Summary
Two patients with metabolic disorders presented with clinical and radiologic features suggestive of sporadic Creutzfeldt-Jakob disease (sCJD). Case 1 was a 50-year-old man with rapid decline in cognitive, behavioral, and motor function following new-onset seizures. MRI was read as consistent with CJD, and he was referred for a treatment trial, but it was determined that he recently experienced rapid correction of hyponatremia resulting in extrapontine myelinolysis. Case 2 was a 66-year-old woman with poorly controlled diabetes mellitus who was found unconscious after a suspected insulin overdose. Examination showed altered mental status and neuroimaging was remarkable for cortical/striatal hyperintensities suggestive of sCJD. On autopsy, she had hypoglycemic/hypoxic nerve cell loss. Although characteristic MRI findings have high sensitivity and specificity for sCJD, potentially reversible metabolic disorders sometimes present rapidly and can resemble sCJD both clinically and radiologically. These cases highlight the importance of establishing a broad differential diagnosis when evaluating a patient with suspected sCJD.
Sporadic Creutzfeldt-Jakob disease (sCJD) is a fatal neurodegenerative prion disease usually presenting as a rapidly progressive dementia (RPD) with cognitive, behavioral, and/or motor dysfunction. Potentially treatable autoimmune and metabolic processes may mimic sCJD both clinically and radiologically.1,2 We have noted in our CJD clinical research program that as many as one-third of cases referred for suspected sCJD have a nonprion diagnosis.3,4 Other prion referral centers have also found many misdiagnoses among suspected CJD cases.2
We discuss 2 patients initially diagnosed with sCJD based on clinical presentation and MRI findings who were later determined to have potentially reversible metabolic causes for their conditions. One patient had extrapontine myelinolysis following rapid sodium correction for hyponatremia and the second had encephalopathy secondary to insulin-induced hypoglycemia and seizure.
Case 1
A 50-year-old right-handed man with a history of chronic alcoholism, hypertension, hypercholesterolemia, and hepatitis C presented with nausea, vomiting, and diarrhea over a 3-day period. His medications included lisinopril, prednisone, rosuvastatin, metroprolol extended release, and hydrocodone. He was diagnosed with the flu by his primary care physician and decreased his alcohol consumption while increasing his water intake to maintain hydration. One week later, he had 2 generalized tonic-clonic seizures and was admitted to a local intensive care unit with encephalopathy and hyponatremia. A brain CT scan was unremarkable. He was encephalopathic for several weeks but was eventually discharged home. He had episodic memory loss, slurred and halting speech, executive dysfunction, gait imbalance, and myoclonus of the hands and trunk. Behavioral symptoms included emotional blunting, violent outbursts, delusions, and hallucinations, resulting in 3 psychiatric admissions over the next few months. Approximately 2 months after symptom onset, his first brain MRI showed T2 fluid-attenuated inversion recovery (FLAIR) and diffusion-weighted imaging (DWI) bilateral striatal hyperintensities with corresponding hypointensity on apparent diffusion coefficient (ADC) map, suggesting restricted diffusion and T1 hyperintensities in bilateral globus pallidi (figure 1, A–D). Based on his symptoms and the brain MRI, he was referred to our CJD treatment trial with a diagnosis of sCJD.
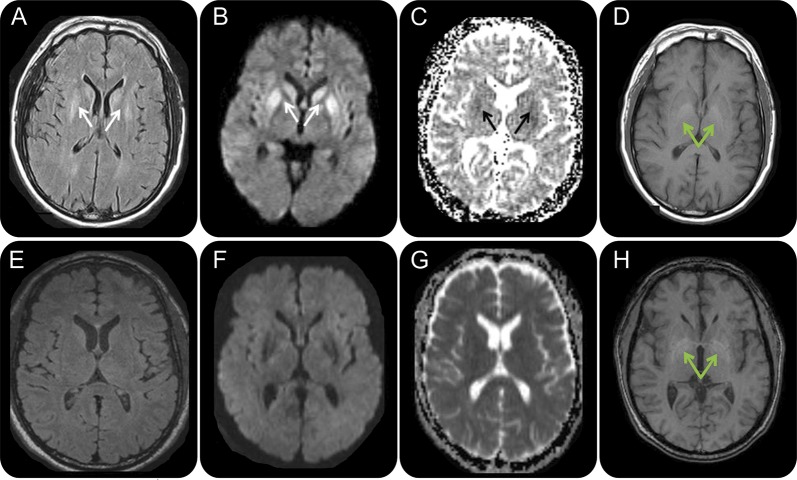
Figure 1. Serial brain MRIs in case 1, a 50-year-old man with extrapontine myelinolysis.
