony stimulating factor
- HIV
Human immunodeficiency virus
- IFN
Interferon
- IRBP
Interphotoreceptor retinoid-binding proteins
- MHC
Major histocompatibility complex
- MLR
Mixed lymphocyte reactions
- mDCs
Myeloid dendritic cells
- MoDCs
Monocyte-derived dendritic cells
- pDCs
Plasmacytoid dendritic cells
- Treg
Regulatory T cells
- Th1
T helper cell 1
- TLR
Toll-like receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
References
- 1.Auffray C, Emre Y, Geissmann F. Homeostasis of dendritic cell pool in lymphoid organs. Nat Immunol. 2008;9:584–586. doi: 10.1038/ni0608-584. [DOI] [PubMed] [Google Scholar]
- 2.Butler TL, McMenamin PG. Resident and infiltrating immune cells in the uveal tract in the early and late stages of experimental autoimmune uveoretinitis. Invest Ophthalmol Vis Sci. 1996;37:2195–2210. [PubMed] [Google Scholar]
- 3.Chan CC, Benezra D, Rodrigues MM, et al. Immunohistochemistry and electron microscopy of choroidal infiltrates and Dalen-Fuchs nodules in sympathetic ophthalmia. Ophthalmology. 1985;92:580–590. doi: 10.1016/s0161-6420(85)34006-x. [DOI] [PubMed] [Google Scholar]
- 4.Chang JH, McCluskey P, Wakefield D. Expression of toll-like receptor 4 and its associated lipopolysaccharide receptor complex by resident antigen-presenting cells in the human uvea. Invest Ophthalmol Vis Sci. 2004;45:1871–1878. doi: 10.1167/iovs.03-1113. [DOI] [PubMed] [Google Scholar]
- 5.Chang JH, McCluskey PJ, Wakefield D. Toll-like receptors in ocular immunity and the immunopathogenesis of inflammatory eye disease. Br J Ophthalmol. 2006;90:103–108. doi: 10.1136/bjo.2005.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang CL, Kandalaft LE, Tanyi J, et al. A dendritic cell vaccine pulsed with autologous hypochlorous acid-oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clin Cancer Res. 2013;19:4801–4815. doi: 10.1158/1078-0432.CCR-13-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhury A, Pakalnis VA, Bowers WE. Characterization and functional activity of dendritic cells from rat choroid. Exp Eye Res. 1994;59:297–304. doi: 10.1006/exer.1994.1111. [DOI] [PubMed] [Google Scholar]
- 8.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 9.Cros J, Cagnard N, Woollard K, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curnow SJ, Scheel-Toellner D, Jenkinson W, et al. Inhibition of T cell apoptosis in the aqueous humor of patients with uveitis by IL-6/soluble IL-6 receptor trans-signaling. J Immunol. 2004;173:5290–5297. doi: 10.4049/jimmunol.173.8.5290. [DOI] [PubMed] [Google Scholar]
- 11.Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–221. doi: 10.1111/j.1749-6632.2009.05133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Smet MD, Yamamoto JH, Mochizuki M, et al. Cellular immune responses of patients with uveitis to retinal antigens and their fragments. Am J Ophthalmol. 1990;110:135–142. doi: 10.1016/s0002-9394(14)76981-8. [DOI] [PubMed] [Google Scholar]
- 13.de Smet MD, Bitar G, Mainigi S, Nussenblatt RB. Human S-antigen determinant recognition in uveitis. Invest Ophthalmol Vis Sci. 2001;42:3233–3238. [PubMed] [Google Scholar]
- 14.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–1696. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Denniston AK, Kottoor SH, Khan I, et al. Endogenous cortisol and TGF-beta in human aqueous humor contribute to ocular immune privilege by regulating dendritic cell function. J Immunol. 2011;186:305–311. doi: 10.4049/jimmunol.1001450. [DOI] [PubMed] [Google Scholar]
- 16.Denniston AK, Tomlins P, Williams GP, et al. Aqueous humor suppression of dendritic cell function helps maintain immune regulation in the eye during human uveitis. Invest Ophthalmol Vis Sci. 2012;53:888–896. doi: 10.1167/iovs.11-8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dick AD. Retinal antigen-specific T cells mediate experimental autoimmune uveoretinitis (EAU) in PVG rat a model for tracking antigen-specific CD4(+) T cells in the inflamed eye. Ocul Immunol Inflamm. 1995;3:261–270. doi: 10.3109/09273949509069120. [DOI] [PubMed] [Google Scholar]
- 18.Dzionek A, Fuchs A, Schmidt P, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 19.Fonteneau JF, Larsson M, Beignon AS, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frassanito MA, Dammacco R, Fusaro T, et al. Combined cyclosporin-A/prednisone therapy of patients with active uveitis suppresses IFN-gamma production and the function of dendritic cells. Clin Exp Immunol. 2003;133:233–239. doi: 10.1046/j.1365-2249.2003.02214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilliet M, Boonstra A, Paturel C, et al. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heuss ND, Lehmann U, Norbury CC, et al. Local activation of dendritic cells alters the pathogenesis of autoimmune disease in the retina. J Immunol. 2012;188:1191–1200. doi: 10.4049/jimmunol.1101621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hou W, Wu Y, Sun S, et al. Pertussis toxin enhances Th1 responses by stimulation of dendritic cells. J Immunol. 2003;170:1728–1736. doi: 10.4049/jimmunol.170.4.1728. [DOI] [PubMed] [Google Scholar]
