Abstract
Specification of the trophectoderm (TE) and inner cell mass (ICM) lineages in the mouse blastocyst correlates with cell position, as TE derives from outer cells whereas ICM from inner cells. Differences in position are reflected by cell polarization and Hippo signaling. Only in outer cells, the apical-basal cell polarity is established, and Hippo signaling is inhibited in such a manner that LATS1 and 2 (LATS1/2) kinases are prevented from phosphorylating YAP, a key transcriptional co-activator of the TE-specifying gene Cdx2. However, the molecular mechanisms that regulate these events are not fully understood. Here, we showed that inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling and disruption of apical-basal polarity. Embryos treated with ROCK inhibitor Y-27632 exhibited elevated expression of ICM marker NANOG and reduced expression of CDX2 at the blastocyst stage. Y-27632-treated embryos failed to accumulate YAP in the nucleus, although it was rescued by concomitant inhibition of LATS1/2. Segregation between apical and basal polarity regulators, namely PARD6B, PRKCZ, SCRIB, and LLGL1, was dampened by Y-27632 treatment, whereas some of the polarization events at the late 8-cell stage such as compaction and apical localization of p-ERM and tyrosinated tubulin occurred normally. Similar abnormalities of Hippo signaling and apical-basal polarization were also observed in embryos that were treated with RHO GTPases inhibitor. These results suggest that RHO-ROCK signaling plays an essential role in regulating Hippo signaling and cell polarization to enable proper specification of the ICM and TE lineages.
Keywords: Inner cell mass, trophectoderm, cell polarity, cell lineage, YAP, CDX2
Introduction
The first cell differentiation in mammalian development is the establishment of the two cell lineages, inner cell mass (ICM) and trophectoderm (TE). ICM is a population of pluripotent stem cells that give rise to all cell types in the body, whereas TE contributes only to extraembryonic tissues, namely trophoblasts of the placenta. When the embryo reaches the blastocyst stage, ICM and TE are distinguishable by their morphology as well as gene expression patterns (Fujimori, 2010; Marikawa and Alarcon, 2012; Stephenson et al., 2012; Zernicka-Goetz et al., 2009). TE forms a monolayer of epithelium surrounding a fluid-filled cavity, and ICM forms a single, ovoid-shaped cell aggregate that is situated within the cavity. TE expresses homeodomain-containing CDX2 and GATA family member GATA3, whereas ICM expresses POU-domain-containing POU5F1 and homeodomain-containing NANOG. These transcription factors play essential roles in maintaining the characteristics of each lineage, as demonstrated by gene knockout and knockdown studies (Chambers et al., 2003; Home et al., 2009; Mitsui et al., 2003; Nichols et al., 1998; Strumpf et al., 2005). While the nature of the initial signals that specify the two cell lineages during early development is somewhat controversial (Blij et al., 2012; Hiiragi et al., 2006; Takaoka and Hamada, 2012; Zernicka-Goetz, 2013), it is evident that the position of cells within an embryo is critical to direct lineage specification toward TE or ICM. At around 32-cell stage, cells located in the outer layer of the embryo are committed to differentiating into TE, whereas those positioned inside are destined to become ICM, as demonstrated by cell dissociation and cell repositioning experiments (Marikawa and Alarcon, 2012; Sasaki, 2010; Stephenson et al., 2012; Suwinska et al., 2008; Zernicka-Goetz et al., 2009). Thus, elucidation of the molecular mechanisms of how a cell interprets positional information to execute lineage-specific gene expression programs as well as to adopt lineage-specific morphology should help in understanding how the mammalian embryo regulates its first cell differentiation event.
Hippo signaling controls organ size through cell proliferation and apoptosis, and many components of the signaling pathway are conserved in various animals, ranging from fruitfly to mammals (Johnson and Halder, 2014; Yu and Guan, 2013). Key components of the Hippo signaling pathway play pivotal roles in cell-lineage specification in the mouse blastocyst. TEA-domain transcription factor TEAD4 is a downstream effector of Hippo signaling and is essential for TE formation (Nishioka et al., 2008; Yagi et al., 2007). While ubiquitously expressed in the embryo, TEAD4 activates Cdx2 expression specifically in TE, because its transcriptional coactivator Yes-associated protein (YAP) accumulates in the nucleus only in the outer cells (Hirate et al., 2012; Nishioka et al., 2009). YAP is retained in the cytoplasm in inner cells, owing to its phosphorylation by kinases LATS1 and 2 (LATS1/2) (Hao et al., 2008). Loss of function of LATS1/2 in mouse embryos leads to ubiquitous nuclear YAP localization and Cdx2 expression throughout the embryo, including inner cells, while they form the blastocyst cavity (Lorthongpanich et al., 2013; Nishioka et al., 2009). Recent studies have also revealed other regulators of Hippo signaling that act upstream of LATS, namely AMOT and NF2, and play critical roles in lineage formation in the mouse blastocyst (Cockburn et al., 2013; Hirate et al., 2013; Leung and Zernicka-Goetz, 2013). Thus, differential control of Hippo signaling between inner and outer cells is a crucial element for the specification of ICM and TE in the mouse blastocyst, i.e., its inhibition induces TE lineage whereas its activation promotes ICM lineage.