FLAIR (A, E), DWI (B, F), ADC map (C, G), and T1 (D, H) sequences, in radiologic orientation, 2 months (A–D) and 3 months (E–H) after onset. Initial MRI 2 months after onset (A–D) shows symmetric bilateral striatal FLAIR/DWI hyperintensities (A, B; solid white arrows) with corresponding hypointensities on the ADC map suggesting restricted diffusion (C; solid black arrows). Bilateral globus pallidus hyperintensities were present on T1-weighted imaging (D; green arrows). MRI 1 month later (E–H), 3 months after onset, shows resolution of the prior FLAIR, DWI, and ADC map abnormalities but no change in the globus pallidus T1 hyperintensities (H; green arrows). ADC = apparent diffusion coefficient, DWI = diffusion-weighted imaging; FLAIR = fluid-attenuated inversion recovery.
At our center, 3 months after initial onset and presentation, surprisingly he had improved remarkably, demonstrating intact alertness, language, and gait but with questionable simultagnosia, mild dysarthria, and slowed and irregular finger taps on neurologic examination. Cognitive evaluation revealed 28/30 on the Mini-Mental State Examination (MMSE) (missing points for place and county), diminished phonemic verbal fluency (10 d words in 1 minute), normal semantic fluency (21 animals with 2 repetitions in 1 minute), slowed processing speed, and working memory deficits. He repeated 6 digits forward and 4 digits backward, and on the Symbol Digit Modalities test he scored 35 correct in 90 seconds, both of which were below expectation for his age and education. On the Alzheimer's Disease Assessment Scale–Cognition (ADAS-Cog), he showed slight impairment on memory and visuospatial tasks with mildly slowed processing speed.
At our center, a thorough laboratory evaluation for causes of RPD4–6 was unrevealing. This included erythrocyte sedimentation rate, antinuclear antibody, anti-neutrophil cytoplasmic antibodies (ANCA), rheumatoid factor (RF), paraneoplastic and related antibodies (CV2/CRMP-5, ACh receptor, PCA-Tr, ANNA-3, PCA-2, anti-Hu [ANNA-1], and amphiphysin), anti–voltage-gated potassium channel complex [VGKCc] antibody, anti-thyroperoxidase antibody, anti-thyroglobulin antibody (TG), anti-gliadin antibodies, anti-tissue transglutaminase (TTG) antibodies, Lyme antibody, rapid plasma reagin (RPR), and 24-hour urine for heavy metals. CSF analysis showed 61 red blood cells (RBCs) and no white blood cells (WBCs), with normal protein and glucose levels. CSF 14-3-3 protein Western blot (WB) was ambiguous (National Prion Disease Pathology Surveillance Center [NPDPSC], Cleveland, OH), neuron-specific enolase (NSE) was 13 ng/mL (<15 normal; Mayo Laboratories, Rochester, MN), and total tau was normal at 414 pg/mL (Athena Diagnostics, Worcester, MA). His second brain MRI, 1 month after the first, showed resolution of the prior hyperintensities with no restricted diffusion (figure 1, E–G). The T1 hyperintensities of the globus pallidi remained, however (figure 1H). Given his clinical and radiologic improvement, sCJD was thought to be highly unlikely. A thorough review of outside medical records showed that at his initial hospitalization postseizure, his admission sodium was 106 mEq/L, which declined to 102 within 3.5 hours and was corrected to 130 in less than 36 hours. The FLAIR and DWI findings, with resolution, along with the clinical history of rapidly corrected hyponatremia, were therefore felt to be consistent with extrapontine myelinolysis (EPM), his ultimate clinical diagnosis.
