Abstract
Objective
To estimate the association between pregnancy glucose values in women without recognized pregestational diabetes or gestational diabetes (GDM) and cardiometabolic risk in their children.
Study design
This longitudinal cohort study of 211 Mexican-American mother-child pairs participating in CHAMACOS (Center for the Health Assessment of Mothers and Children of Salinas) used multiple logistic regression to estimate the children’s risk of non-fasting total cholesterol, non-fasting triglycerides, blood pressure and waist circumference g≥75th percentile at 7 years of age associated with a 1 mmol/L (18 mg/dl) increase in maternal pregnancy glucose level, measured 1-hour after a 50-g oral glucose load.
Results
The odds ratios for children belonging to the upper quartile of diastolic blood pressure (DBP), systolic blood pressure (SBP), and waist circumference (WC) associated with a 1 mmol/L increase in pregnancy glucose level were 1.39 (95% CI 1.10-1.75), 1.38 (95% CI 1.10-1.73) and 1. 25 (95% CI 1.02-1.54), respectively. Pre-pregnancy obesity was independently associated with increased odds of children belonging to the upper quartile of WC; maternal sugar-sweetened beverage consumption and gestational weight gain prior to the glucose test were not independently associated with any of the cardiometabolic outcomes.
Conclusion
In Mexican-American women without recognized pregestational diabetes or GDM, we found an association between increasing pregnancy glucose values and the children’s DBP, SBP and WC at 7 years of age. Whether interventions to reduce pregnancy glucose values, even if below levels diagnostic of overt disease, will mitigate subsequent high BP and abdominal obesity in late childhood remains to be determined.
Keywords: Pregnancy glucose, developmental programming, cardiometabolic risk
Similar to other ethnic groups in the United States (U.S.), cardiovascular disease is the leading cause of death among Mexican-Americans (1). Compared with non-Hispanic whites, Mexican-American adults are at greater risk of cardiovascular disease mortality (2), as well as several cardiovascular disease risk factors, including metabolic syndrome (2) and diabetes (1), which are likely related to the high prevalence of obesity in this population (1).
Mexican-American children are more likely to be overweight or obese than non-Hispanic white children (4). Among Mexican-American children, 30% of 2- to 5-year olds and 43% of 6- to 11-year olds are overweight or obese, with the corresponding prevalence in non-Hispanic whites at 23% and 32%, respectively (3).
A widely accepted hypothesis is that exposure to abnormal maternal fuel metabolism in utero, resulting from maternal diabetes at one end of the spectrum and maternal under-nutrition at the other, programs a fetus for later life morbidity, including obesity, diabetes, hypertension and heart disease (5, 6). In women without recognized pregestational diabetes or GDM, an increasing trend in offspring weight-for-age across increasing quartiles of pregnancy glucose has also been reported (10), yet there is a paucity of data on the association between in utero exposure to levels of maternal glycemia below those diagnostic of disease and childhood cardiometabolic risk. In women free of gestational diabetes, there appears to be a continuous association between increasing maternal glucose levels and the risk of several perinatal complications (11, 12), thus it is plausible that increasing pregnancy glucose levels below those diagnostic of disease could also be associated with longer-term adverse outcomes in the offspring.
The current study examines the association between increasing pregnancy glucose levels in women without recognized pregestational diabetes or GDM and cardiometabolic risk factors in their children at 7 years of age.
Methods
The mothers and children were participants in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS Study), a longitudinal birth cohort of low-income Mexican-Americans. Pregnant women were eligible for the CHAMACOS study if they sought prenatal care at six health clinics between October 1999 and October 2000, were less than 20 weeks gestation, 18 years of age or older, eligible for state-sponsored health care (Medi-Cal) and intended to deliver at Natividad Medical Center (a county hospital in Monterey County, CA, USA). A total of 601 women were enrolled and 485 followed until the delivery of a full term (≥ 37 weeks gestation), liveborn singleton.