Another key element for linking the positional information (i.e., inside versus outside) to lineage specification (i.e., ICM versus TE) is the establishment of apical-basal cell polarity. By the end of the 8-cell stage, the event known as compaction occurs, in which the overall appearance of the embryo becomes smooth due to enhanced cell-cell adhesion. During compaction, all eight cells start to exhibit polarity along the apical and basal axis. However, the subsequent cleavages to 16- to 32-cell stages generate inner and outer cell populations, and only outer cells further establish distinct apical and basal polarity, while inner cells remain nonpolarized (Eckert and Fleming, 2008; Stephenson et al., 2012). Various molecules have been identified that are localized to the apical or basal membrane in the outer cells, many of which are homologs of evolutionary conserved cell polarity regulators. For example, PARD3 (a par-3 homolog), PARD6B (a par-6 homolog) and PRKCI/PRKCZ (atypical protein kinase C or aPKC) are localized to the apical membrane, whereas SCRIB (a scribble homolog), LLGL1 (a lethal giant larva homolog) and MARK2 (a par-1 homolog) are confined to the basal membrane (Alarcon, 2010; Dard et al., 2009; Plusa et al., 2005; Tao et al., 2012; Vinot et al., 2005). Knockdown of PARD6B causes cavitation failure due to defective tight junction formation. Also, the expression of CDX2 is diminished while NANOG expression is elevated in PARD6B-knockdown embryos, indicating that PARD6B is essential for TE specification (Alarcon, 2010). Furthermore, a recent study has shown that knockdown of PARD6B impairs nuclear localization of YAP in outer cells (Hirate et al., 2013), suggesting that the activity of Hippo signaling is controlled by cell polarity regulators. Thus, delineating the molecular players that impact Hippo signaling as well as the apical-basal polarity is the key to understand the mechanisms of cell-lineage specification in the mouse blastocyst.
In the present study, we investigated the role of RHO-ROCK (Rho-associated kinase) signaling in lineage specification, specifically focusing on its link to Hippo signaling and apical-basal polarization. ROCK is a serine-threonine kinase and is activated by its association with RHO small GTPases (Amano et al., 2010; Amin et al., 2013; Nishioka et al., 2012; Thumkeo et al., 2013). ROCK phosphorylates a number of protein targets and regulates various cellular processes, such as cell migration, cytokinesis, and neurite elongation. It has been shown previously that inhibition of ROCK during mouse preimplantation development using a specific inhibitor, Y-27632, interferes with blastocyst cavity formation (Kawagishi et al., 2004), raising the possibility that RHO-ROCK signaling is required for TE lineage formation. Nonetheless, the impact of RHO-ROCK signaling inhibition on cell-lineage specification has not been explored. Moreover, recent studies with cultured cells showing that inhibition of RHO alters LATS1/2 activity and YAP localization (Mo et al., 2012; Yu et al., 2012; Zhao et al., 2012) warrant further investigations on the relationship between RHO-ROCK and Hippo signaling in mouse preimplantation embryos. Here, we report that inhibition of RHO-ROCK signaling enhances the ICM lineage and suppresses the TE lineage formation by impacting Hippo signaling and proper apical-basal cell polarization.
Materials and methods
Animals and collection of preimplantation embryos and oocytes
F1 (C57BL/6 × DBA/2) mice from the National Cancer Institute were used. Female mice were injected with pregnant mare serum gonadotropin and human chorionic gonadotropin (hCG) (EMD Millipore) at 48 hours (h) apart. For collection of preimplantation embryos, female mice were mated with males after injection with hCG, and one-cell (pronuclear) and two-cell stage embryos were collected from oviducts, using standard protocols (Nagy et al., 2003). For collection of oocytes, female mice were sacrificed about 18 hours after hCG injection and oocyte-cumulus cell complex was recovered from oviducts. Cumulus cells were removed by treatment with 75 U/mL hyaluronidase in FHM HEPES-buffered medium (EMD Millipore), and zona pellucida was digested with proteases (0.5% Pronase; Roche) in FHM. Oocytes were washed in FHM several times and carefully examined to confirm no cumulus cells remained. The protocol for animal handling and use was reviewed and approved by the University of Hawaii Institutional Animal Care and Use Committee.
Embryo treatment with pharmacological inhibitors
Stocks of ROCK inhibitor Y-27632 (20 mM, EMD Millipore) and RHO inhibitor I (100 µg/mL, Cytoskeleton) were dissolved in dimethyl sulfoxide and water, respectively, and stored at −20°C until ready for use. Embryos were treated in 20 µL droplets of Y-27632 (20 µM) and RHO inhibitor I (1 µg/mL) that were freshly diluted in KSOM-AA culture medium (MR-121-D, EMD Millipore), covered with mineral oil, and pre-equilibrated at 37°C with 5% CO2 in air. Control treatment was in 20 µL droplets that were prepared by adding dimethyl sulfoxide or water to KSOM-AA at a volume equal to that of the inhibitor.
Time-lapse cinematography
Embryo development was recorded in real time, as previously described (Alarcon, 2010). Briefly, embryos were cultured in a heated stage of an Axiovert 200 inverted microscope (Carl Zeiss), which was enclosed in an incubation chamber (PeCon). Temperature and CO2 concentration were regulated by Tempcontrol 37-2 and CO2-Controller (PeCon). Images were captured every 15 minutes, using AxioCam MRm digital camera, which was controlled by AxioVision software (Carl Zeiss). The incubation chamber was covered with a black plastic sheet during time-lapse recording.