Case 2
A 66-year-old right-handed woman with a history of poorly controlled insulin-dependent diabetes mellitus, hypothyroidism, and primary biliary cirrhosis was found unconscious from a suspected insulin overdose by her neighbor after reportedly being at baseline the day before. The patient had a history of poor compliance with respect to her insulin, and a friend had expressed concerns about her ability to self-administer the drug. Her blood glucose level in the field was 99 mg/dL according to the emergency medical technician report. Her other medications included insulin glargine, insulin aspart, levothyroxine, spironolactone, rifaximin, ursodiol, propranolol, and alendronate. She eventually regained consciousness at an outside hospital where she remained for 2 weeks due to persistent altered mental status. During her first 3 days of admission, her blood sugar ranged from 96 to 366 mg/dL and then subsequently dropped to hypoglycemic levels of 25 and 42 on the fourth and fifth days of her hospitalization. Neurologic examination was nonfocal, but she manifested persistent signs of confusion characterized by using her telephone as a television remote control, difficulty managing her insulin treatments, and increased sweets consumption. Laboratory workup was either normal or negative for the following: ANCA, RF, paraneoplastic and related antibodies (CV2/CRMP5, ACh receptor, PCA-1, PCA-2, PCA-Tr, anti-Hu [ANNA-1], ANNA-2, ANNA-3, AGNA-1, striational, N-type Ca Channel, P/Q-type Ca channel, AcChR ganglionic neuronal, VGKCc, and amphiphysin), TG antibody, anti-gliadin antibodies, TTG antibodies, B12, and RPR. Her thyroid-stimulating hormone level was elevated at 11.2 mIU/L (normal 0.4–4.0). Initial CSF studies on admission showed 79 RBCs, 7 WBCs (95% neutrophils), normal protein and glucose, and a negative screen for infectious causes. Repeat lumbar puncture 5 days later revealed 0 RBCs, 1 WBC, normal protein and glucose, and elevated CSF biomarkers, including a positive 14-3-3 protein WB (NPDPSC), NSE of 78 ng/mL (>35 consistent with CJD per laboratory, Mayo Laboratories), and total tau of 17,585 pg/mL (>1,200 consistent with CJD per laboratory, Athena Diagnostics). Phospho-tau was increased at 88.4 pg/mL (>61 elevated) and Aβ42 was normal at 828.8 pg/mL; this pattern was interpreted by the laboratory to be consistent with CJD or other RPD and not with Alzheimer disease (Athena Diagnostics). Brain MRI FLAIR, DWI, and ADC map sequences revealed cortical ribboning, right caudate FLAIR hyperintensities, restricted diffusion, and mild generalized atrophy (figure 2, A–C). She had gradual improvement in her cognitive function during the hospitalization, so she was discharged home to the care of a friend 2 weeks after admission. She still displayed intermittent confusion and disorientation, however, even to self. For example, after being dropped off for a follow-up brain MRI, she became lost and denied her actual name when questioned by security personnel.
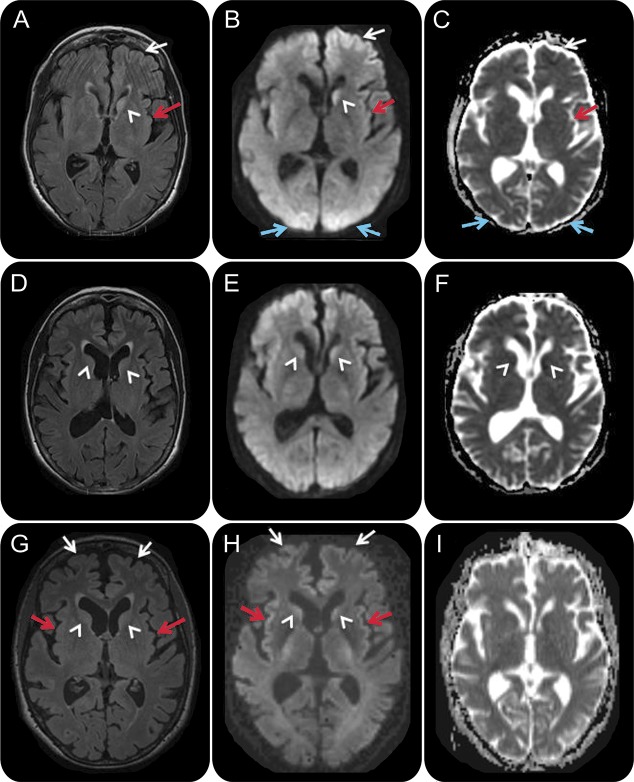
Figure 2. Serial MRIs in case 2, a 66-year-old woman with hypoxic/hypoglycemic encephalopathy.