Measurements of pregestational and gestational plasma glucose, as well as diabetes and GDM diagnoses, were abstracted from the medical record by a registered nurse. This analysis includes women without type 1 diabetes, type 2 diabetes, or GDM that had a plasma glucose value measured 1-hour after a 50-g oral glucose challenge test (screening GCT) performed within the recommended window of 24 to 28 weeks gestation (13). Of the 485 women delivering full term, liveborn singletons, we excluded 11 with recognized pregestational diabetes; one with possible unrecognized pregestational diabetes (glucose level >200 mg/dl [11.1 mmol/L] on more than one occasion during pregnancy) and five cases of GDM, identified by the results of a diagnostic 100-g, 3-hour oral glucose tolerance test (OGTT) following an abnormal GCT. During this period in this setting, the diagnosis of GDM was based on the National Diabetes Data Group criteria (13) (50-g, 1-h screening GCT level ≥140 mg/dl [7.8 mmol/L] and at least 2 plasma glucose measurements on the diagnostic 100-g, 3-h OGTT, performed the morning after an overnight fast, that meet or exceed the following thresholds: fasting ≥105 mg/dl [5.8 mmol/L], 1-hour ≥190 mg/dl [10.5 mmol/L], 2-hour ≥165 mg/dl [9.1 mmol/L], and 3-hour ≥145 mg/dl [8.0 mmol/L]). Women with glucose values below these thresholds did not receive treatment. Also excluded was: one woman with an abnormal value on the screening GCT (200 mg/dl [11.1 mmol/L]) but no follow up diagnostic OGTT; 23 women with diagnoses of GDM in their medical record who did not meet the diagnostic criteria because they likely received treatment for pregnancy hyperglycemia; and 113 woman whose screening tests were not performed within the recommended window of 24 to 28 weeks gestation (13). None of the remaining 331 women met the lower plasma glucose thresholds for GDM of the American Diabetes Association (14). Among these 331 women, 211 of their children had measurements at 7 years of age of non-fasting total cholesterol and triglyceride levels, blood pressure or waist circumference.
Non-fasting blood samples were collected from the children at 7 years of age between March 2007 and November 2008. Blood samples were immediately processed, with sera stored at −80° C until shipment on dry ice to the U.S. Centers for Disease Control and Prevention (CDC) (Atlanta, GA), where they were analyzed. Measurement of triglycerides (mg/dl) and cholesterol (mg/dl) in serum were made using standard enzymatic methods (Roche Chemicals, Indianapolis, IN) (15).
Blood pressure measurements (BP; mmHg) were made after the child had been sitting quietly for a minimum of 2 minutes; children were sitting with their arm relaxed either in their lap or on a low table. BP was measured up to 4 times on the same arm using a Dinamap 9300 (Critikon Corp., Tampa, FL), an automatic blood pressure machine that allows inflation pressure to be set at an appropriate level for children. One child had only one BP measurement. Trials were averaged for children with 2 BP measurements (n= 3); we averaged the last two trials for those with 3 BP measurements (n= 174). If any readings were unusually high (for boys: SBP >115 mmHg or DBP >76 mmHg, for girls: SBP >113 mmHg or DBP >75 mmHg), the cuff was removed and the child rested for at least 5 minutes prior to a fourth measurement. For children with 4 measurements available (n= 11), we excluded the first measurement and averaged the 2 trials in which mean arterial pressure (MAP) values were closest to each other.
Waist circumference (cm) was measured with a tape against the skin at above the crest of the ileum while the children were standing upright. Measurements were recorded to the nearest 0.1 cm after the child exhaled. Waist circumference was measured in triplicate, with the tape loosened prior to repeating each measurement; we took the mean of the 3 waist circumference trials.
From questionnaires administered to the mother during pregnancy, we obtained data on: smoking status (yes or no), poverty [living above versus at or below the federal poverty line, which represents an annual income of $17,650 for a family of four (16)], and sugar-sweetened beverage consumption. Sugar-sweetened beverage consumption prior to the screening test was used as a proxy for dietary added sugars and ascertained from the women at the end of the second trimester (mean gestational age= 26.7 weeks, SD= 2.0); women were asked how often in the last three months they drank non-diet soda, fruit juice and fruit drinks, the frequency of consumption was coded times per week. Pre-pregnancy weight was obtained from several sources, according to the following hierarchy: 1) as recorded in the medical record (n= 189), 2) self-reported on the pregnancy questionnaire (n= 16), 3) from an early prenatal weight measurement (<13 weeks gestational age; n= 2), or 4) calculated by a regression line that utilized all prenatal weight measurements and corresponding gestational ages (n= 4). In the sub-set of women with pre-pregnancy weight data available in the medical record, the pregnancy questionnaire and from an early prenatal weight measurement (n= 108), pre-pregnancy weight from the medical record was significantly and positively correlated with the early prenatal weight (Spearman’s rho =0.96, p-value < 0.0001); self-reported pre-pregnancy weight was also significantly and positively correlated with the early prenatal weight (Spearman’s rho =0.96, p-value < 0.001). From the medical record, we abstracted gestational weight gained just prior to the glucose test (kg) and gestational age at this pregnancy weight measurement (weeks), as well as infant birthweight (grams) and gestational age at birth (weeks); maternal height was measured by study staff. Only gestational weight gain occurring prior to the exposure could confound the association of interest (17), thus pre-pregnancy weight was subtracted from the nearest prenatal weight measurement taken prior to the glucose test to calculate the amount of weight gained up until the time of the glucose test.