Immunofluorescent staining
Procedure was performed, as previously described (Alarcon, 2010). Embryos were incubated in primary antibody at 4°C and in secondary antibody at the ambient temperature (22°-26°C). Primary antibodies were mouse anti-CDX2 (1:200; CDX2-88, BioGenex), rabbit anti-NANOG (1:800; RCAB0002P-F, Cosmo Bio), mouse anti-YAP1 (1:100; 2F12, Novus Biologicals), mouse anti-hemagglutinin (HA) peptide (1:100; 12CA5, Roche), rabbit anti-p-ERM (1:200; #3141S), rabbit anti-CDH1 (1:500; 24E10) and rabbit anti-PCM1 (1:500; G2000) from Cell Signaling Technology, rabbit anti-LLGL1 (1:100; M-102), rabbit anti-PARD6B (1:100; M-64), mouse anti-PRKCZ (1:100; H-1) and rabbit anti-SCRIB (1:100; H-300) from Santa Cruz Biotechnology, rabbit anti-TJP1 (1:200; 61–7300, Life Technologies), mouse anti-β-tubulin (1:10,000; TUB2.1, Sigma-Aldrich), and rat anti-tyrosinated tubulin YL1/2 (1:5000; #ab6160, Abcam). Secondary antibodies were goat anti-mouse and goat anti-rabbit conjugated with Alexa Fluor 488 (both at 1:1000; Life Technologies), goat anti-mouse conjugated with Alexa Fluor 546 (1:1000; Life Technologies), and goat anti-rat conjugated with rhodamine (1:500; Jackson ImmunoResearch). F-actin filaments were visualized by adding phalloidin conjugated with Alexa 546 (Life Technologies) at a final concentration of 33 nM in the secondary antibody solution. Embryos were mounted in ProLong Gold medium containing 4’,6-diamidino-2-phenylindole (DAPI) to visualize nuclei (Life Technologies).
Confocal microscopy and image analysis
Embryos were imaged using FV1000 confocal laser scanning microscope (Olympus), by capturing serial optical sections at 2 µm intervals under a 40× oil objective lens. To compare localization patterns of the target proteins between control and the inhibitor-treated embryos, we paid specific attention to the following points during image capture. Embryos from the same experiment, consisting of control and experimental treatments, were imaged in a single session using the identical configuration, namely scan speed, excitation laser power, and detector high voltage (HV) values. At the beginning of each imaging session, the optimal value of detector HV for each laser channel was established by scanning two or three control specimen with varying values using the saturation-warning function. This was to set the detector HV value as high as possible without saturating pixels for most strongly stained areas (e.g., nuclei of outer cells in the case of CDX2 staining). Z-axis projections of serial optical sections and examination of every optical section to count nuclei were performed using Fluoview Viewer software (Olympus). To score nuclei that were positive for CDX2, NANOG, or YAP staining, DAPI staining was concomitantly visualized to locate the position of individual nuclei. When CDX2, NANOG, or YAP staining was more pronounced relative to DAPI staining based on merged colors, it was scored positive. Specifically, light blue (green plus dark blue) or purple (red plus dark blue) nuclei in merged images were scored positive, whereas dark blue nuclei were scored negative. In most cases, distinction between positive and negative nuclei was evident without quantitation. In some experiments, localization of proteins along the apical-basal cell polarity was assessed using the ImageJ program (National Institutes of Health). After conversion to TIFF format, optical sections were opened in ImageJ and pixel intensities along a straight line across the apical and basal sides of an outer cell were examined using the Plot Profile Tool.
Reverse transcription-polymerase chain reaction (RT-PCR) analyses
Total RNA was extracted from each sample of 15–25 embryos or 100–150 oocytes with TRI reagent (Sigma-Aldrich) and used for cDNA synthesis. Quantitative PCR was performed, using iCycler Thermal Cycler with MyiQ Single Color Real-Time PCR Detection System (Bio-Rad), as previously described (Alarcon, 2010). Amplification was done with iQ SYBR Green Supermix (Bio-Rad) as follows: initial denaturation at 94°C for 5 min, followed by up to 45 cycles of 94°C for 15 sec, 60°C for 20 sec, and 72°C for 40 sec. For standard RT-PCR to detect Rock1 and Rock2 mRNAs in the oocyte, amplification was performed with JumpStart REDTaq DNA Polymerase (Sigma-Aldrich) with the same denaturation, annealing, and extension conditions as quantitative PCR. Amplified products were resolved in 1% agarose gels, and visualized by staining with ethidium bromide. The following primers were used: Cdx2, F-GAC TTC CTG TCC CTT CCC TCG TCT, R-CCT CCC GAC TTC CCT TCA CCA TAC; Gata3, F-CAT GCT CTG TGA ATC AGT CCC TGT, R-AAC CCT CCA GAG TAC ATC CAC CTT; Nanog, F-ATA ACT TCG GGG AGG ACT TTC TGC, R-CCC TGA CTT TAA GCC CAG ATG TTG; Pou5f1, F-AGG CAG GAG CAC GAG TGG AAA GCA, R-GGA GGG CTT CGG GCA CTT CAG AAA; Sox2, F-CCA TGC AGG TTG ATA TCG TTG GTA, R-GCC AGC CTG ATT CCA ATA AGA GAG; Tead4, F-CTC AAG GCT TTC TGG TGT CTG CTT, R-CCT TGT CCC TCA CCT CTG TAG CAT; Actb, F-GAG AGG GAA ATC GTG CGT GAC ATC, R-CAG CTC AGT AAC AGT CCG CCT AGA; Rock1-N, F-ATC ATG TCG ACT GGG GAC AGT TTT, R-ATG CAC CTC TGC CGA TTA CCT TTA (for amplification of sequences encoding the N-terminal end of ROCK1 [262bp]; spanning the 1st and 2nd introns); Rock1-M, F-AGG AAA GCA AGA AAG CTG CTT CAA, R-CAT TCA GCT CCT TCT GGT GTT TCA (for amplification of sequences encoding the middle part of ROCK1 [429bp]; spanning the 23rd, 24th, and 25th introns); Rock1-C, F-GAT GCC ATG TTA AGT GCC ACA GAG, R-TTT TTG TGC CAA AAC AAG GAC AGA (for amplification of sequences encoding the C-terminal end of ROCK1 [437bp]; spanning the 31st and 32nd introns); Rock2-N, F-CGG CCG TCA GAG GAA GCT GGA G, R-CTG AAC TTC ACC AAA AGC ACC TCT (for amplification of sequences encoding the N-terminal end of ROCK2 [250bp]; spanning the 1st and 2nd introns); Rock2-M, F-GAA CTG CAA GAC CAA CTT GAA GCA, R-TGC AAG ATT TGC AAC ATC GCT AGT (for amplification of sequences encoding the middle portion of ROCK2 [378bp]; spanning the 21st, 22nd, and 23rd introns); and Rock2-C, F-CAC ATG TTT AAG CCT CCT CCT GCT, R-CGC ACG TGT GGT GTA TGT ATG TGT (for amplification of sequences encoding the C-terminal end of ROCK2 [443bp]; spanning the 31st and 32nd introns). Actb was used to normalize gene expression levels for quantitative PCR. Gene expression analyses were carried out, using three independent sets of samples.