FLAIR (A, D, G), DWI (B, E, H), and ADC map (C, F, I) sequences, in radiologic orientation, 2 days (A–C), 3 weeks (D–F), and 1 month (G–I) after onset. Initial MRI (A–C) shows left frontal (white arrows), left insular (red arrows), bilateral medial occipital (blue arrows), and left caudate (arrowhead) FLAIR/DWI hyperintensity with restricted diffusion, which is subtle but definitely appreciable. Repeat MRI about 3 weeks later (D–F) showed possible reduced FLAIR/DWI hyperintensity in the bilateral caudate heads (D–F; arrowhead) and medial occipital regions, and possible increased right caudate FLAIR hyperintensity and restricted diffusion (D–F; white arrowhead). A third MRI 1 week later, 1 month after onset (G–I), revealed more intense FLAIR/DWI insular (G, H; red arrows) and frontal cortical hyperintensities (G, H; white arrows) and possible restricted diffusion and FLAIR hyperintensity still present in the caudate heads (G, H; arrowheads). In retrospect, the resolution of occipital cortical ribboning in such a short time argues against a diagnosis of sporadic Creutzfeldt-Jakob disease. ADC = apparent diffusion coefficient, DWI = diffusion-weighted imaging; FLAIR = fluid-attenuated inversion recovery.
A second MRI approximately 3 weeks from onset showed continued cortical ribboning but reduced left caudate DWI and T2/FLAIR hyperintensity and possible new reduced diffusion in the right caudate head and anterior putamen (figure 2, D–F). Based on the rapid onset of cognitive impairment, elevated CSF biomarkers, and characteristic MRI findings, she was diagnosed with suspected sCJD and referred to our sCJD treatment trial.
At our center 1 month after onset, her friend reported continued cognitive and behavioral improvement since discharge, such that she was about 85% back to her baseline. Neurologic examination was only remarkable for bilateral snout reflex and a wide-based gait. Despite a normal MMSE score (30/30), formal neuropsychological testing revealed both verbal and visual memory impairment along with frontal-executive dysfunction. CSF showed 1 RBC, no WBCs, normal protein and glucose, positive 14-3-3 protein on WB (NPDPSC), and elevated NSE of 79 ng/mL (>35 consistent with CJD; Mayo Laboratories). A third MRI, 1 month after symptom onset, showed possibly more intense DWI cortical ribboning and greater right caudate head involvement with no significant change in left caudate head and maintained sparing of the putamen (figure 2, G–I). Given her overall clinical improvement and her radiologic findings, she was diagnosed with hypoglycemia-related encephalopathy with MRI abnormalities secondary to seizure vs hypoglycemia.
She returned to her home under the care of her friends. Unfortunately, she experienced a second hypoglycemic episode within several weeks, resulting in coma followed by death 5 weeks later. Brain autopsy revealed hypoxic/hypoglycemic nerve cell loss, reactive astrocytosis (glial fibrillary acidic protein stain), and activation of microglia (CD68 antibodies) in multiple brain regions, including all cortical regions, CA1/4 hippocampus, entorhinal cortex, deep nuclei, cerebellar Purkinje cells, and dentate nucleus. There was also evidence in similar regions for acute ischemic necrosis and Alzheimer type II gliosis (consistent with hypoxia) in gray matter. Although there were many cortical regions of vacuolation (spongiform change), the pattern of vacuolation was felt to be different from that seen in prion disease. Furthermore, 3F4 antibody staining by hydrolytic autoclaving method revealed no evidence of prions.7,8 The findings were not considered consistent with prion disease.
