Abstract
Deficits in voluntary activation of the quadriceps muscle are characteristic of knee osteoarthritis (OA), contributing to the quadriceps weakness that is also a hallmark of the disease. The mechanisms underlying this central activation deficit (CAD) are unknown, although cortical mechanisms may be involved. Here, we utilize transcranial magnetic stimulation (TMS) to assess corticospinal and intracortical excitability in patients with knee osteoarthritis (OA) and in a comparably aged group of healthy older adults, to quantify group differences and to examine associations between TMS measures and pain, quadriceps strength, and CAD. Seventeen patients with knee OA and 20 healthy controls completed testing. Motor evoked potentials (MEPs) were measured at the quadriceps by superficial electromyographic (EMG) recordings. Corticospinal excitability was assessed by measuring resting motor threshold (RMT) to TMS stimulation of the quadriceps representation at primary motor cortex, and intracortical excitability was assessed via paired pulse paradigms for short interval intracortical inhibition (SICI) and intracortical facilitation (ICF). No statistically significant differences between patients with knee OA and healthy controls were found for RMT, SICI or ICF measures (p>0.05). For patients with knee OA, there were significant associations observed between pain and RMT, as well as between pain and ICF. No associations were observed between CAD and measures of corticospinal or intracortical excitability. These data suggest against direct involvement of corticospinal or intracortical pathways within primary motor cortex in the mechanisms of CAD. However, pain is implicated in the neural mechanisms of quadriceps motor control in patients with knee OA.
Keywords: transcranial magnetic stimulation, motor evoked potential, quadriceps, knee osteoarthritis
1. Introduction
Quadriceps weakness is a hallmark of knee osteoarthritis (OA), with strong associations to clinical parameters such as pain (O'Reilly et al. 1998; Peat et al. 2001), self-reported function (McAlindon et al. 1993), and physical performance (Liikavainio et al. 2007; Sharma et al. 2003). The etiology of quadriceps weakness in knee OA remains unclear, although the factors involved are likely complex and multifactorial, potentially encompassing mechanisms such as disuse atrophy, reflex inhibition due to pain and inflammation, and decreased cortical drive (Hurley 1999; Mizner et al. 2005b; Palmieri-Smith et al. 2007). The central nervous system has been particularly implicated in quadriceps weakness in knee OA, as patients have been shown to exhibit a sharply diminished ability to voluntarily activate the quadriceps muscle (Fitzgerald et al. 2004; Lewek et al. 2004). This phenomenon, known as central activation deficit (CAD) is commonly assessed using peripheral, superimposed, electrical stimulation delivered directly to the muscle during a maximal voluntary isometric contraction, to measure whether additional force can be generated. The well-documented and dramatic strength loss that is often seen immediately after knee surgery is largely explained by pronounced CAD, (Mizner et al. 2005b), and CAD is associated with measures of physical performance in a variety of clinical populations, including patients with knee OA and patients following ligament injury/reconstruction and meniscectomy (Fitzgerald et al. 2004; Shakespeare et al. 1985; Urbach et al. 2001; Urbach et al. 1999). Although CAD clearly plays a clinically important role in knee OA, we do not yet understand the neurophysiological pathways involved—from motor cortical activity through corticospinal activity to the neuromuscular junction. Thus the origins of CAD remain to be elucidated.
Transcranial magnetic stimulation (TMS) of the motor cortex has been used as a tool to understand intracortical and corticospinal pathways involved with neuromuscular function. Specifically, single pulse TMS has been used to examine the threshold required to produce motor evoked potentials (MEPs) in a variety of muscles groups. Resting motor threshold (RMT) is thought to act as a general measure of corticospinal excitability. Paired-pulse TMS paradigms have been used to access pathways involved with intracortical inhibition (ICI) or intracortical facilitation (ICF) within the motor cortex. By manipulating the interstimulus interval in these paired-pulse paradigms and observing effects on the resulting MEPs, pathways that appear to be preferentially governed by gamma-aminobutyric acid (GABA) mediated inhibition or N-Methyl-D-aspartate (NMDA) mediated facilitation can be studied (Ziemann et al. 1998; Ziemann et al. 1996). These TMS measures have previously been used to assess changes in neuromuscular function associated with normal aging (Stevens-Lapsley et al. 2013), as well as to characterize the pathophysiology of neuromuscular deficits in clinical populations such as stroke and multiple sclerosis (Caramia et al. 2004; Wittenberg et al. 2007). For example, patients in the “relapsing” phase of multiple sclerosis have been shown to demonstrate higher motor thresholds and abnormalities in ICI compared to patients in the “remitting” phase (Caramia et al. 2004).
Our previous work has explored corticospinal and intracortical excitability of the quadriceps in physically active younger and older adults (Stevens-Lapsley et al. 2013). A recent case report used TMS to examine MEPs in a patient with unilateral knee OA (Hunt et al. 2011), noting increases in both MEP amplitude and quadriceps strength over the course of a resistance-training program. Other studies have examined changes in quadriceps strength and activation following the application of single pulse or repetitive TMS to the motor cortex (Gibbons et al. 2010). However, the bulk of TMS research has been conducted in the upper extremity, and much remains to be learned regarding TMS assessment or modulation of quadriceps function, particularly in clinical populations. In this study we investigated TMS measures of quadriceps neurophysiology in a population of patients with end-stage knee OA, as well as in a comparably-aged group of healthy adults. Our overall aims were to determine 1) if differences exist in corticospinal and motor cortical excitability between patients with knee OA and healthy controls and 2) if there are correlations between TMS measures and clinically relevant measures of quadriceps strength, CAD, and knee pain. We hypothesized that patients with knee OA would demonstrate diminished corticospinal excitability (increased resting motor threshold) as well as diminished intracortical excitability (increased ICI and decreased ICF) compared to healthy controls, thus contributing to increased CAD. We further hypothesized that, in patients with knee OA, TMS measures would be significantly correlated with quadriceps strength, CAD and knee pain.