Because there is no standard definition for abnormal cardiometabolic risk factors in children, we classified each cardiometabolic risk factor as increased if it was at or above the upper quartile for that outcome. The 75th percentile was determined from sex- and age-specific, nationally representative waist circumference data for Mexican-American youth (18) and the study cohort sex-specific distributions; for all other cardiometabolic risk factors, the study cohort distribution was used to determine the 75th percentile.
Statistical Analyses
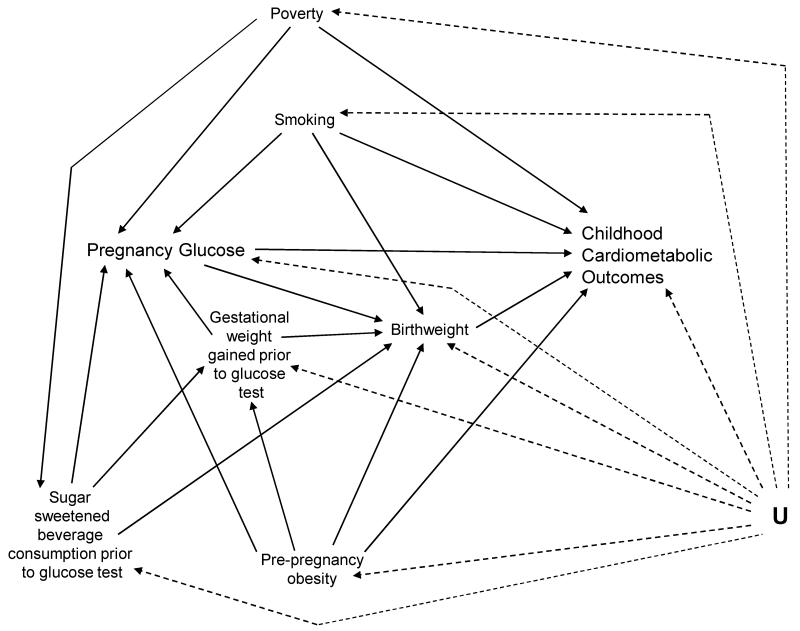
We used separate multiple logistic regression models to estimate the children’s risk of each cardiometabolic risk factor, defined as ≥75th percentile, associated with a 1 mmol/L (equivalent to 18 mg/dl) increase in pregnancy glucose, measured 1-hour after a 50-g oral glucose tolerance load. The results were compared with models that utilized the 50th and 90th percentiles as cut points, as well as linear regression analyses of continuous outcomes. A directed acyclic graph (DAG; Figure) (19) guided the selection of adjustment variables. Logistic regression models were adjusted for pre-pregnancy obesity (BMI ≥ 30 kg/m2), soda consumption (times per week) prior to the glucose test (continuous), gestational weight gained prior to the glucose test (continuous), gestational age at the weight measurement (continuous), smoking during pregnancy, poverty, and infant birthweight (continuous). Routine adjustment for gestational age at birth has recently been called into question (20); therefore, we present the results of models with and without adjustment for gestational age at birth.
Figure.
Directed Acyclic Graph (DAG). There is no arrow from poverty to birthweight due to the Latina birthweight paradox. In this Mexican-American immigrant population, there is no association between poverty and pre-pregnancy obesity, or between poverty and smoking.