Synthetic RNA and shRNA plasmid injection
RNA encoding the kinase-dead mutant of LATS2 (LATS2-KD), phosphorylation-defective mutant YAP with hemagglutinin-epitope tag (YAP-S112A-HA), and β-globin were synthesized from cDNAs cloned into the pcDNA3.1-poly(A)83 plasmid (kind gifts of Drs. Hiroshi Sasaki and Yoshikazu Hirate, Kumamoto University, Japan). The efficacy of these constructs was previously validated (Nishioka et al., 2009). Purified RNAs were injected at the two-cell stage in both blastomeres, as previously described (Nishioka et al., 2009). Prevalidated Lats1 and Lats2 short hairpin RNA (shRNA) plasmids were obtained (TRCN0000022941 for Lats1 and TRCN0000022705 for Lats2, Sigma-Aldrich). To confirm efficacy in our hands, P19 mouse embryonal carcinoma cells (American Type Culture Collection) were transfected with the Lats1 and Lats2 shRNA plasmids and gene expression analysis was conducted, as previously described (Alarcon, 2010). Enhanced green fluorescent protein (Egfp) shRNA plasmid (SHC005, Sigma-Aldrich) was used as control. Purified shRNA plasmids were injected into one-cell embryos, as previously described (Alarcon, 2010).
Statistics
Data were analyzed using Student t-test and chi-square test, and statistically significant differences were defined as p < 0.05.
Results
Inhibition of ROCK activity impairs TE formation
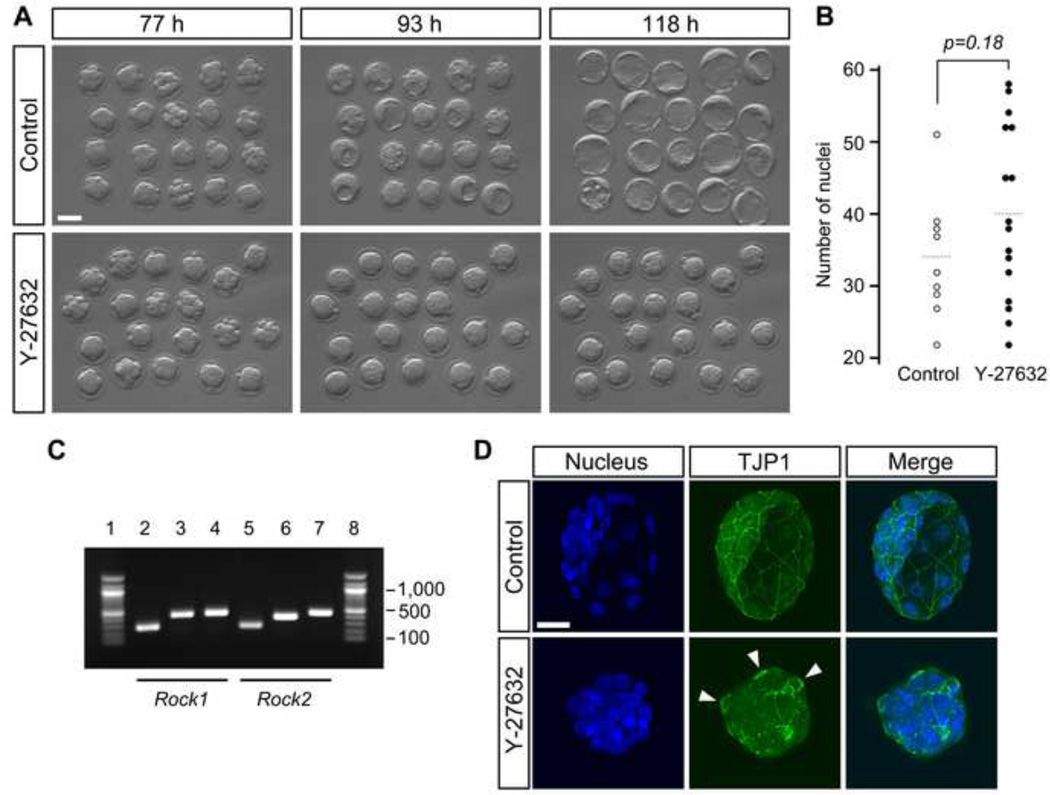
A previous study has reported that inhibition of ROCK activity using Y-27632 during preimplantation development interferes with the formation of the blastocyst cavity (Kawagishi et al., 2004). To confirm this, we cultured embryos from the 2-cell stage (E1.5) in the presence of Y-27632 in the culture medium, and monitored their morphological changes by time-lapse cinematography for 3 days (up to E4.5). Timing of the initial cleavages to the 4- and 8-cell stages, and the compaction of blastomeres at the late 8-cell stage were indistinguishable between Y-27632-treated and non-treated control embryos (Fig. 1A; Supplemental movies). However, none of the Y-27632-treated embryos (n=20) formed a visible blastocyst cavity throughout the entire period of time-lapse recording, whereas all of the control embryos (n=20) generated a robustly expanding cavity. Y-27632-treated embryos maintained an overall shape of compacted morula with no sign of cell detachment or dissociation to indicate cell death. The surface of Y-27632-treated embryos exhibited bulging periodically, which is indicative of continuous cell divisions. Consistent with this, total cell numbers were not significantly different between Y-27632-treated and control embryos (Fig. 1B). Thus, ROCK inhibition impaired blastocyst cavity formation without compromising overall cell proliferation.