DISCUSSION
The diagnosis of sCJD is particularly challenging due to the variety of mimics.2–5,9 Brain MRI is a critical diagnostic tool with higher sensitivity and specificity than traditional biomarkers such as CSF 14-3-3 protein and EEG.10,11 FLAIR and DWI sequences typically demonstrate hyperintensities involving the cortex, striatum, and/or thalamus.12 The combination of DWI and FLAIR sequences has at least 91%–92% sensitivity and 94%–95% specificity for sCJD.10–13 Furthermore, the addition of ADC sequences may further increase imaging sensitivity.11 In case 1, the patient had isolated striatal FLAIR/DWI MRI lesions (found in about 5% of patients with sCJD),11,12 whereas the second patient showed signal changes in both cortical and subcortical structures (found in about two-thirds of patients with sCJD,11,12 although this varies based on sCJD molecular subtype).14 Both patients were initially misdiagnosed with sCJD and later found to have reversible dementias resulting from 2 separate metabolic disturbances, EPM and hypoglycemia.
EPM results from overly rapid correction of hyponatremia, sparing the pons and affecting the basal ganglia, internal capsule, white matter, corpus callosum, hippocampus, and cerebral cortex.15 Postmortem studies have revealed circumscribed striatal demyelination.16 Presenting symptoms of dysarthria, rigidity, bradykinesia, dystonia, and akinetic mutism may be confused with sCJD.17,18 Acutely, MRI shows T2 hyperintensities that resolve over months and T1 hypointensities that convert from initial decreased signal to increased signal during the subacute stage.15,17,18 The T1 hyperintensities are thought to result from damage to the vascular endothelium or lipid deposition secondary to myelin injury.17 Symmetric striatal FLAIR/DWI hyperintensities have been reported in a patient similar to case 1, a chronic alcohol abuser with osmotic demyelination from rapid correction of hyponatremia.19
Sustained hypoglycemia may result in clinical syndromes characterized by weakness, confusion, seizures, and coma,20 and imaging findings of cortical (insular, temporal, and occipital), basal ganglia, and hippocampal FLAIR/DWI hyperintensities with restricted diffusion and relative sparing of the thalamus.19,21 Although our second patient's symptoms were confined to memory and executive function, her MRI revealed both cortical and subcortical FLAIR and DWI hyperintensities that were suggestive of prion disease. Persistent MRI changes lasting 8 days to 1 year involving the cerebral cortex, caudate, lenticular nucleus, substantia nigra, and hippocampus have been described in persistent vegetative state due to hypoglycemia.22 In addition, white matter abnormalities can be found in structures such as the internal capsule and corpus callosum.20 Another, perhaps even more likely, explanation for her MRI findings is seizures, which are well-known to cause temporally limited restricted diffusion in the cortex and deep nuclei.23,24
There were features of both cases that, especially in retrospect, were inconsistent with the diagnosis of sCJD. The very acute nature of the onset in both suggested against a sCJD diagnosis, although acute onset has been reported in sCJD.25–28 Case 1 had profound hyponatremia on admission, which, along with his pattern of alcohol abuse, should have raised the possibility of a metabolic etiology. Case 2 showed considerable clinical improvement after her initial admission, a course that would be extremely unlikely in the context of prion disease, which is usually characterized by intractable decline. Case 2's MRI findings were not as prominent as those often seen in CJD,10,11 especially in the deep nuclei; nevertheless, they were felt to be somewhat concerning for CJD by physicians very experienced with prion disease. The cortical ribboning in the occipital cortex, however, resolved between the first and second scans, whereas generally in sCJD the DWI abnormalities progressively worsen over time (except in prolonged cases when these abnormalities may disappear due to brain atrophy). Lastly, in case 2 some of the regions with more consistent cortical ribboning serially, including the frontal and insular cortices, are regions that are sometimes problematic for CJD diagnosis, as they are known to be susceptible to artifact.13,14 Despite these considerations, the clinical and radiologic findings in these cases probably were sufficient to warrant the inclusion of sCJD in the differential diagnosis. Furthermore, the CSF findings in case 2, especially the extraordinarily high level of total tau and all CSF biomarkers testing positive, were even more suggestive of prion disease according to some studies,29,30 although this is controversial.
Both cases illustrate the clinical and radiologic overlap of metabolic disorders with sCJD as a cause of RPD. Our experience has shown that an extensive metabolic workup, repeated clinical follow-up, and serial MRIs are particularly useful for distinguishing a reversible metabolic encephalopathy from a fatal prion illness. Thus, clinicians should always consider the possibility of treatable metabolic etiologies when evaluating a patient with suspected sCJD.