The waist circumference outcome was based on the study cohort’s sex-specific waist circumference distributions, as well as sex-specific distributions from a nationally representative sample of 7 year old Mexican-American children (18); thus, all models, except for the waist circumference models, included additional adjustment for sex. In the fetal origins of disease literature, including adjustment for current body size in analyses of subsequent hypertension has been debated (21). Thus, we conducted the BP analyses with and without additional adjustment for current waist circumference, BMI, and BMI z-score (22) (in separate models). Because we hypothesized that current body size was on the causal pathway from in utero glucose exposure to subsequent hypertension, we present the results of models that excluded any adjustment for current body size.
We assessed potential effect modification in the relationship between pregnancy glucose level and each childhood cardiometabolic outcome by maternal pre-pregnancy obesity, birthweight and child’s sex. Cross products were added, one at a time, to fully adjusted models; no cross products were statistically significant (all p-values ≥ 0.10).
To assess whether loss to follow-up resulted in bias, we compared those included in the study sample to those who were lost to follow-up in regards to the following: maternal educational attainment, marital status, parity, pre-pregnancy obesity, poverty and smoking during pregnancy. We also conducted analyses weighted by the inverse probability that a mother-child pair would remain in the cohort and attend the 7-year follow-up visit. SuperLearner (23), a prediction algorithm, was used to predict whether a pair was assessed at 7 years; prediction was based on the variables listed above, as well as infant birthweight and gestational age at birth.
All analyses were conducted in SAS (version 9.1, SAS Institute Inc., Cary, NC); SuperLearner was run in R (version 2.12.1, The R Foundation for Statistical Computing, Vienna, Austria). Study participants provided written informed consent and all research activities were approved by the University of California–Berkeley Committee for the Protection of Human Subjects.
Results
Mother-child pairs who were lost to follow-up (n= 120) did not differ from the study sample (n=211) in terms of maternal educational attainment, marital status, parity, pre-pregnancy obesity, and poverty; women who smoked during pregnancy were more likely to be lost to follow-up (p= 0.01). Characteristics of the 211 mother-child pairs are presented in Table I. Over three-quarters of the women attained less than a high school education. Half of the women had been in the U.S. for 5 years or less when they became pregnant and 64% were at or below the poverty line. The mean glucose value at the screening test was 107.1 mg/dl (SD 27.0) [5.9 mmol/L (SD 1.5)] and mean pre-pregnancy BMI 26.9 kg/m2 (SD 4.9). The average gestational age at the weight measurement prior to the screening test was 22.1 weeks (SD 5.5 weeks) and women gained, on average, 4.9 kg prior to the glucose test (SD 4.2). Thirty-eight percent of the children were obese at age 7 years [BMI z-score ≥ 95th percentile (22)]. The children’s mean total cholesterol was 170.1 mg/dl (SD 29.7; n= 174), triglycerides 131.1 mg/dl (SD 82.7; n= 174), boys’ waist circumference 66.2 cm (SD 9.2; n=98), girls’ waist circumference 67.3 cm (SD 9.9; n= 112), DSP 52.9 mmHg (SD 5.6; n= 189), and SBP 96.1 mmHg (SD 9.0; n= 189).
Table 1.
Cohort characteristics of 211 Mexican-American mother-child pairs from the CHAMACHOS cohort, 1999-2000.