Fig. 1.
Inhibition of ROCK activity interferes with blastocyst cavity formation. Embryos were cultured in the absence (control) or presence of Y-27632 from 2-cell to blastocyst stage (E1.5–E4.5). (A) Development was recorded by time-lapse cinematography. Snap-shot images of control and inhibitor-treated embryos correspond to developmental stages (from left to right) 8-cell (uncompacted and compacted), morula and early cavitating (E3.5), and late blastocyst with expanded cavity (E4.5). h, hours post-hCG injection. (B) Comparison of total cell numbers. Circles represent the number of DAPI-stained nuclei in individual embryos, and horizontal dashed bars represent the mean value for each group. There is no statistically significant difference (Student t-test) between control (n=9) and inhibitor-treated (n=16) embryos. (C) Presence of maternal mRNA for Rock1 and Rock2 in the oocyte, as determined by RT-PCR. Lanes 1 and 8 are DNA size marker (100bp ladder). Lanes 2, 3, and 4 are PCR products of the Rock1 primers (N, M, and C, respectively). Lanes 5, 6, and 7 are PCR products of the Rock2 primers (N, M, and C, respectively). (D) Disruption of tight junctions by ROCK inhibition. Representative embryos (E4.5) immunostained for TJP1 (green) are shown. In the control embryo, TJP1 appears as a continuous line in sites of cell-cell contact between outer cells, whereas in the treated embryo, TJP1 appears discontinuous or absent, and thick rings (arrowheads) of TJP1 are sometimes observed. Images are Z-axis projections of optical sections captured by confocal microscopy. Blue, DAPI. Scale bars: (A) 50 µm, (D) 20 µm.
Mutant embryos that are homozygous null for both Rock1 and Rock2 genes (Rock1−/−; Rock2−/−) die in utero (Kamijo et al., 2011). However, they appear to form normal blastocysts with an expanded cavity, which is in striking contrast to the Y-27632-treated embryos shown in the present study. It is possible that the mRNA and/or protein products of Rock1 and/or Rock2 are maternally supplied, i.e., stored in the oocyte, which may be sufficient to support early development of zygotic null (Rock1−/−; Rock2−/−) embryos up to the blastocyst stage. Thus, we examined whether Rock1 or Rock2 mRNA was present in unfertilized oocytes by RT-PCR. To detect mRNA specifically in the oocyte, ovulated mature oocytes were treated with proteases to completely remove zona pellucida along with residual cumulus cells. We also used intron-spanning primer pairs to confirm that PCR products were originated from mRNA rather than unprocessed transcripts or genomic DNA. Three different pairs of primers were used for each of Rock1 and Rock2 that correspond to regions encoding the N-terminal, middle, and C-terminal portions of the protein. All the primer pairs tested amplified PCR products of predicted size (Fig. 1C). This result suggests that mRNAs encoding full-length ROCK1 and ROCK2 are present in the oocyte as maternal supplies.
The formation of the blastocyst cavity requires paracellular sealing between outer cells, which depends on the establishment of tight junctions (Eckert and Fleming, 2008; Marikawa and Alarcon, 2012). To assess whether the failure of cavity formation in Y-27632-treated embryos is due to defective tight junctions, we examined the localization of a key component, TJP1 (ZO-1). At the blastocyst stage, continuous lines of TJP1 staining were observed along the boundaries between outer cells in control embryos (n=9) (Fig. 1D). In contrast, the staining was either discontinuous or absent in Y-27632-treated embryos (n=18), suggesting that defective tight junction formation contributed to the cavitation failure.
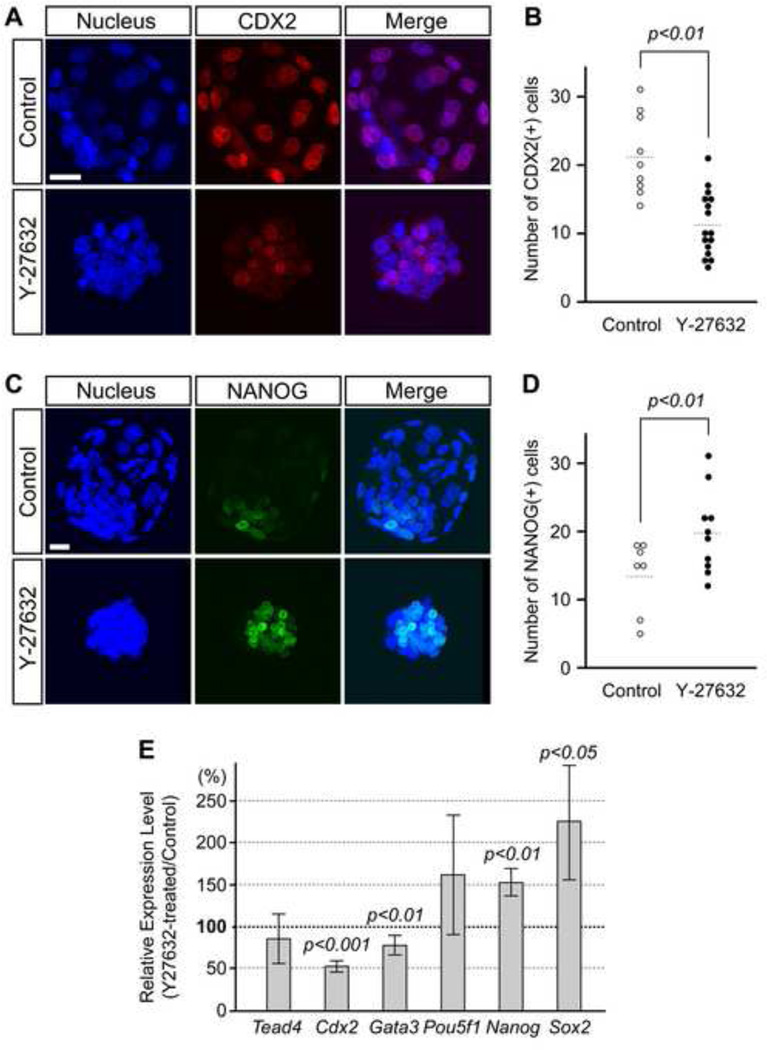
Because tight junction formation is a key feature of TE, we investigated whether the lineage segregation between TE and ICM is compromised by ROCK inhibition. The TE and ICM lineages were assessed by expressions of TE-specific transcription factor CDX2 and ICM-specific transcription factor NANOG (Chambers et al., 2003; Mitsui et al., 2003; Strumpf et al., 2005). The number of CDX2-positive nuclei was significantly lower in Y-27632-treated embryos compared to the control (Fig. 2A, B). In contrast, the number of NANOG-positive nuclei was significantly increased by Y-27632 treatment (Fig. 2C, D). Notably, control embryos expressed NANOG almost exclusively in inner cells, whereas Y-27632-treated embryos expressed NANOG in both inner and outer cells (Fig. 2C). Furthermore, quantitative RT-PCR analyses demonstrated that Y-27632 treatment significantly reduced the transcript levels of Cdx2 and Gata3, but significantly increased those of Nanog and Sox2, another key regulator of ICM pluripotency (Fig. 2E). These results suggest that the TE lineage is diminished, whereas the ICM lineage is promoted by ROCK inhibition.