2. Methods
2.1. Participants
Patients with knee OA were recruited from orthopedic practices in the Denver metropolitan area. Patients were included if they were awaiting unilateral total knee arthroplasty for OA. Patients were excluded if they had a body mass index (BMI) of greater than 35 m/kg2, history of diabetes, cardiovascular disease, peripheral neuropathy, neurological or psychiatric disease, lower quarter pain problems (other than knee osteoarthritis), or if they were taking medications known to alter cortical excitability. The same exclusion criteria applied to healthy controls with neither a history of knee trauma nor current knee pain. Additionally, all participants were required to meet the TMS safety criteria as outlined by the National Institutes of Neurological Disorders and Stroke (Wassermann 1998). This study was approved by the Colorado Multiple Institutional Review Board, and all participants provided written, informed consent before participation.
2.2. Electromyography Recording
Participants were tested in a seated position, with approximately 45° of hip flexion as previously described (Stevens-Lapsley et al. 2013). Briefly, surface electromyography (EMG) was collected via 3 cm Ag-Ag/Cl electrodes (ConMed, Utica, NY, USA), with two electrodes placed midway between the iliac crest and the lateral joint line of the knee to record vastus lateralus (VL) activity. Ground electrodes were placed on the contralateral patella for all participants. The EMG signal was collected using a Biopac MP100 unit and AcqKnowledge (v3.8.1) software (Biopac System Inc., Goleta, CA) and amplified at a gain of 2,000 before being filtered online with a high pass of 10 Hz and low pass of 500 Hz. Visual inspection of EMG signal was performed during all TMS stimulations to confirm that testing was performed on a resting muscle. The right leg was assessed in all healthy controls, and the involved lower extremity was assessed in patients with knee OA.
2.3. Transcranial Magnetic Stimulation
Motor evoked potentials were produced and recorded by stimulating the motor cortex contralateral to the VL being tested. Stimuli were induced by a double cone coil connected to two Magstim 2002 units joined by a BiStim2 module (The Magstim Company, Whitland, UK). A posterioranterior orientation of the double cone coil was used to access the anatomical location of quadriceps representation on the motor homunculus. Each participant wore a lycra cap displaying a grid, which was used to locate and deliver stimuli at a number of locations until the optimal stimulating point (largest and most consistent observation of quadriceps MEPs) was found. The optimal stimulating point was marked and all subsequent testing was performed directly over this location. Stimuli were separated by at least three seconds, with the exception of paired pulse pulses during intracortical paradigms.
The resting motor threshold (RMT) was defined for all participants as the minimum stimulator intensity required to produce 5 of 10 MEPs whose peak-to-peak amplitudes exceeded 50 microvolts and were at least two standard deviations above resting EMG signal, as previously described (Stevens-Lapsley et al. 2013). Once RMT was determined, paired pulse measurements were used to quantify intracortical excitability using a sub-threshold conditioning stimulus (80% RMT) followed by a suprathreshold test stimulus (120% RMT). Pulses separated by 3 ms have been shown to produce an inhibitory effect on the test stimulus—known as short intracortical inhibition (SICI)—and pulses separated by 15 ms have been shown to produce a facilitory effect on the test stimulus (ICF) (Chen et al. 1998; Kujirai et al. 1993). Ten SICI followed by 10 ICF stimuli were administered, and one set of 10 stimuli at 120% RMT was then delivered for the purposes of normalizing paired-pulse MEPs.
2.4. Isometric Quadriceps Torque and Activation Testing
Isometric quadriceps torque and voluntary quadriceps activation testing using a twitch interpolation technique were performed, as previously described. (Behm et al. 2001; Behm et al. 1996; Mintken et al. 2007). A KIN-COM electromechanical dynamometer (Chattanooga Group, Inc., Chattanooga, TN) was used to measure quadriceps torque. Data were collected using a Biopac Data Acquisition System (MP 100, BIOPAC Systems, Inc., Goleta, CA) and subsequently analyzed with AcqKnowledge software, Version 3.8.2 (BIOPAC Systems, Inc., Goleta, CA). Participants were positioned in the KIN-COM dynamometer with 60° of knee flexion and asked to perform a maximal voluntary isometric contraction (MVIC) of the quadriceps muscle. Participants were provided both visual and verbal feedback for 3 trials unless the first 2 trials were consistent within 5 percent of one another. The trial with the largest MVIC torque output was used for data analysis. A high voltage constant current stimulator (model DS7AH, Digitimer Ltd., Hertfordshire, England) was used for voluntary muscle activation testing via self-adherent, flexible electrodes (7.6 × 12.7 cm, Supertrodes, SME, Inc).[ref] Voluntary activation of the quadriceps muscle was assessed using the twitch interpolation technique, where a supramaximal stimulus was applied directly to the contracting quadriceps muscle during an MVIC and again immediately afterwards, when the muscle was at rest (stimulus parameters: 1-pulse, 500-μs pulse duration, 400V) (Mizner et al. 2005b; Mizner et al. 2003; Stevens et al. 2003). The increase in torque observed as a result of the stimulus applied during the MVIC was then normalized to the torque observed during the resting stimulus. This value represented the decrease in activation capacity for the quadriceps, which was subtracted from 1.0 to obtain the CAD. Thus, CAD values less than 1.0 represent incomplete motor unit recruitment or failure to achieve maximal discharge rate from recruited motor units (Behm et al. 2001).