| Characteristic | n | % |
|---|---|---|
| Pre-pregnancy BMI | ||
| Underweight (<18.5 kg/m2) | 2 | 1.0 |
| Normal (18.5-24.9 kg/m2) | 81 | 38.4 |
| Overweight (25.0-29.9 kg/m2) | 85 | 40.3 |
| Obese (≥30.0 kg/m2) | 43 | 20.4 |
| Years in the U.S. | ||
| ≤ 5 years | 109 | 51.7 |
| > 5 years | 102 | 48.3 |
| Maternal education | ||
| ≤ 6th grade | 97 | 46.0 |
| 7-12th grade | 68 | 32.2 |
| ≥ high school graduate | 46 | 21.8 |
|
At or below the poverty line
Parity |
135 | 64.0 |
| 0 | 67 | 31.8 |
| 1 | 67 | 31.8 |
| 2 | 50 | 23.7 |
| 3+ | 27 | 12.8 |
| Smoked during pregnancy | 8 | 3.8 |
|
Sugar-sweetened beverage consumption prior to the glucose test (n= 208) |
||
| <1 per day | 43 | 20.7 |
| 1 per day | 61 | 29.3 |
| 2 per day | 44 | 21.2 |
| 3 per day | 37 | 17.8 |
| 4+ per day | 23 | 11.1 |
| Maternal age at delivery | ||
| 18-24 years | 94 | 44.6 |
| 25-29 years | 77 | 36.5 |
| 30-34 years | 24 | 11.4 |
| 35-45 years | 16 | 7.6 |
| Child’s sex | ||
| Boys | 99 | 46.9 |
| Girls | 112 | 53.1 |
| Child’s BMI z-score ≥ 95%* (n= 210) | 79 | 37.6 |
| Non-fasting cholesterol ≥ 75%a (186 mg/dl; n= 174) | 44 | 25.3 |
| Non-fasting triglycerides ≥ 75%a (160 mg/dl; n= 174) | 44 | 25.3 |
| Waist Circumference ≥ 75% a | ||
| Boys (71.5 cm; n= 98) | 24 | 24.5 |
| Girls (75.3 cm; n= 112) | 28 | 25.0 |
| Waist Circumference ≥ 75% b | ||
| Boys (63.4 cm; n= 98) | 50 | 51.0 |
| Girls (63.0 cm; n= 112) | 62 | 55.4 |
| Diastolic Blood Pressure ≥ 75%a (56.5 mmHg; n= 189) | 48 | 25.4 |
| Systolic Blood Pressure≥ 75%a (101 mmHg; n= 189) | 56 | 29.6 |
| Child’s sugar-sweetened beverage consumption | ||
| <1 per day | 57 | 27.0 |
| 1 per day | 76 | 36.0 |
| 2 per day | 11 | 5.3 |
| 3 per day | 34 | 16.1 |
| 4+ per day | 33 | 15.6 |
| Child’s average TV watching | ||
| <1 hour per day | 41 | 19.4 |
| 1-2 hours per day | 61 | 28.9 |
| >2 hours per day | 109 | 51.7 |
| mean | SD | |
|---|---|---|
|
|
||
| Glucose screening value (mmol/L) | 5.9 | 1.5 |
| Gestational age at screening test (weeks) | 26.3 | 1.1 |
|
Gestational age at soda consumption assessment (n= 201; weeks) |
26.7 | 2.0 |
| Gestational weight gained prior to the glucose test (kg) | 4.9 | 4.2 |
| Gestational age at weight measurement (weeks) | 22.1 | 5.5 |
| Infant Birthweight (kg) | 3.5 | 0.4 |
| Gestational age at delivery (weeks) | 39.2 | 1.2 |
| Child’s absolute age at 7 year follow-up visit (months) | 85.1 | 2.4 |
BMI z-score calculated from sex-specific, BMI-for-age cut points issued by the CDC (National Center for Health Statistics. CDC growth charts. 2005. United States.)
Based on the study cohort distribution
Based on a nationally representative sample of 7 year old Mexican-American children
The 75th percentiles used to define each cardiometabolic risk factor are displayed in Table I. The study cohort-specific 75th percentiles for waist circumference were 71.5 cm and 75.3 cm for boys and girls, respectively, thereby exceeding the nationally representative 75th percentiles for 7-year old Mexican-Americans by 8.1 cm and 12.3 cm, respectively; 51% of the boys and 55% of the girls met or exceeded the nationally representative cut points for Mexican-American children.
Odds Ratios and 95% confidence intervals for the association between a 1 mmol/L increase in pregnancy glucose level and the presence of each childhood cardiometabolic risk factor are presented in Table II. The odds ratios for children belonging to the upper quartile of DBP and SBP associated with a 1 mmol/L increase in pregnancy glucose level were 1.39 (95% CI 1.10-1.75) and 1.38 (95% CI 1.10-1.73), respectively. Using the nationally representative cut point, the odds of children in the upper quartile of waist circumference were 1. 25 (95% CI 1.02-1.54) times higher for those exposed to a 1 mmol/L increase in maternal glucose level; the estimate for waist circumference defined by the cohort-specific cut point was comparable [1.19 (95% CI 0.95-1.49)].
Table 2.