Fig. 2.
Inhibition of ROCK activity promotes ICM and diminishes TE gene expressions. Embryos were cultured in the absence (control) or presence of Y-27632 from 2-cell to blastocyst stage (E1.5-E4.5). (A) Distribution of the TE lineage marker, CDX2 (red), in representative control and treated embryos (E4.5). (B) Comparison of the number of CDX2-positive cells. Inhibitor-treated embryos (n=16) have significantly less CDX2-expressing cells (Student t-test) than control embryos (n=9). Circles represent the number of CDX2-positive cells in individual embryos, and horizontal dashed bars represent the mean value for each group. (C) Distribution of the pluripotency marker, NANOG (green), in representative control and treated embryos (E4.5). (D) Comparison of the number of NANOG-positive cells. Inhibitor-treated embryos (n=10) have significantly more NANOG-expressing cells (Student t-test) than control embryos (n=7). Circles represent the number of NANOG-positive cells in individual embryos, and horizontal dashed bars represent the mean value for each group. Images (A, C) are Z-axis projections of optical sections captured by confocal microscopy. Blue, DAPI. Scale bars: 20 µm. (E) Quantitative RT-PCR analyses of Y-27632-treated embryos at the early blastocyst stage (100 h post-hCG). Relative expression levels of Tead4, Cdx2, Gata3, Pou5f1, Nanog, and Sox2 are shown as percentages of their levels in the inhibitor-treated embryos relative to those in control embryos. In each set of experiments, the expression level of each gene is normalized by that of Actb. Bars indicate mean ± standard deviation. P values for Cdx2, Gata3, Nanog, and Sox2 are based on Student t-test, indicating that the change in these genes by Y-27632 treatment is statistically significant.
When Y-27632-treated embryos were further cultured beyond E4.5, some embryos started to form a small cavity whereas the other embryos partly degenerated with apparently dead cells being extruded on the surface. It is possible that increased cell death was due to reduction in CDX2 expression, because Cdx2 null embryos exhibit increased apoptosis at the blastocyst stage (Strumpf et al., 2005).
Inhibition of ROCK results in activation of Hippo signaling
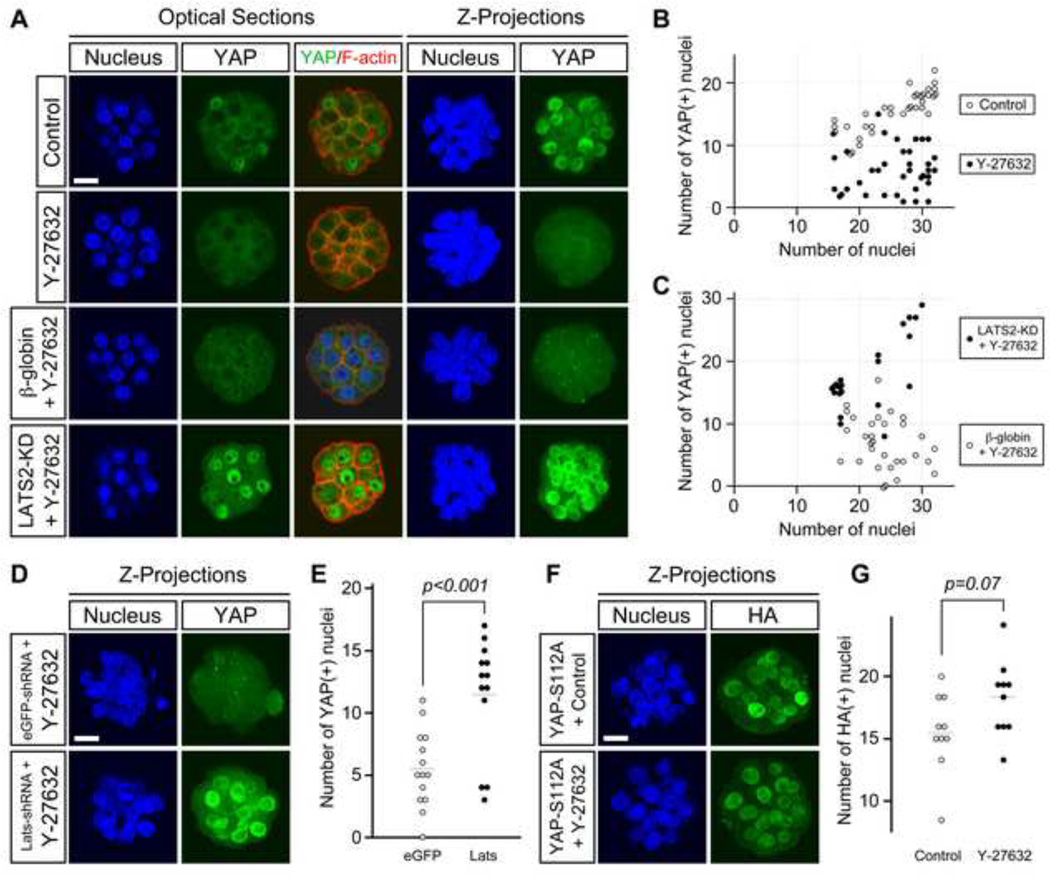
Expression of CDX2 in the mouse blastocyst is dependent on nuclear localization of YAP, which together with TEAD4 acts as a transcriptional activator (Nishioka et al., 2008, 2009; Yagi et al., 2007). Because CDX2 expression was reduced in Y-27632-treated embryos, we examined whether nuclear accumulation of YAP was also diminished. Between 16- and 32-cell stages, distinct nuclear YAP was observed in the outer cells of control embryos (Fig. 3A), whereas fewer nuclear YAP was observed in Y-27632-treated embryos. The average number of YAP-positive nuclei per embryo was significantly lower (p < 0.001) in Y-27632-treated embryos (6.1 ± 3.7; n=37) than in control embryos (15.5 ± 3.3; n=34), while the average number of total nuclei per embryo was comparable between the former (25.5 ± 5.3) and the latter (25.7 ± 5.4) groups (Fig. 3B). This suggests that inhibition of ROCK diminishes nuclear accumulation of YAP.
Fig. 3.