STUDY FUNDING
This study was funded by NIH/NIA grants P01 AG02160, R01 AG031189, and K23 AG021989 and the Michael J. Homer Family Fund.
DISCLOSURES
M.H. Rosenbloom reports no disclosures. M.C. Tartaglia serves on a DSMB of UCSF Investigator-initiated trial in AD and receives research support from CIHR 325708 and 133355 Alzheimer's Society of Canada. S.A. Forner, K.K. Wong, A. Kuo, D.Y. Johnson, V. Colacurcio, and B.D. Andrews report no disclosures. B.L. Miller receives grant support from the NIH/NIA; serves as a consultant for TauRx, Allon Therapeutics, and Siemens Medical Solutions; has received a research grant from Novartis; serves as Scientific Advisor for Tau Consortium, Medical Advisor for the John Douglas French Foundation, Director of The Larry Hillblom Foundation, and on the scientific advisory boards for National Institute for Health Research in Dementia, FasterCures, A Center of the Milken Institute, and Tangled Bank Studios; serves as editor of Neurocase and on the editorial advisory boards of Cambridge University Press and Guilford Publications, Inc.; and receives publishing royalties for Behavioral Neurology of Dementia (Cambridge, 2009), Handbook of Neurology (Elsevier, 2009), and The Human Frontal Lobes (Guilford, 2008). S.J. DeArmond reports no disclosures. M.D. Geschwind serves on the editorial board of Dementia & Neuropsychologia; receives grant support from the NIH/NIA, the Michael J. Homer Family Fund, the Tau Consortium, and CurePSP; and serves/has served as a consultant for Best Doctors, Guidepoint Global, Quest Diagnostics (contract, pending), Lundbeck Inc., MedaCorp, Gerson-Lehman Group, The Council of Advisors, and Neurophage. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Geschwind MD, Tan KM, Lennon VA, et al. Voltage-gated potassium channel autoimmunity mimicking Creutzfeldt-Jakob disease. Arch Neurol 2008;65:1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chitravas N, Jung RS, Kofskey DM, et al. Treatable neurological disorders misdiagnosed as Creutzfeldt-Jakob disease. Ann Neurol 2011;70:437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geschwind MD, Haman A, Miller BL. Rapidly progressive dementia. Neurol Clin 2007;25:783–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geschwind MD, Shu H, Haman A, Sejvar JJ, Miller BL. Rapidly progressive dementia. Ann Neurol 2008;64:97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson RW, Torres-Chae CC, Kuo AL, et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch Neurol 2012;69:1578–1582. 10.1001/2013.jamaneurol.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geschwind MD. Rapidly progressive dementia: prion diseases and other rapid dementias. Continuum (Minneap Minn) 2010;16:31–56. [DOI] [PubMed] [Google Scholar]
- 7.Kitamoto T, Shin RW, Doh-ura K, et al. Abnormal isoform of prion proteins accumulates in the synaptic structures of the central nervous system in patients with Creutzfeldt-Jakob disease. Am J Pathol 1992;140:1285–1294. [PMC free article] [PubMed] [Google Scholar]
- 8.Haritani M, Spencer YI, Wells GA. Hydrated autoclave pretreatment enhancement of prion protein immunoreactivity in formalin-fixed bovine spongiform encephalopathy-affected brain. Acta Neuropathol 1994;87:86–90. [DOI] [PubMed] [Google Scholar]
- 9.Tschampa HJ, Neumann M, Zerr I, et al. Patients with Alzheimer's disease and dementia with Lewy bodies mistaken for Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry 2001;71:33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shiga Y, Miyazawa K, Sato S, et al. Diffusion-weighted MRI abnormalities as an early diagnostic marker for Creutzfeldt-Jakob disease. Neurology 2004;I63:443–449. [DOI] [PubMed] [Google Scholar]