2.5. Pain and Self-reported Function
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) was used to evaluate self-report of knee-specific impairment in patients with knee OA. It assesses the pain, joint stiffness, and perceived disability associated with OA to determine the overall impact on a patients’ perceived function. The pain subscale is a 5-item, 20-point scale that assesses pain in everyday situations (e.g. walking on flat surfaces). The stiffness subscale is a 2-item, 10-point scale that assesses perceived knee stiffness after walking and at the end of the day. The disability subscale is 17-item, 68-point scale that assesses perceived physical function in a variety of everyday situations (e.g. getting in and out of a car). The WOMAC is a valid, reliable, and responsive instrument that is commonly used to assess pain and disability in studies of knee OA (Bellamy et al. 1988; Jinks et al. 2002; Nebel et al. 2009).
2.6. Data Processing and Analysis
Data for each TMS paradigm were averaged within each individual and then averaged across individuals to calculate group means. Paired pulse values are reported as the ratio of the mean MEP amplitude following the delivery of the test pulse, compared to the mean unconditioned single pulse (120% RMT) MEP amplitude averaged within and then across participants. Group differences in unconditioned pulse (120% RMT) amplitude were also examined to ensure that comparisons of paired pulse amplitudes would not be confounded by differences in this parameter.
Outcome measures and demographic variables (age and BMI) between patients with knee OA and healthy controls were compared using independent samples t-tests assuming unequal variance. Difference in sex distribution between groups was assessed using a chi-square test for proportions. Pearson correlation coefficients were calculated between TMS measures (RMT, ICI and ICF) and outcomes of clinical relevance (quadriceps torque, CAD and pain) for patients with knee OA. Statistical significance was set at an alpha level of 0.05. Data are reported as mean ± standard error of the mean (SEM) unless otherwise indicated. All statistical analyses were performed with Statistical Package for the Social Sciences, Version 16.0 (SPSS Inc, Chicago, IL).
3. Results
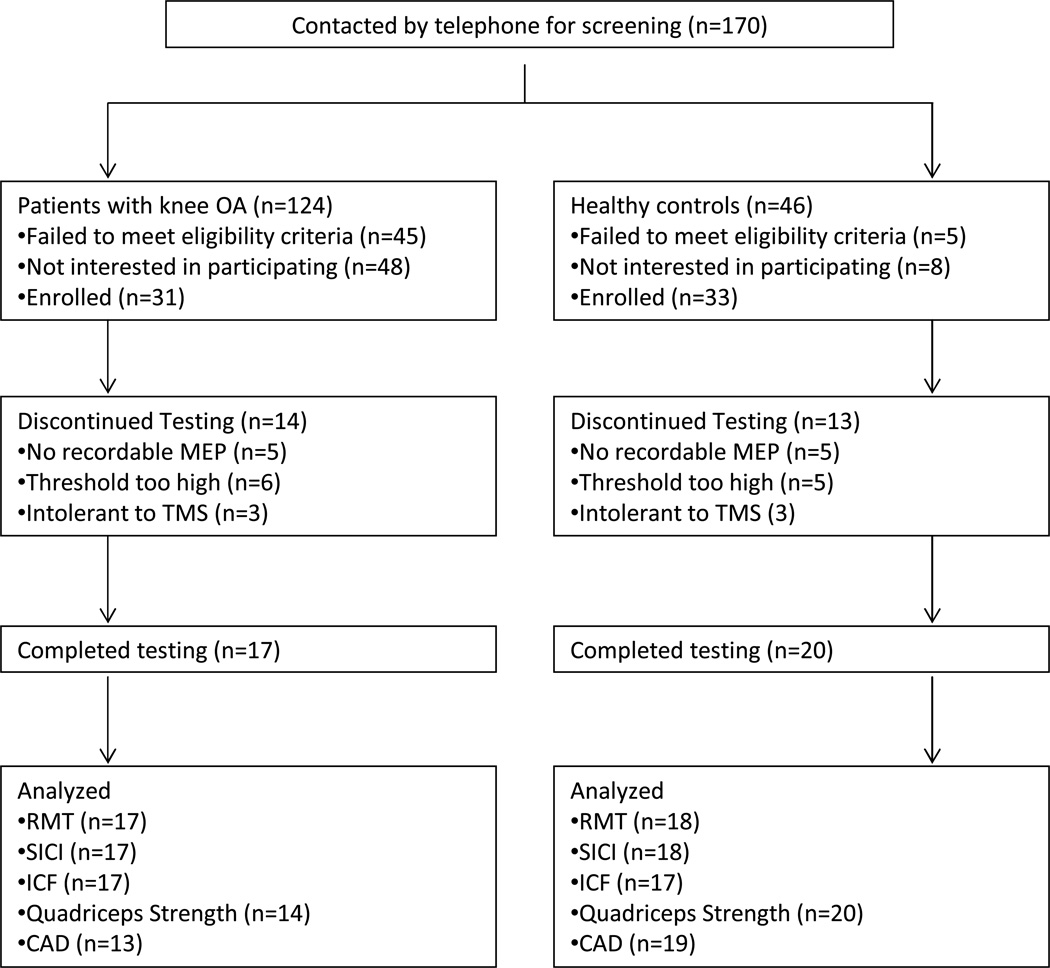
Thirty-one patients with knee OA and 33 healthy controls met the inclusion criteria. Seventeen patients with knee OA (age: 63.9 ± 1.8 years; body mass index [BMI]: 28.3 ± 1.0 kg/m2; 8 males and 9 females) and 20 healthy controls (age: 58.3 ± 2.5 years; BMI: 25.0 ± 2.5 kg/m2; 10 males and 10 females) completed testing. Demographics of study participants are shown in Table 1, and a detailed description of screening, inclusion and exclusion from testing or data analysis is shown in Fig. 1.
Table 1.
Demographics of study participants (Mean ± SEM).
| Patients with knee OA (n=17) |
Healthy Controls (n=20) |
p | |
|---|---|---|---|
| Sex (% female) | 53 | 50 | 0.860 |
| Age (yrs) | 63.9 ± 1.8 | 58.3 ± 2.5 | 0.085 |
| BMI (Kg/m2) | 28.3 ± 1.0 | 25.0 ± 2.5 | 0.018 |
Fig. 1.
