Abstract
Light and dark 14CO2 assimilation, pulse-chase (14CO2 followed by 12CO2) labeling experiments both in the light and in the dark, photorespiratory activity and some enzymes (ribulose 1,5-bisphosphate (RuBP) carboxylase, phosphoenolpyruvate (PEP) carboxylase, and NADP-malic enzyme) were followed in sections of 2.5 centimeters from the base (younger tissue) to the tip (oldest tissue) of the green maize leaf. Tissue was taken from the third leaf of 12- to 16-day-old plants consisting of sections 0 to 2.5 centimeters (base), 4.5 to 7.0 centimeters (center) and 9.0 to 11.5 centimeters (top) measured from the base. Some of these properties were also determined in the intact leaves of 4-day-old maize plants.
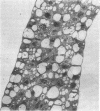
Electron microscopy indicated a Kranz anatomy in all sections. Differentiation into mesophyll granal chloroplasts and bundle sheath agranal chloroplasts had taken place only in the center and top pieces.
All of the sections contained PEP carboxylase, RuBP carboxylase, and NADP-malic enzyme. The ratio of PEP:RuBP carboxylase increased from 3.03 (top) to 4.66 (base) whereas the PEP carboxylase:NADP-malic enzyme ratio rose from 2.87 (top) to 9.57 (base).
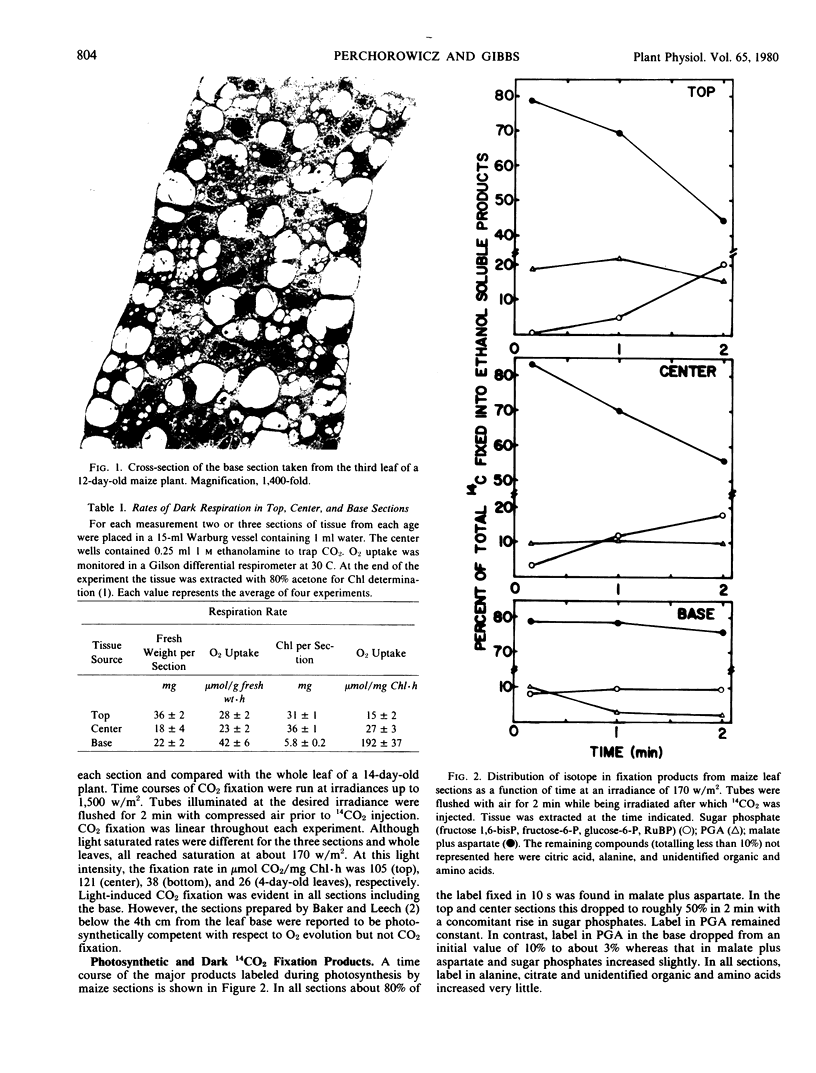
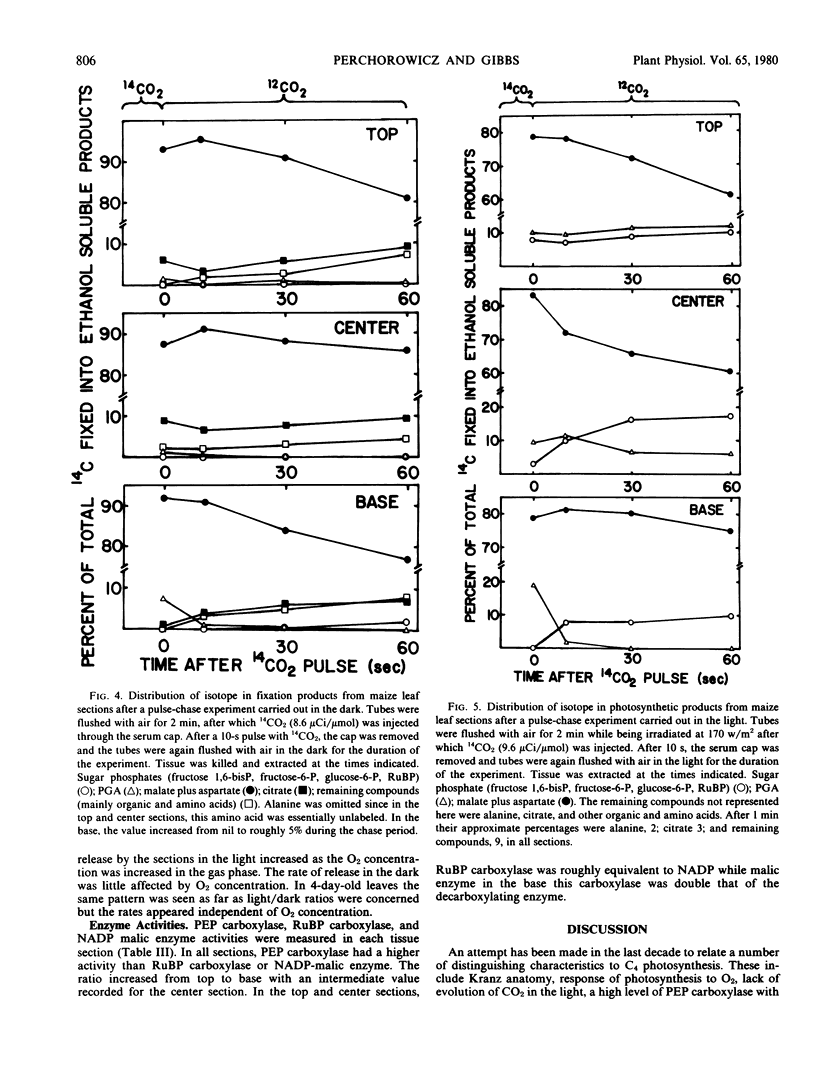
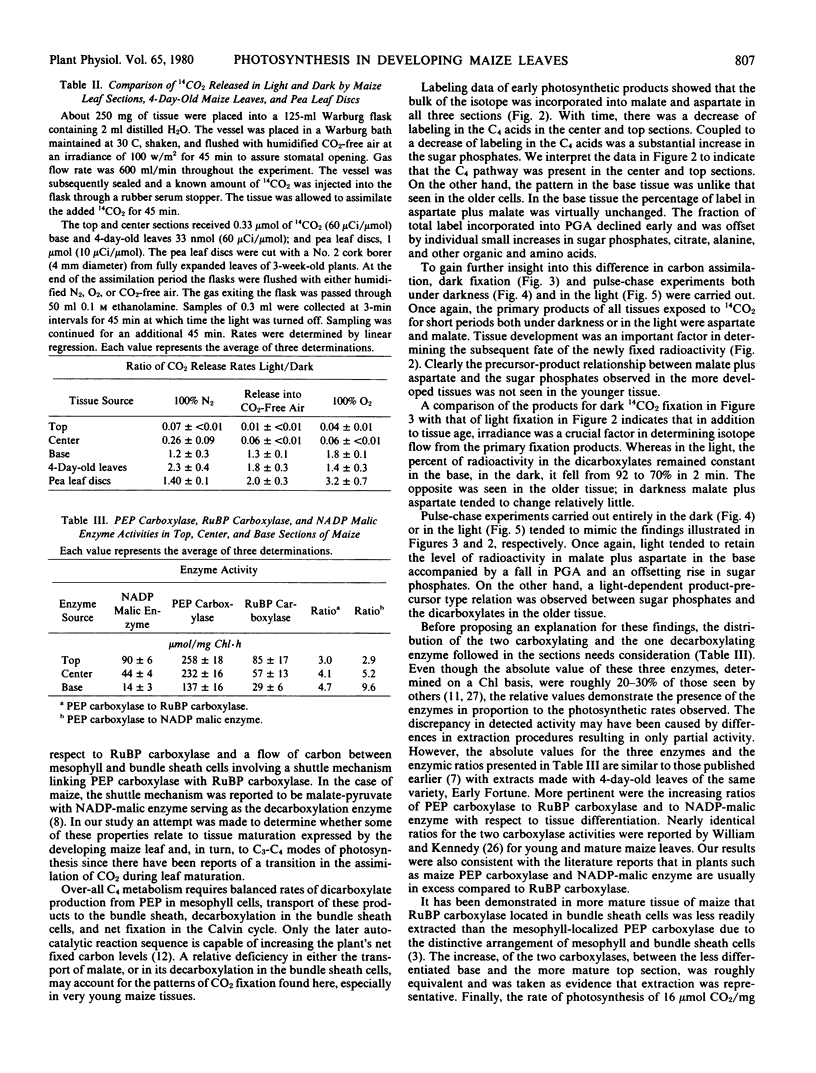
Under conditions of light or dark, the majority of the newly incorporated 14CO2 was found in malate and aspartate in all sections and in 4-day-old leaves. The 14C-labeling pattern typical of C4 plants was present in the center and top sections and