ROCK activity is essential for nuclear localization of YAP through interference with its phosphorylation by LATS1/2. (A) Embryos were cultured in the absence or presence of Y-27632 from 2- to 32-cell stage (E1.5-E3.5). Nuclear YAP (green) diminishes in the outer cells of inhibitor-treated embryos. Overexpression of dominant-negative LATS (LATS2-KD) rescues nuclear localization of YAP in treated embryos, whereas overexpression of β-globin does not. Cell boundaries are demarcated by F-actin (red) staining. (B) The numbers of total nuclei and YAP-positive nuclei in untreated (control) and inhibitor-treated embryos. (C) The numbers of total nuclei and YAP-positive nuclei in inhibitor-treated embryos that have been overexpressed with LATS2-KD and β-globin. (B–C) Circles correspond to individual embryos, which are presented in the graphs according to the total number of nuclei (x-axis) and the number of YAP-positive nuclei (y-axis). (D) Distribution of nuclear YAP (green) in treated embryos that have been injected with Egfp shRNA plasmid or a mixture of Lats1-specific and Lats2-specific shRNA plasmids. Lats1/Lats2 knockdown rescues nuclear accumulation of YAP. (E) Comparison of the number of YAP-positive nuclei. Inhibitor-treated embryos injected with Lats1/Lats2 shRNA plasmids (n=13) have significantly more YAP-positive nuclei (Student t-test) than inhibitor-treated embryos injected with Egfp shRNA plasmid (n=14). Circles represent the number of YAP-positive nuclei in individual embryos, and horizontal dashed bars represent the mean value for each group. (F) Distribution of phosphorylation-defective YAP (YAP-S112A) tagged with HA epitope (green) in untreated and inhibitor-treated embryos. Nuclear localization of YAP-S112A occurs in both treated and control embryos. (G) Comparison of the number of nuclear YAP-S112A. There is no statistically significant difference (Student t-test) between control (n=10) and inhibitor-treated (n=10) embryos. Circles represent the number of HA-positive nuclei in individual embryos, and horizontal dashed bars represent the mean value for each group. Images (A, D, F) were captured by confocal microscopy. Blue, DAPI. Scale bars: 20 µm.
In Hippo signaling, nuclear accumulation of YAP is negatively regulated through its phosphorylation by LATS1/2 (Avruch et al., 2012; Hao et al., 2008; Hong and Guan, 2012). Phosphorylated YAP is either sequestered in the cytoplasm by 14-3-3 (Zhao et al., 2007) or degraded through SCF/β-TrCP E3 ubiquitin ligase (Zhao et al., 2010). Because nuclear accumulation of YAP was reduced in Y-27632-treated embryos, it is possible that ROCK inhibition activated Hippo signaling and promoted LATS1/2 to phosphorylate YAP. Alternatively, it is possible that ROCK inhibition interfered with YAP nuclear accumulation through other mechanisms that are independent from LATS1/2. For example, machineries involved in nuclear import of YAP may be compromised by Y-27632 treatment. To distinguish between these possibilities, we conducted the following three experiments.
In the first experiment, the LATS2 kinase-dead (KD) mutant was overexpressed in embryos, which were then cultured in the presence of Y-27632. LATS2-KD acts as a dominant-negative construct that interferes with YAP phosphorylation by LATS1/2 (Nishioka et al., 2009). Overexpression of LATS2-KD was achieved by the injection of synthetic mRNA at the 2-cell stage into both blastomeres. As a control, mRNA encoding β-globin was injected, followed by Y-27632 treatment. The average number of YAP-positive nuclei per embryo was significantly higher (p < 0.001) in LATS2-KD-injected embryos (17.8 ± 5.9; n=21) than in β-globin-injected embryos (6.9 ± 4.2; n=31) (Fig. 3A, C). The average number of total nuclei per embryo was not significantly different between the former (20.8 ± 5.1) and the latter (24.0 ± 4.1) groups. Thus, nuclear accumulation of YAP was rescued in Y-27632-treated embryos by overexpression of LATS2-KD.