Flow chart for participant inclusion and exclusion and available data for each testing paradigm
RMT= resting motor threshold; SICI= short interval intracortical inhibition; ICF= intracortical facilitation
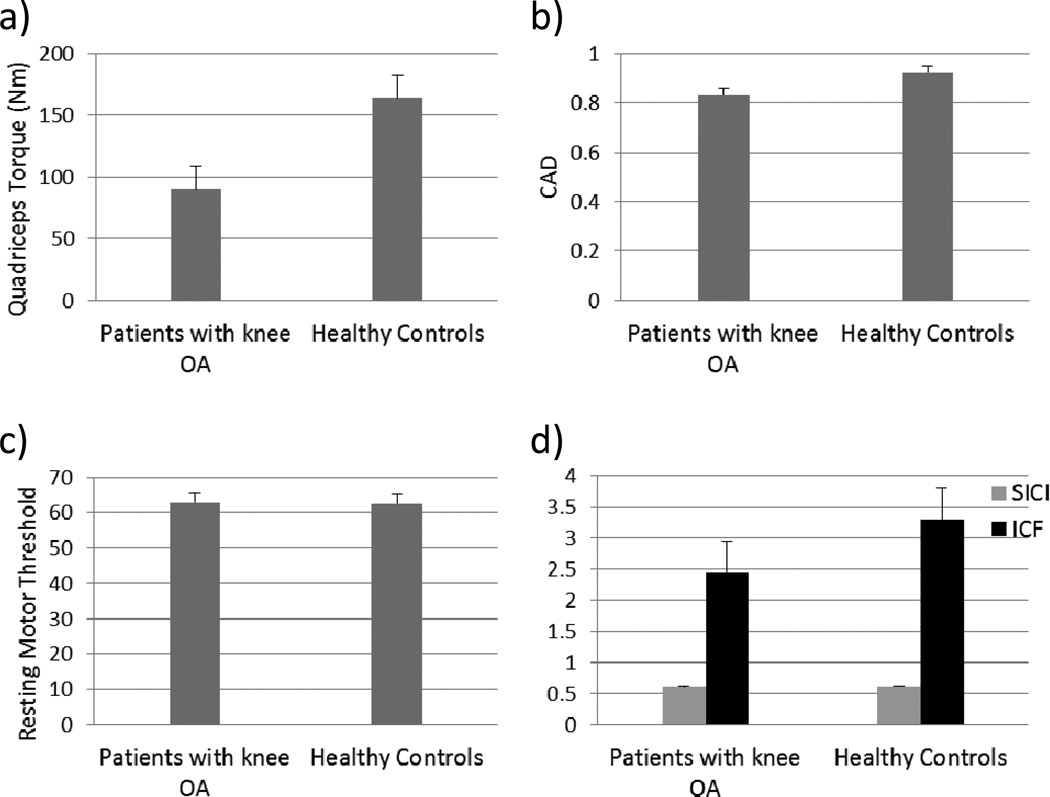
As expected, patients with knee OA demonstrated diminished quadriceps torque (Table 2), compared to healthy controls (p=0.004). Differences were also noted in CAD, although they failed to reach statistical significance (p=0.059). Contrary to our hypothesis, there were no significant differences observed in corticospinal excitability (Table 2) between patients with knee OA and healthy controls, as measured by RMT (P=0.931). Furthermore, although the paired pulse paradigms used to assess SICI and ICF produced inhibition and facilitation as expected (Table 2), there were no significant differences in intracortical excitability for SICI (p=0.946) or ICF (p=0.255) between patients with knee OA and healthy controls (Fig. 2). There were also no group differences observed in unconditioned pulse (120%) amplitude (Table 2).
Table 2.
Muscle function, spinal reflex excitability, corticospinal excitability and intracortical excitability for participants undergoing TKA and healthy older adults (mean ± SEM).
| Patients with Knee OA |
Healthy Controls |
p | |
|---|---|---|---|
| Quadriceps torque (Nm) | 89.9 ± 13.1 | 163.7 ± 18.6 | 0.004 |
| Voluntary activation | 0.83 ± 0.04 | 0.92 ± 0.02 | 0.059 |
| UPA (mV) | 0.31 ± 0.06 | 0.28 ± 0.04 | 0.600 |
| Corticospinal excitability | |||
| RMT (%) | 62.9 ± 2.1 | 62.6 ± 3.1 | 0.931 |
| Intracortical measures | |||
| SICI | 0.61 ± 0.10 | 0.62 ± 0.10 | 0.946 |
| ICF | 2.44 ± 0.46 | 3.29 ± 0.55 | 0.255 |
UPA; Unconditioned Pulse Amplitude
RMT; Resting Motor Threshold (% machine output)
SICI; Short Intracortical Inhibition
ICF; Intracortical Facilitation
Fig. 2.
Bar charts for group comparisons in a) quadriceps torque, b) voluntary activation, as measured by normalized twitch interpolation technique, c) corticospinal excitability, as measured by RMT of quadriceps, and d) intracortical inhibition and intracortical facilitation, as measured by SICI and ICF paradigms, respectively. Data are shown as mean ± SEM
RMT; Resting Motor Threshold
SICI; Short Intracortical Inhibition
ICF; Intracortical Facilitation
For patients with knee OA, RMT was found to be significantly correlated with measures of strength and pain (Table 3). There was a significant negative association between RMT and pain (r = −0.575; p = 0.016). We also observed a significant positive association between RMT and quadriceps torque (r = 0.691, p = 0.009). Because of the potential for incomplete muscle relaxation among patients with high pain levels to influence RMT, we examined both the bivariate correlation between RMT and pain, as well as this correlation with the addition of resting EMG signal (in the 25 ms prior to TMS stimulation) as a covariate. The correlation between RMT and pain remained significant even after controlling for resting EMG signal, thus ruling out a potential confounding effect of incomplete muscle relaxation in patients with higher pain levels.