to a lesser extent in the 4-day-old leaves. In the base tissue, the percentage of radioactivity in malate and aspartate remained relatively constant both during photosynthesis and pulse-chase experiments. In contrast, radioactivity in glycerate-3-phosphate decreased with time coupled to an increase in sugar phosphates. To account for the isotopic pattern in the base tissue, parallel fixation by PEP carboxylase and RuBP carboxylase was proposed with the photosynthetic carbon reduction cycle functioning to some extent independently within the bundle sheath chloroplasts. The apparent lack of cooperation between the mesophyll and bundle sheath cells may have been due to inadequate levels of NADP-malic enzyme required for shuttling carbon as CO2 from the PEP carboxylase products to the Calvin cycle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N. R., Leech R. M. Development of Photosystem I and Photosystem II Activities in Leaves of Light-grown Maize (Zea mays). Plant Physiol. 1977 Oct;60(4):640–644. doi: 10.1104/pp.60.4.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROWLEY G. J., MOSES V., ULLRICH J. A VERSATILE SOLVENT TO REPLACE PHENOL FOR THE PAPER CHROMATOGRAPHY OF RADIOACTIVE INTERMEDIARY METABOLITES. J Chromatogr. 1963 Oct;12:219–228. doi: 10.1016/s0021-9673(01)83673-6. [DOI] [PubMed] [Google Scholar]
- Chollet R. Evaluation of the light/dark C assay of photorespiration: tobacco leaf disk studies with glycidate and glyoxylate. Plant Physiol. 1978 Jun;61(6):929–932. doi: 10.1104/pp.61.