- 11.Vitali P, Maccagnano E, Caverzasi E, et al. Diffusion-weighted MRI hyperintensity patterns differentiate CJD from other rapid dementias. Neurology 2011;76:1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young GS, Geschwind MD, Fischbein NJ, et al. Diffusion-weighted and fluid-attenuated inversion recovery imaging in Creutzfeldt-Jakob disease: high sensitivity and specificity for diagnosis. AJNR Am J Neuroradiol 2005;26:1551–1562. [PMC free article] [PubMed] [Google Scholar]
- 13.Tschampa HJ, Kallenberg K, Kretzschmar HA, et al. Pattern of cortical changes in sporadic Creutzfeldt-Jakob disease. AJNR Am J Neuroradiol 2007;28:1114–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zerr I, Kallenberg K, Summers DM, et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 2009;132:2659–2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bradley WG. Neurology in Clinical Practice, 5th ed Philadelphia, PA: Butterworth-Heinemann/Elsevier; 2008. [Google Scholar]
- 16.Health law—genetics—Congress restricts use of genetic information by insurers and employers. Genetic Information Nondiscrimination Act of 2008, Pub. L. No. 110–233, 122 Stat. 881 (to be codified in scattered sections of 26, 29, and 42 U.S.C.). Harv Law Rev 2009;122:1038–1045. [PubMed] [Google Scholar]
- 17.Waragai M, Satoh T. Serial MRI of extrapontine myelinolysis of the basal ganglia: a case report. J Neurol Sci 1998;161:173–175. [DOI] [PubMed] [Google Scholar]
- 18.Seok JI, Youn J, Chung EJ, Lee WY. Sequential observation of movement disorders and brain images in the case of central pontine myelinolysis and extrapontine myelinolysis. Parkinsonism Relat Disord 2006;12:462–464. [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, Eesa M, Scott JN. Toxic and acquired metabolic encephalopathies: MRI appearance. AJR Am J Roentgenol 2009;193:879–886. [DOI] [PubMed] [Google Scholar]
- 20.Allison M. Industry welcomes Genetic Information Nondiscrimination Act. Nat Biotechnol 2008;26:596–597. [DOI] [PubMed] [Google Scholar]
- 21.Cubo E, Andres MT, Rojo A, Guerrero A, Urra DG, Mendez R. Neuroimaging of hypoglycemia [in Spanish]. Rev Neurol 1998;26:774–776. [PubMed] [Google Scholar]
- 22.Appelbaum PS. Law & psychiatry: Genetic discrimination in mental disorders: the impact of the genetic information nondiscrimination act. Psychiatr Serv 2010;61:338–340. [DOI] [PubMed] [Google Scholar]
- 23.Becze E. Know your patients' rights under the Genetic Information Nondiscrimination Act. ONS Connect 2011;26:14–15. [PubMed] [Google Scholar]
- 24.Asmonga D. Getting to know GINA. An overview of the Genetic Information Nondiscrimination Act. J AHIMA 2008;79:18, 20, 22. [PubMed] [Google Scholar]
- 25.Obi T, Takatsu M, Kitamoto T, Mizoguchi K, Nishimura Y. A case of Creutzfeldt-Jakob disease (CJD) started with monoparesis of the left arm [in Japanese]. Rinsho Shinkeigaku 1996;36:1245–1248. [PubMed] [Google Scholar]
- 26.Hohler AD, Flynn FG. Onset of Creutzfeldt-Jakob disease mimicking an acute cerebrovascular event. Neurology 2006;67:538–539. [DOI] [PubMed] [Google Scholar]
- 27.Blasco Olcina R, Yaya Huaman R, Garces Sanchez M, Villanueva Haba VE, Bataller Alberola L, Baquero Toledo M. Creutzfeldt-Jakob disease with unilateral onset: clinical profile and neuroimaging [in Spanish]. Neurologia 2001;16:381–384. [PubMed] [Google Scholar]
- 28.Lyytinen J, Sairanen T, Valanne L, Salmi T, Paetau A, Pekkonen E. Progressive Stroke-Like Symptoms in a Patient with Sporadic Creutzfeldt-Jakob Disease. Case Rep Neurol 2010;2:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamlin C, Puoti G, Berri S, et al. A comparison of tau and 14-3-3 protein in the diagnosis of Creutzfeldt-Jakob disease. Neurology 2012;79:547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chohan G, Pennington C, Mackenzie JM, et al. The role of cerebrospinal fluid 14-3-3 and other proteins in the diagnosis of sporadic Creutzfeldt-Jakob disease in the UK: a 10-year review. J Neurol Neurosurg Psychiatry 2010;81:1243–1248. [DOI] [PubMed] [Google Scholar]