Table 3.
Pearson correlation coefficients for associations between TMS measures and measures of CAD, quadriceps strength, and self-reported pain, stiffness and disability. The correlation between quadriceps torque and pain is also shown.
| WOMAC | |||||
|---|---|---|---|---|---|
| Voluntary Activation |
Quadriceps Torque (Nm) |
Pain | Stiffness | Disability | |
| RMT | 0.285 | 0.691** | −0.575* | −0.431 | −0.480 |
| SICI | −0.278 | 0.322 | −0.306 | −0.114 | −0.158 |
| ICF | −0.334 | 0.394 | −0.495* | −0.548* | −0.439 |
| Quadriceps Torque (Nm) | −0.821** | ||||
RMT; Resting Motor Threshold
SICI; Short Intracortical Inhibition
ICF; Intracortical Facilitation
correlation is significant at p=0.05
correlation is significant at p=0.01
There were significant associations found between ICF and measures of self-reported pain (r = − 0.495, p = 0.043) and stiffness (r = −0.548, p = 0.023), although the relationship between ICF and quadriceps torque was not statistically significant (r = 0.394, p = 0.183). There were no significant associations observed between CAD and measures of corticospinal or intracortical excitability (Table 3). Measures of intracortical inhibition (SICI) were not significantly associated with quadriceps torque, pain, or self-reported function.
4. Discussion
To our knowledge, this is the first study to use TMS to examine intracortical and corticospinal mechanisms for quadriceps CAD in patients with knee OA. Contrary to our hypothesis, we did not find significant differences in RMT, SICI or ICF between patients with knee OA and healthy controls. We also did not observe a significant association between any of the TMS measures and CAD. This suggests against a direct role of primary motor cortex in the mechanisms of CAD. However, for patients with knee OA, we found significant associations between measures of corticospinal and intracortical excitability and measures of strength and pain. There was a positive correlation between RMT and strength, such that patients with lower RMT (higher overall corticospinal excitability) demonstrated reduced quadriceps torque. There was also a significant negative correlation between RMT and pain, indicating that patients with higher levels of pain tended to demonstrate greater excitability of the quadriceps corticospinal system. We also found a significant negative correlation between ICF and pain, suggesting that patients with higher levels of pain tended to demonstrate reduced intracortical facilitation. In this study, MEPs during the SICI paradigm did not appear to be associated with strength or pain.
This study adds to our knowledge of the complex processes involved with pain and neuromuscular control of the quadriceps in patients with knee OA. The diagnosis, “knee OA” is clinically defined by the presence of pain (Zhang et al. 2010), and both quadriceps strength and voluntary activation deficits often co-occur with this diagnosis (O'Reilly et al. 1998; Petterson et al. 2008; Pietrosimone et al. 2011). Our data provide further evidence supporting a strong association between quadriceps torque and pain (Table 3). Our data also link corticospinal excitability (assessed by RMT) with both quadriceps torque and pain. However, our measures of corticospinal and intracortical excitability were not associated with central activation deficits (CAD), nor were there group differences in any TMS measures. This suggests against a major role for primary motor cortex in the mechanisms of voluntary activation deficits in patients with knee OA. The motor output of the quadriceps can be conceptualized as the sum of excitatory and inhibitory processes throughout the neuraxis, and our measure of CAD also reflects a balance of multiple neurophysiological processes. The lack of an association between intracortical measures and CAD may therefore implicate areas other than primary motor cortex in the etiology of CAD. Specifically, further research on measures of spinal excitability, such as Hoffman’s reflex, could provide insight into the relative contributions of cortical and spinal excitability in quadriceps activation for patients with knee OA.
Although we did not observe group differences in measures of intracortical facilitation or inhibition, the inverse relationship observed between pain and ICF suggests that motor cortex activity may indeed change with disease severity in knee OA. There is now evidence in favor of sensory and premotor cortical changes in this population, with recent studies demonstrating poor performance of a motor imagery task and reduced tactile acuity at the knee among patients with knee OA (Stanton et al. 2013; Stanton et al. 2012). Future research could seek to assess motor cortex somatotopy in knee OA (via cortical mapping) to determine if sensorimotor cortical reorganization relates to cortical excitability and changes in motor control.
When considering the behavior of TMS measures across chronic pain populations, group differences may or may not manifest, depending on the nociceptive mechanisms involved. For example, no significant differences in motor threshold were found between a group of patients with CRPS and healthy controls, but a subset of patients with allodynia were found to exhibit significantly lower motor thresholds (Schwenkreis et al. 2003). On the other hand, patients with low back pain have been shown to exhibit elevated active motor thresholds of the erector spinae muscles—or less overall corticospinal excitability—compared to healthy controls (Strutton et al. 2005). In a recent study comparing patients with upper extremity neuralgia to patients with OA of the hand and healthy controls, no differences in TMS measures were found between patients with OA and healthy older adults. However, cortical disinhibition (increased intracortical facilitation and reduced intracortical inhibition) was found to occur in patients with neuralgia, relative to both controls and patients with OA (Schwenkreis et al. 2010). Furthermore, among patients with neuralgia, cortical disinhibition appeared to be related to pain intensity rather than severity of nerve damage, suggesting a link between neuropathic pain mechanisms and changes in motor cortex function.