- 24.Howard OM, Dong HF, Su SB, et al. Autoantigens signal through chemokine receptors: uveitis antigens induce CXCR3- and CXCR5-expressing lymphocytes and immature dendritic cells to migrate. Blood. 2005;105:4207–4214. doi: 10.1182/blood-2004-07-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaitin DA, Kenigsberg E, Keren-Shaul H, et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 2014;343:776–779. doi: 10.1126/science.1247651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kavousanaki M, Makrigiannakis A, Boumpas D, Verginis P. Novel role of plasmacytoid dendritic cells in humans: induction of interleukin-10-producing Treg cells by plasmacytoid dendritic cells in patients with rheumatoid arthritis responding to therapy. Arthritis Rheum. 2010;62:53–63. doi: 10.1002/art.25037. [DOI] [PubMed] [Google Scholar]
- 28.Kim TW, Kang JS, Kong JM, et al. Maturation profiles of peripheral blood dendritic cells in patients with endogenous uveitis. Immunol Lett. 2012;142:14–19. doi: 10.1016/j.imlet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Krutzik SR, Tan B, Li H, et al. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuwajima S, Sato T, Ishida K, et al. Interleukin 15-dependent crosstalk between conventional and plasmacytoid dendritic cells is essential for CpG-induced immune activation. Nat Immunol. 2006;7:740–746. doi: 10.1038/ni1348. [DOI] [PubMed] [Google Scholar]
- 31.Lanzavecchia A, Sallusto F. Regulation of T cell immunity by dendritic cells. Cell. 2001;106:263–266. doi: 10.1016/s0092-8674(01)00455-x. [DOI] [PubMed] [Google Scholar]
- 32.Larson T, Nussenblatt RB, Sen HN. Emerging drugs for uveitis. Expert Opin Emerg Drugs. 2011;16:309–322. doi: 10.1517/14728214.2011.537824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells. Semin Immunol. 2005;17:313–318. doi: 10.1016/j.smim.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Liang D, Zuo A, Shao H, et al. Role of CD25+ dendritic cells in the generation of Th17 autoreactive T cells in autoimmune experimental uveitis. J Immunol. 2012;188:5785–5791. doi: 10.4049/jimmunol.1200109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lou Y, Liu C, Kim GJ, et al. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol. 2007;178:1534–1541. doi: 10.4049/jimmunol.178.3.1534. [DOI] [PubMed] [Google Scholar]
- 36.Lutz MB, Kukutsch N, Ogilvie AL, et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald KP, Munster DJ, Clark GJ, et al. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 38.Maraskovsky E, Brasel K, Teepe M, et al. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathew S, Bauer KL, Fischoeder A, et al. The anergic state in sarcoidosis is associated with diminished dendritic cell function. J Immunol. 2008;181:746–755. doi: 10.4049/jimmunol.181.1.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattapallil MJ, Silver PB, Mattapallil JJ, et al. Uveitis-associated epitopes of retinal antigens are pathogenic in the humanized mouse model of uveitis and identify autoaggressive T cells. J Immunol. 2011;187:1977–1985. doi: 10.4049/jimmunol.1101247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McMenamin PG, Crewe J, Kijlstra A. Resident and infiltrating cells in the rat iris during the early stages of experimental melanin protein-induced uveitis (EMIU) Ocul Immunol Inflamm. 1997;5:223–233. doi: 10.3109/09273949709085063. [DOI] [PubMed] [Google Scholar]
- 42.Mellman I, Nussenzweig M. Retrospective. Ralph M. Steinman (1943–2011) Science. 2011;334:466. doi: 10.1126/science.1215136. [DOI] [PubMed] [Google Scholar]
- 43.Miller JC, Brown BD, Shay T, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muhaya M, Calder VL, Towler HM, et al. Characterization of phenotype and cytokine profiles of T cell lines derived from vitreous humour in ocular inflammation in man. Clin Exp Immunol. 1999;116:410–414. doi: 10.1046/j.1365-2249.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oh K, Kim YS, Lee DS. Maturation-resistant dendritic cells ameliorate experimental autoimmune uveoretinitis. Immune Netw. 2011;11:399–405. doi: 10.4110/in.2011.11.6.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pay S, Pekel A, Simsek I, et al. Pronounced interferon-alpha production from plasmacytoid dendritic cells in patients with Behcet's disease following CpG D ODN stimulation. Clin Exp Rheumatol. 2009;27:S37–S42. [PubMed] [Google Scholar]