Odds Ratios (95% Confidence Intervals) for each offspring cardiometabolic risk factor meeting or exceeding the 75th percentile at 7 years of age associated with a 1 mmol/L increase in maternal pregnancy glucose level, CHAMACOS study, 1999-2008.
| Cardio-metabolic risk factor ≥ 75th percentile |
Unadjusted | Model 1a | Model 2d | Model 3c |
|---|---|---|---|---|
| Non-fasting total cholesterol | 1.06 (0.85 - 1.33) |
1.12 (0.88-1.42) |
1.17 (0.91-1.49) |
1.13 (0.88-1.45) |
| Non-fasting triglycerides | 1.16 (0.93 - 1.45) |
1.19 (0.94-1.51) |
1.19 (0.93-1.51) |
1.17 (0.91-1.49) |
| Waist Circumference d | 1.17 (0.95-1.44) |
1.14 (0.92-1.42) |
1.15 (0.92-1.42) |
1.19 (0.95-1.49) |
| Waist Circumference e |
1.25
(1.04 - 1.52) |
1.26
(1.04-1.54) |
1.24
(1.01-1.52) |
1.2
(1.02-1.54) |
| Diastolic blood pressure |
1.29
(1.04 - 1.60) |
1.35
(1.08-1.69) |
1.35
(1.08-1.69) |
1.39
(1.10-1.75) |
| Systolic blood pressure |
1.31
(1.07 - 1.61) |
1.37
(1.10-1.71) |
1.38
(1.11-1.72) |
1.38
(1.10-1.73) |
Model 1 adjusted for child’s sex; maternal pre-pregnancy obesity (BMI ≥ 30 kg/m2), sugar sweetened beverage consumption during pregnancy (times per week, continuous), gestational weight gained prior to the glucose test (continuous), gestational age at weight measurement (continuous), smoking (yes/no), and poverty (at/below poverty line vs. above).
Model 2 adjusted for Model 1 covariates, plus infant birthweight (continuous).
Model 3 adjusted for Model 2 covariates, plus infant’s gestational age at birth (continuous).
Based on the study cohort sex-specific distributions; child’s sex dropped from the adjusted models
Based on a nationally representative sample of 7 year old Mexican-American children, sex-specific; child’s sex dropped from the adjusted models
Adjustment for child’s sex, infant birthweight and gestational age at birth; maternal pre-pregnancy obesity, sugar-sweetened beverage consumption prior to the glucose test, gestational weight gained prior to the glucose test, gestational age at weight measurement, smoking, and poverty did not appreciably alter the risk estimates (Table II). In the model for waist circumference defined by the nationally representative cut points, the odds of children belonging to the upper quartile of waist circumference were almost 3 times higher (OR= 2.78 [95% CI 1.24 - 6.22]) for the children of obese women as compared with the children of non-obese women; the estimate was comparable when waist circumference was defined by the cohort-specific cut point (OR= 3.06 [95% CI 1.39-6.74]). Female sex was associated with a decreased odds of belonging to the upper quartile of non-fasting total cholesterol (OR= 0.42 [95% CI 0.19-0.93]); no other covariates demonstrated an association with the cardiometabolic outcomes.
Models that excluded adjustment for infant birthweight and/or gestational age at birth gave comparable estimates (Table II). The results of models for BP that included additional adjustment for current body size were comparable with those presented in Table II (data not shown). Models utilizing the 50th and 90th percentiles to define the cardiometabolic risk factors yielded similar results; linear regression analyses also generated similar findings (data not shown). Models adjusted for pre-pregnancy BMI (continuous) instead of obesity likewise gave comparable estimates (data not shown). Lastly, estimates obtained from the inverse probability weighted analyses were similar to those presented in Tables II (data not shown).
Discussion
Our results are consistent with the findings of previous studies among women with diabetes and GDM (7-9). A retrospective cohort study of mother-child pairs belonging to an HMO in Colorado reported that 82 youth, 6 to 13 years of age, exposed to maternal GDM in utero had larger waist circumference than 379 of their unexposed peers (9). Analyses stratified by Hispanic ethnicity were also presented: Hispanic youth exposed to maternal GDM in utero had a significantly higher waist circumference (7.1 cm) as compared with Hispanic youth that were not exposed to GDM, yet the comparable estimate among non-Hispanic white youth (3.4 cm) did not attain statistical significance. A prospective cohort study in China compared 63 youth, 7 to 10 years of age, who had been exposed to either maternal gestational impaired glucose tolerance (defined as fasting plasma glucose <7.0 mmol/L and 2 hour plasma glucose level ≥7.8-11.1 mmol/L) or GDM in utero to 101 youth whose mothers had normal glucose tolerance during pregnancy (7); youth exposed to maternal GDM or IGT in utero had higher SBP and DBP. Previous studies have also reported associations between maternal pregnancy hyperglycemia and lower levels of high-density lipoprotein cholesterol in the children, but not non-fasting total cholesterol or triglycerides (7, 8). Given that our results for non-fasting total cholesterol and triglycerides are consistent with these findings, we speculate that the lack of association in the current study was not due to the use of non-fasting blood samples.