Our results are partially consistent with these reports. We found no differences in TMS measures between patients with knee OA and healthy older adults. However, we found a significant negative correlation between intracortical facilitation and pain among patients with knee OA. The study by Schwenkreis et al. (2010) did not examine within-group correlations between TMS measures and pain among patients with OA, so it is not known whether their findings of pain-related cortical disinhibition among patients with upper extremity neuralgia would be replicated or contradicted in the OA group. Nevertheless, our results support the overall finding that mechanisms governing the relationship between pain and motor cortex function differ based on pain mechanisms. Patients with nerve injury or central neuropathic pain appear to exhibit cortical disinhibition, while patients with OA (with pain resulting from direct nociceptor stimulation) appear not to differ significantly from healthy controls in measures of cortical/corticospinal excitability. While our results provide evidence that pain-dependent cortical/corticospinal changes may occur in knee OA, the magnitude of these changes appears reduced relative to more severe neuropathic pain conditions.
The functional connectivity between the pain system and other sensorimotor systems is likely to differ across pain pathologies (Lefaucheur et al. 2006; Schwenkreis et al. 2010). Pain conditions that are associated with profound central changes (e.g. complex regional pain syndrome) appear to involve widespread cortical changes (Juottonen et al. 2002; Maihofner et al. 2007; Schwenkreis et al. 2003), whereas conditions such as peripheral nerve injury—with consistent ectopic peripheral bombardment of the nociceptive system—appear to demonstrate hemisphere-specific motor cortical changes (Schwenkreis et al. 2010). The type and severity of nociceptive activity in knee OA may not be sufficient to induce the same cortical changes observed in these neurologically driven pain pathologies.
In addition, for patients with knee OA, motor demands placed on the quadriceps may necessitate a different balance of cortical and corticospinal excitability than has been observed in other pain conditions. Multiple studies have revealed an inverse relationship between quadriceps strength and pain in knee OA (Hall et al. 2006; O'Reilly et al. 1998; Segal et al. 2010). At the same time, a certain amount of quadriceps force may be required for performance of common functional tasks, in spite of pain (Mizner et al. 2005a; Sharma et al. 2003; Yoshida et al. 2008). The results of our study suggest that an increased quadriceps corticospinal excitability is associated with higher pain levels in patients with knee OA, which may represent a neurophysiological adaptation to reduced strength in this important force generator. Somewhat paradoxically, we also observed an inverse relationship between ICF and pain, which suggests that systemic neurophysiological changes are not built simply around the goal of improving quadriceps force production.
The finding that pain is associated with both reduced intracortical facilitation and elevated corticospinal excitability seems contradictory. However, it is important to remember that these measures offer relatively static assessments of motor neurophysiology in a resting muscle, while the functional requirements of the knee joint and the pain associated with knee OA are complex and dynamic. According to the recent work of Hodges and Tucker (in their theory of motor adaptation to pain), pain-related changes in motor activity may not consistently involve excitation or inhibition but may instead occur in variable patterns that ultimately serve to protect the painful body part and reduce the overall experience of pain (Hodges and Tucker 2011). In the case of knee OA, this could conceivably involve an attempt to maximize quadriceps strength during dynamic functional tasks (to attenuate forces through the knee joint) while—at the same time—attempting to reduce resting knee joint compressive forces through selective inhibition of the quadriceps, perhaps via reduced ICF at the level of primary motor cortex. In other chronic pain conditions, including upper extremity neuralgia, the balance between functional motor demands and pain—and the influence of motor activity on nociception—may simply require different patterns of intracortical and corticospinal excitability. Longitudinal studies will be required to parse the time-course of these changes and help elucidate directional or causal relationships between pain and altered cortical excitability. At this point, it remains unclear whether the observed neurophysiological changes contribute to disease pathology or represent an adaptive response to pain.
The time period immediately following knee replacement surgery tends to be characterized by both a dramatic increase in CAD as well as a predictable resolution of pain. Our results are consistent with the idea that pain influences cortical activity and corticospinal excitability. Longitudinal studies of patients undergoing knee replacement surgery may therefore help to clarify the complex associations between pain, CAD and TMS measures of CNS excitability in patients with knee OA.
This study has several limitations that are important to note. Due to our exclusion criteria and the difficulty in obtaining TMS measures in all participants, these data reflect only a subset of all potential healthy controls and patients with knee OA. Although the rates of missing data are similar between groups, we cannot rule out the possibility of a selection bias that influences group comparisons and within-group correlations. Intra- and inter-individual variability is high in TMS, with reports of up to 50% variability, depending on the measurement technique (Wassermann 2002). Our study did not control for factors such as caffeine and alcohol intake, circadian rhythm, circulating hormone levels, or other influences on neural function that are thought to contribute to the variability in TMS measures (Wassermann 2002).
As mentioned, resting motor threshold—a surrogate of corticospinal excitability—reflects a balance of multiple processes occurring throughout the neuraxis and therefore cannot directly implicate spinal or cortical processes. Future studies should consider implementing measures of spinal excitability (e.g. Hoffman’s reflex) or other measures of corticospinal excitability, such as stimulus response curves, to provide additional or confirmatory information on the anatomic locations responsible for disease-specific or pain-specific changes in motor neurophysiology. We also did not perform measures of systemic arousal in this study (e.g. vascular responses to stress) so we cannot rule out a possible confounding effect of “arousal” in the relationship between pain and motor threshold. However, regardless of the mechanisms by which pain and motor neurophysiology are associated, we feel the relationship is of potential significance to our understanding of knee OA pathophysiology. Specifically, future work may seek to examine this relationship, perhaps by including measures of arousal or other important covariates to help clarify mechanisms and elucidate potential targets for intervention.
5. Conclusion
Corticospinal and intracortical excitability were similar between patients with knee OA and healthy controls. For patients with knee OA, there were significant associations observed between pain and RMT, as well as between pain and ICF, suggesting a possible role for pain in the neural mechanisms of quadriceps motor control in knee OA. No associations were observed between CAD and measures of corticospinal or intracortical excitability. Given the importance of quadriceps strength to mobility and physical performance in patients with knee OA, future studies should focus on elucidating the complex mechanisms involved in CAD, perhaps by further investigating the role of pain in quadriceps motor neurophysiology.