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs M., Latzko E., O'Neal D., Hew C. S. Photosynthetic carbon fixation by isolated maize chloroplasts. Biochem Biophys Res Commun. 1970 Sep 30;40(6):1356–1361. doi: 10.1016/0006-291x(70)90015-x. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy R. A. Photorespiration in c(3) and c(4) plant tissue cultures: significance of kranz anatomy to low photorespiration in c(4) plants. Plant Physiol. 1976 Oct;58(4):573–575. doi: 10.1104/pp.58.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R. M., Rumsby M. G., Thomson W. W. Plastid differentiation, acyl lipid, and Fatty Acid changes in developing green maize leaves. Plant Physiol. 1973 Sep;52(3):240–245. doi: 10.1104/pp.52.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neal D., Hew C. S., Latzko E., Gibbs M. Photosynthetic carbon metabolism of isolated corn chloroplasts. Plant Physiol. 1972 Apr;49(4):607–614. doi: 10.1104/pp.49.4.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C. K., Edwards G. E. Intracellular localization of certain photosynthetic enzymes in bundle sheath cells of plants possessing the C4 pathway of photosynthesis. Arch Biochem Biophys. 1975 Nov;171(1):214–225. doi: 10.1016/0003-9861(75)90026-0. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D., Goodchild D. J. Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 Sep;114(3):489–498. doi: 10.1042/bj1140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Zelitch I. Investigation on photorespiration with a sensitive C-assay. Plant Physiol. 1968 Nov;43(11):1829–1837. doi: 10.1104/pp.43.11.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]