Increasing levels of maternal glycemia are associated with increased neonatal adiposity (25) and this disproportionately high fat mass relative to lean body mass is likely to persist, as fetal development is the critical period for muscle growth (26). The unfavorable body composition that accompanies exposure to increased glucose levels in utero likely leads to the increased cardiometabolic risk observed in this and other studies (7-9). The increased growth velocity observed among children exposed to increasing pregnancy glucose levels in utero (27) could also partially explain observations of higher BP in childhood. The U.S. Collaborative Perinatal Project reported that children who had crossed into higher weight percentiles throughout their childhood growth displayed higher BP at 7 years of age (28). Therefore, developmental programming for increased childhood fat mass and growth velocity could potentially lie on the causal pathways connecting pregnancy glucose levels to increased BP in late childhood.
The prospective design is a clear strength of the current study and essential for examining the effect of any in utero exposure on subsequent childhood obesity. The amount of bias incurred from the use of self-reported pre-pregnancy weight for a few women also appears to be minimal. Yet, as in any observational study, there are several important limitations to consider. Our estimates likely contain some degree of bias due to residual and unmeasured confounding; shared lifestyle characteristics and/or genetic factors, for example, may have contributed to some or all of the associations described. In addition, the 90th percentile has been used to define children’s cardiometabolic outcomes in previous publications but could not be used in this study due to sparse data; specifically, maximum likelihood estimates were not available for the smoking covariate in fully adjusted models for cholesterol, triglycerides, and SBP defined by this cut point.
These findings suggest that lifestyle interventions promoting healthy diet and physical activity to improve pregnancy glucose levels, as well as abdominal obesity and elevated BP in late childhood should be evaluated as potential strategies to prevent the development of cardiometabolic disease.
Acknowledgments
We thank our staff and community partners, our study participants, and our field staff. We are especially grateful to Dr Raul Aguilar, Dr Jonathan Chevrier, Katherine Kogut, Kristin Tyler, and Michelle G. Vedar of the Center for Environmental Research and Children’s Health at the University of California, Berkeley for guidance on the analysis and presentation of these data.
Supported by dissertation assistance to S.E. from the Russell M. Grossman Endowment, the Reshetko Family Scholarship, EPA (RD 83171001 to B.E.), and NIEHS (PO1ES009605 to B.E.). Contents do not necessarily represent the official views of funders. Study sponsors played no role in determining the study design; the collection, analysis, and interpretation of data; writing of the manuscript; or the decision to submit for publication.