Acknowledgement
We thank Katrina Maluf PhD, Kristin Thomas, and Ryan Marker for their contributions to this investigation.
Role of the funding source
Support for this study was provided by the National Institutes of Health (R01-HD065900; K23-AG029978, KL2-TR000156, T32 AG000279), and the Foundation for Physical Therapy (PODS I award). Sponsors had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Conflict of interest statement: The authors declare that they have no conflict of interest.
References
- Behm D, Power K, Drinkwater E. Comparison of interpolation and central activation ratios as measures of muscle inactivation. Muscle Nerve. 2001;24:925–934. doi: 10.1002/mus.1090. doi:10.1002/mus.1090 [pii] [DOI] [PubMed] [Google Scholar]
- Behm DG, St-Pierre DM, Perez D. Muscle inactivation: assessment of interpolated twitch technique. Journal of applied physiology. 1996;81:2267–2273. doi: 10.1152/jappl.1996.81.5.2267. [DOI] [PubMed] [Google Scholar]
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- Caramia MD, et al. Brain excitability changes in the relapsing and remitting phases of multiple sclerosis: a study with transcranial magnetic stimulation. Clin Neurophysiol. 2004;115:956–965. doi: 10.1016/j.clinph.2003.11.024. doi:10.1016/j.clinph.2003.11.024 S1388245703004383 [pii] [DOI] [PubMed] [Google Scholar]
- Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998;80:2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Fitzgerald GK, Piva SR, Irrgang JJ, Bouzubar F, Starz TW. Quadriceps activation failure as a moderator of the relationship between quadriceps strength and physical function in individuals with knee osteoarthritis. Arthrit Rheum-Arthr. 2004;51:40–48. doi: 10.1002/art.20084. doi. [DOI] [PubMed] [Google Scholar]
- Gibbons CE, Pietrosimone BG, Hart JM, Saliba SA, Ingersoll CD. Transcranial magnetic stimulation and volitional quadriceps activation. J Athl Train. 2010;45:570–579. doi: 10.4085/1062-6050-45.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MC, Mockett SP, Doherty M. Relative impact of radiographic osteoarthritis and pain on quadriceps strength, proprioception, static postural sway and lower limb function. Ann Rheum Dis. 2006;65:865–870. doi: 10.1136/ard.2005.043653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges PW, Tucker K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain. 2011;152:S90–S98. doi: 10.1016/j.pain.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Hunt MA, Zabukovec JR, Peters S, Pollock CL, Linsdell MA, Boyd LA. Reduced quadriceps motor-evoked potentials in an individual with unilateral knee osteoarthritis: a case report Case Report. Rheumatol. 2011;2011:537420. doi: 10.1155/2011/537420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley MV. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 1999;25:283–298. vi. doi: 10.1016/s0889-857x(05)70068-5. [DOI] [PubMed] [Google Scholar]
- Jinks C, Jordan K, Croft P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain. 2002;100:55–64. doi: 10.1016/s0304-3959(02)00239-7. doi:S0304395902002397 [pii] [DOI] [PubMed] [Google Scholar]
- Juottonen K, Gockel M, Silen T, Hurri H, Hari R, Forss N. Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain. 2002;98:315–323. doi: 10.1016/S0304-3959(02)00119-7. [DOI] [PubMed] [Google Scholar]
- Kujirai T, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic. pain Neurology. 2006;67:1568–1574. doi: 10.1212/01.wnl.0000242731.10074.3c. [DOI] [PubMed] [Google Scholar]
- Lewek MD, Rudolph KS, Snyder-Mackler L. Quadriceps femoris muscle weakness and activation failure in patients with symptomatic knee osteoarthritis. J Orthopaed Res. 2004;22:110–115. doi: 10.1016/S0736-0266(03)00154-2. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liikavainio T, et al. Loading and gait symmetry during level and stair walking in asymptomatic subjects with knee osteoarthritis: importance of quadriceps femoris in reducing impact force during heel strike? Knee. 2007;14:231–238. doi: 10.1016/j.knee.2007.03.001. doi:S0968-0160(07)00029-4 [pii].10.1016/j.knee.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Maihofner C, et al. The motor system shows adaptive changes in complex regional pain syndrome. Brain. 2007;130:2671–2687. doi: 10.1093/brain/awm131. [DOI] [PubMed] [Google Scholar]
- McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis. 1993;52:258–262. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintken PE, Carpenter KJ, Eckhoff D, Kohrt WM, Stevens JE. Early neuromuscular electrical stimulation to optimize quadriceps muscle function following total knee arthroplasty: a case report. J Orthop Sports Phys Ther. 2007;37:364–371. doi: 10.2519/jospt.2007.2541. [DOI] [PubMed] [Google Scholar]
- Mizner RL, Petterson SC, Stevens JE, Axe MJ, Snyder-Mackler L. Preoperative quadriceps strength predicts functional ability one year after total knee arthroplasty. J Rheumatol. 2005a;32:1533–1539. doi:0315162X-32-1533 [pii] [PubMed] [Google Scholar]
- Mizner RL, Petterson SC, Stevens JE, Vandenborne K, Snyder-Mackler L. Early quadriceps strength loss after total knee arthroplasty - The contributions of muscle atrophy and failure of voluntary muscle activation. Journal of Bone and Joint Surgery-American. 2005b;87A:1047–1053. doi: 10.2106/JBJS.D.01992. doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizner RL, Stevens JE, Snyder-Mackler L. Voluntary activation and decreased force production of the quadriceps femoris muscle after total knee arthroplasty. Phys Ther. 2003;83:359–365. [PubMed] [Google Scholar]