- 47.Piccioli D, Sammicheli C, Tavarini S, et al. Human plasmacytoid dendritic cells are unresponsive to bacterial stimulation and require a novel type of cooperation with myeloid dendritic cells for maturation. Blood. 2009;113:4232–4239. doi: 10.1182/blood-2008-10-186890. [DOI] [PubMed] [Google Scholar]
- 48.Plskova J, Greiner K, Muckersie E, et al. Interferon-alpha: a key factor in autoimmune disease? Invest Ophthalmol Vis Sci. 2006;47:3946–3950. doi: 10.1167/iovs.06-0058. [DOI] [PubMed] [Google Scholar]
- 49.Randolph GJ, Inaba K, Robbiani DF, et al. Differentiation of phagocytic monocytes into lymph node dendritic cells in vivo. Immunity. 1999;11:753–761. doi: 10.1016/s1074-7613(00)80149-1. [DOI] [PubMed] [Google Scholar]
- 50.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 51.Sathe P, Pooley J, Vremec D, et al. The acquisition of antigen cross-presentation function by newly formed dendritic cells. J Immunol. 2011;186:5184–5192. doi: 10.4049/jimmunol.1002683. [DOI] [PubMed] [Google Scholar]
- 52.Satpathy AT, Kc W, Albring JC, et al. Zbtb46 expression distinguishes classical dendritic cells and their committed progenitors from other immune lineages. J Exp Med. 2012;209:1135–1152. doi: 10.1084/jem.20120030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlitzer A, Loschko J, Mair K, et al. Identification of CCR9- murine plasmacytoid DC precursors with plasticity to differentiate into conventional DCs. Blood. 2011;117:6562–6570. doi: 10.1182/blood-2010-12-326678. [DOI] [PubMed] [Google Scholar]
- 54.Schlitzer A, Heiseke AF, Einwachter H, et al. Tissue-specific differentiation of a circulating CCR9- pDC-like common dendritic cell precursor. Blood. 2012;119:6063–6071. doi: 10.1182/blood-2012-03-418400. [DOI] [PubMed] [Google Scholar]
- 55.Schreibelt G, Klinkenberg LJ, Cruz LJ, et al. The C-type lectin receptor CLEC9A mediates antigen uptake and (cross-)presentation by human blood BDCA3+ myeloid dendritic cells. Blood. 2012;119:2284–2292. doi: 10.1182/blood-2011-08-373944. [DOI] [PubMed] [Google Scholar]
- 56.Segura E, Wong J, Villadangos JA. Cutting edge: B220+CCR9− dendritic cells are not plasmacytoid dendritic cells but are precursors of conventional dendritic cells. J Immunol. 2009;183:1514–1517. doi: 10.4049/jimmunol.0901524. [DOI] [PubMed] [Google Scholar]
- 57.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 58.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki J, Yoshimura T, Simeonova M, et al. Aminoimidazole carboxamide ribonucleotide ameliorates experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53:4158–4169. doi: 10.1167/iovs.11-9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang J, Zhu W, Silver PB, et al. Autoimmune uveitis elicited with antigen-pulsed dendritic cells has a distinct clinical signature and is driven by unique effector mechanisms: initial encounter with autoantigen defines disease phenotype. J Immunol. 2007;178:5578–5587. doi: 10.4049/jimmunol.178.9.5578. [DOI] [PubMed] [Google Scholar]
- 62.Tang J, Zhou R, Luger D, et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol. 2009;182:4624–4632. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toma-Hirano M, Namiki S, Miyatake S, et al. Type I interferon regulates pDC maturation and Ly49Q expression. Eur J Immunol. 2007;37:2707–2714. doi: 10.1002/eji.200737173. [DOI] [PubMed] [Google Scholar]
- 64.Usui Y, Takeuchi M, Hattori T, et al. Suppression of experimental autoimmune uveoretinitis by regulatory dendritic cells in mice. Arch Ophthalmol. 2009;127:514–519. doi: 10.1001/archophthalmol.2009.34. [DOI] [PubMed] [Google Scholar]
- 65.Villadangos JA, Shortman K. Found in translation: the human equivalent of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1131–1134. doi: 10.1084/jem.20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Waskow C, Liu K, Darrasse-Jeze G, et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu Y, Zhan Y, Lew AM, et al. Differential development of murine dendritic cells by GM-CSF versus Flt3 ligand has implications for inflammation and trafficking. J Immunol. 2007;179:7577–7584. doi: 10.4049/jimmunol.179.11.7577. [DOI] [PubMed] [Google Scholar]
- 68.Yang GX, Lian ZX, Kikuchi K, et al. Plasmacytoid dendritic cells of different origins have distinct characteristics and function: studies of lymphoid progenitors versus myeloid progenitors. J Immunol. 2005;175:7281–7287. doi: 10.4049/jimmunol.175.11.7281. [DOI] [PubMed] [Google Scholar]
- 69.Yang Y, Qi J, Wang Q, et al. Berberine suppresses Th17 and dendritic cell responses. Invest Ophthalmol Vis Sci. 2013;54:2516–2522. doi: 10.1167/iovs.12-11217. [DOI] [PubMed] [Google Scholar]
- 70.Ziegler-Heitbrock L, Ancuta P, Crowe S, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 71.Zuniga EI, McGavern DB, Pruneda-Paz JL, et al. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat Immunol. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]