Abbreviations
- GDM
Gestational diabetes mellitus
- CHAMACOS
Center for the Health Assessment of Mothers and Children of Salinas
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- (1).Sundquist J, Winkleby MA. Cardiovascular risk factors in Mexican American adults: a transcultural analysis of NHANES III, 1988-1994. Am J Public Health. 1999;89:723–30. doi: 10.2105/ajph.89.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Davidson JA, Kannel WB, Lopez-Candales A, Morales L, Moreno PR, Ovalle F, et al. Avoiding the looming Latino/Hispanic cardiovascular health crisis: a call to action. J Cardiometab Syndr. 2007;2:238–43. doi: 10.1111/j.1559-4564.2007.07534.x. [DOI] [PubMed] [Google Scholar]
- (3).Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. JAMA. 2010;20:303, 242–9. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- (4).US Census Bureau . The American Community, Hispanics: 2004. Washington DC: 2007. [Google Scholar]
- (5).Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29:1023–35. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- (6).Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23:588S–95S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- (7).Tam WH, Ma RC, Yang X, Ko GT, Tong PC, Cockram CS, et al. Glucose intolerance and cardiometabolic risk in children exposed to maternal gestational diabetes mellitus in utero. Pediatrics. 2008;122:1229–34. doi: 10.1542/peds.2008-0158. [DOI] [PubMed] [Google Scholar]
- (8).Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin A(1)c and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab. 2005;90:3225–9. doi: 10.1210/jc.2005-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Crume TL, Ogden L, West NA, Vehik KS, Scherzinger A, Daniels S, et al. Association of exposure to diabetes in utero with adiposity and fat distribution in a multiethnic population of youth: the Exploring Perinatal Outcomes among Children (EPOCH) Study. Diabetologia. 2011;54:87–92. doi: 10.1007/s00125-010-1925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–92. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- (11).Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- (12).Ehrlich SF, Crites YM, Hedderson MM, Darbinian JA, Ferrara A. The risk of large for gestational age across increasing categories of pregnancy glycemia. Am J Obstet Gynecol. 2011:204. doi: 10.1016/j.ajog.2010.10.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- (14).Gestational Diabetes Mellitus. Diabetes Care. 2000;23:S77–S79. [PubMed] [Google Scholar]
- (15).Phillips DL, Pirkle JL, Burse VW, Bernert JT, Jr., Henderson LO, Needham LL. Chlorinated hydrocarbon levels in human serum: effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- (16).US Census Bureau . Poverty Thresholds for 2000 by Size of Family and Number of Related Children Under 18 Years. Washington DC: 2010. [Google Scholar]
- (17).Jewell NP. Statistics for Epidemiology. Chapman & Hall/CRC; 2004. [Google Scholar]
- (18).Fernandez JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr. 2004;145:439–44. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- (19).Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10:37–48. [PubMed] [Google Scholar]
- (20).Wilcox AJ, Weinberg CR, Basso O. On the pitfalls of adjusting for gestational age at birth. Am J Epidemiol. 2011;174:1062–8. doi: 10.1093/aje/kwr230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Tu YK, West R, Ellison GT, Gilthorpe MS. Why evidence for the fetal origins of adult disease might be a statistical artifact: the “reversal paradox” for the relation between birth weight and blood pressure in later life. Am J Epidemiol. 2005;161:27–32. doi: 10.1093/aje/kwi002. [DOI] [PubMed] [Google Scholar]
- (22).National Center for Health Statistics . CDC growth charts. United States: 2005. [Google Scholar]
- (23).van der Laan MJ, Polley EC, Hubbard AE. Super learner. Stat Appl Genet Mol Biol. 2007;6 doi: 10.2202/1544-6115.1309. Article25. [DOI] [PubMed] [Google Scholar]
- (24).Dabelea D, Pettitt DJ, Hanson RL, Imperatore G, Bennett PH, Knowler WC. Birth weight, type 2 diabetes, and insulin resistance in Pima Indian children and young adults. Diabetes Care. 1999;22:944–50. doi: 10.2337/diacare.22.6.944. [DOI] [PubMed] [Google Scholar]
- (25).Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes. 2009;58:453–9. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Widdowson EM, Crabb DE, Milner RD. Cellular development of some human organs before birth. Arch Dis Child. 1972;47:652–5. doi: 10.1136/adc.47.254.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Crume TL, Ogden L, Daniels S, Hamman RF, Norris JM, Dabelea D. The Impact of In Utero Exposure to Diabetes on Childhood Body Mass Index Growth Trajectories: The EPOCH Study. J Pediatr. 2011 doi: 10.1016/j.jpeds.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Hemachandra AH, Howards PP, Furth SL, Klebanoff MA. Birth weight, postnatal growth, and risk for high blood pressure at 7 years of age: results from the Collaborative Perinatal Project. Pediatrics. 2007;119:e1264–e1270. doi: 10.1542/peds.2005-2486. [DOI] [PubMed] [Google Scholar]
- (29).Lane DA, Gill P. Ethnicity and tracking blood pressure in children. J Hum Hypertens. 2004;18:223–8. doi: 10.1038/sj.jhh.1001674. [DOI] [PubMed] [Google Scholar]
- (30).Hirschler V, Aranda C, Calcagno ML, Maccalini G, Jadzinsky M. Can waist circumference identify children with the metabolic syndrome? Arch Pediatr Adolesc Med. 2005;159:740–4. doi: 10.1001/archpedi.159.8.740. [DOI] [PubMed] [Google Scholar]