- Nebel MB, et al. The relationship of self-reported pain and functional impairment to gait mechanics in overweight and obese persons with knee osteoarthritis. Arch Phys Med Rehabil. 2009;90:1874–1879. doi: 10.1016/j.apmr.2009.07.010. doi:S0003-9993(09)00670-4 [pii] 10.1016/j.apmr.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly SC, Jones A, Muir KR, Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis. 1998;57:588–594. doi: 10.1136/ard.57.10.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri-Smith RM, Kreinbrink J, Ashton-Miller JA, Wojtys EM. Quadriceps inhibition induced by an experimental knee joint effusion affects knee joint mechanics during a single-legged drop landing. Am J Sports Med. 2007;35:1269–1275. doi: 10.1177/0363546506296417. doi:0363546506296417 [pii].10.1177/0363546506296417. [DOI] [PubMed] [Google Scholar]
- Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petterson SC, Barrance P, Buchanan T, Binder-Macleod S, Snyder-Mackler L. Mechanisms underlying quadriceps weakness in knee osteoarthritis. Med Sci Sports Exerc. 2008;40:422–427. doi: 10.1249/MSS.0b013e31815ef285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrosimone BG, Hertel J, Ingersoll CD, Hart JM, Saliba SA. Voluntary quadriceps activation deficits in patients with tibiofemoral osteoarthritis: a meta-analysis. PM & R : the journal of injury, function, and rehabilitation. 2011;3:153–162. doi: 10.1016/j.pmrj.2010.07.485. quiz 162. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, et al. Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology. 2003;61:515–519. doi: 10.1212/wnl.61.4.515. [DOI] [PubMed] [Google Scholar]
- Schwenkreis P, Scherens A, Ronnau AK, Hoffken O, Tegenthoff M, Maier C. Cortical disinhibition occurs in chronic neuropathic, but not in chronic nociceptive pain. BMC neuroscience. 2010;11:73. doi: 10.1186/1471-2202-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal NA, et al. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort Osteoarthritis and cartilage / OARS, Osteoarthritis. Research Society. 2010;18:769–775. doi: 10.1016/j.joca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakespeare DT, Stokes M, Sherman KP, Young A. Reflex inhibition of the quadriceps after meniscectomy: lack of association with pain. Clin Physiol. 1985;5:137–144. doi: 10.1111/j.1475-097x.1985.tb00589.x. [DOI] [PubMed] [Google Scholar]
- Sharma L, Cahue S, Song J, Hayes K, Pai YC, Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local mechanical, and neuromuscular factors. Arthritis Rheum. 2003;48:3359–3370. doi: 10.1002/art.11420. [DOI] [PubMed] [Google Scholar]
- Stanton TR, Lin CW, Bray H, Smeets RJ, Taylor D, Law RY, Moseley GL. Tactile acuity is disrupted in osteoarthritis but is unrelated to disruptions in motor imagery performance. Rheumatology (Oxford) 2013;52:1509–1519. doi: 10.1093/rheumatology/ket139. doi:ket139 [pii] 10.1093/rheumatology/ket139. [DOI] [PubMed] [Google Scholar]
- Stanton TR, Lin CW, Smeets RJ, Taylor D, Law R, Lorimer Moseley G. Spatially defined disruption of motor imagery performance in people with osteoarthritis. Rheumatology (Oxford) 2012;51:1455–1464. doi: 10.1093/rheumatology/kes048. doi:kes048 [pii] 10.1093/rheumatology/kes048. [DOI] [PubMed] [Google Scholar]
- Stevens-Lapsley JE, Thomas AC, Hedgecock JB, Kluger BM. Corticospinal and intracortical excitability of the quadriceps in active older and younger healthy adults. Arch Gerontol Geriatr. 2013;56:279–284. doi: 10.1016/j.archger.2012.06.017. doi:S0167-4943(12)00141-0 [pii] 10.1016/j.archger.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JE, Mizner RL, Snyder-Mackler L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J Orthop Res. 2003;21:775–779. doi: 10.1016/S0736-0266(03)00052-4. doi:S0736026603000524. [pii] 10.1016/S0736-0266(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Strutton PH, Theodorou S, Catley M, McGregor AH, Davey NJ. Corticospinal excitability in patients with chronic low back pain. J Spinal Disord Tech. 2005;18:420–424. doi: 10.1097/01.bsd.0000169063.84628.fe. doi:00024720-200510000-00008.[pii] [DOI] [PubMed] [Google Scholar]
- Urbach D, Nebelung W, Becker R, Awiszus F. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris a prospective twitch interpolation study. J Bone Joint Surg Br. 2001;83:1104–1110. doi: 10.1302/0301-620x.83b8.11618. [DOI] [PubMed] [Google Scholar]
- Urbach D, Nebelung W, Weiler HT, Awiszus F. Bilateral deficit of voluntary quadriceps muscle activation after unilateral ACL tear. Med Sci Sports Exerc. 1999;31:1691–1696. doi: 10.1097/00005768-199912000-00001. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002;113:1165–1171. doi: 10.1016/s1388-2457(02)00144-x. doi:S138824570200144X [pii] [DOI] [PubMed] [Google Scholar]
- Wittenberg GF, Bastings EP, Fowlkes AM, Morgan TM, Good DC, Pons TP. Dynamic course of intracortical TMS paired-pulse responses during recovery of motor function after stroke. Neurorehabil Neural Repair. 2007;21:568–573. doi: 10.1177/1545968307302438. doi:1545968307302438 [pii].10.1177/1545968307302438. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Mizner RL, Ramsey DK, Snyder-Mackler L. Examining outcomes from total knee arthroplasty and the relationship between quadriceps strength and knee function over time. Clin Biomech (Bristol, Avon) 2008;23:320–328. doi: 10.1016/j.clinbiomech.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, et al. EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis. 2010;69:483–489. doi: 10.1136/ard.2009.113100. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]