In the second experiment, both Lats1 and Lats2 mRNA were knocked down by coinjection of specific shRNA plasmids at the 1-cell stage, and the injected embryos were then treated with Y-27632 from the 2-cell stage. As a control, shRNA plasmid against the enhanced Green Fluorescent Protein (Egfp) was injected. Knockdown of Lats1 and Lats2 rescued YAP nuclear accumulation in Y-27632-treated embryos, as the average number of YAP-positive nuclei per embryo was significantly higher than in Egfp shRNA-injected embryos (Fig. 3D, E). This result is consistent with the first experiment, supporting that LATS2-KD specifically interfered with the action of LATS1/2 as a dominant-negative construct.
In the third experiment, we examined the localization of the YAP-S112A construct in Y-27632-treated embryos. YAP-S112A is a phosphorylation-defective mutant of YAP, in which the serine residue for LATS1/2-mediated phosphorylation is replaced with alanine (Nishioka et al., 2009). We used this construct to test whether the diminished nuclear localization of YAP by Y-27632 treatment could occur independently from its phosphorylation. Embryos at the 2-cell stage were injected with mRNA encoding YAP-S112A tagged with HA epitope, and then cultured in the absence or presence of Y-27632. The average number of nuclear YAP-S112A, detected by anti-HA antibody, was not significantly different between control and Y-27632-treated groups (Fig. 3F, G), indicating that nuclear translocation of YAP-S112A was not diminished by inhibition of ROCK.
Taken together, these results suggest that ROCK activity is essential for YAP nuclear accumulation through interference of its phosphorylation by LATS1/2 in mouse preimplantation embryos.
Inhibition of ROCK activity causes mislocalization of key components of the apical-basal cell polarity
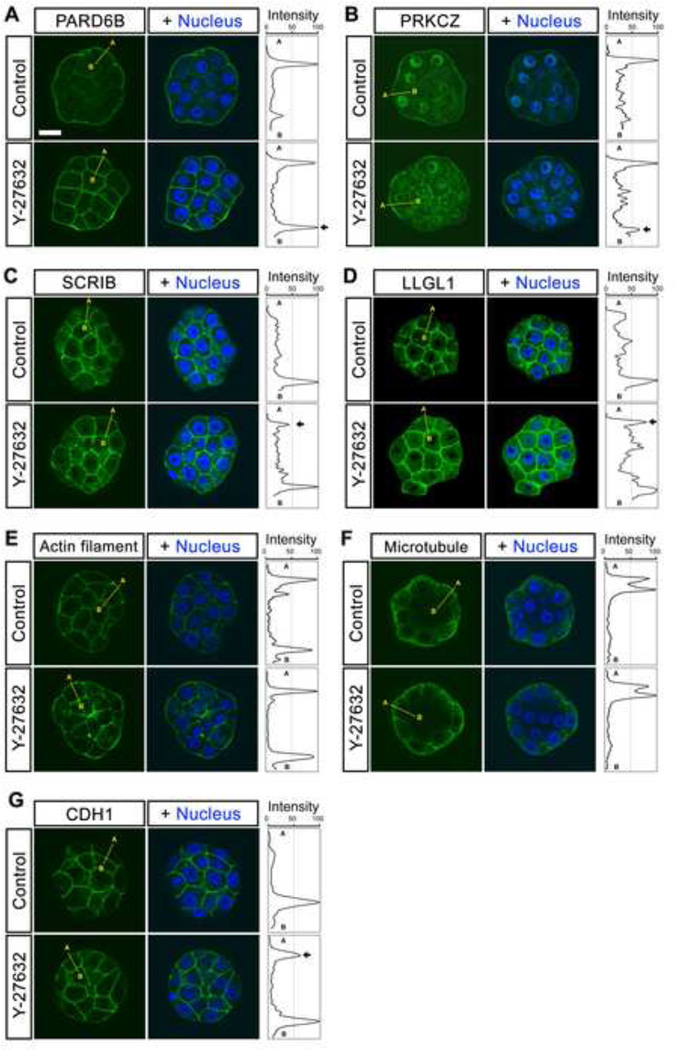
Because activity of Hippo signaling is affected by the apical-basal cell polarity (Genevet and Tapon, 2011; Hirate et al., 2013; Leung and Zernicka-Goetz, 2013; McCaffrey and Macara, 2011; Parsons et al., 2010; Thompson et al., 2013; Zhao et al., 2011), we then investigated the impact of ROCK inhibition on localization of the key polarity regulators. In control embryos between 16- and 32-cell stages, the Par-3/Par-6/aPKC complex and the Scribble/Lgl/Dlg complex were distributed in a mutually exclusive manner. Namely, the components of the former complex, PARD6B (n=10) and PRKCZ (n=14), were preferentially localized to the apical membrane, whereas those of the latter complex, SCRIB (n=10) and LLGL1 (n=11), were localized to the basolateral membranes (Fig. 4A–D), consistent with the previous observations (Alarcon, 2010; Hirate et al., 2013; Plusa et al., 2006; Tao et al., 2012; Vinot et al., 2005). In striking contrast, all of these polarity regulators were mislocalized in Y-27632-treated embryos. PARD6B (n=14) and PRKCZ (n=16) were no longer restricted to the apical membrane, since they were also found in the basolateral membrane domain. Similarly, SCRIB (n=14) and LLGL1 (n=16) were distributed along both the apical and basolateral membrane domains. Thus, the apical and basolateral components were not segregated from each other in Y-27632-treated embryos, suggesting that ROCK activity is essential for proper apical-basal cell polarization.
Fig. 4.
Localization of polarity proteins in ROCK inhibitor-treated embryos. Embryos were cultured in the absence or presence of Y-27632 from 2- to 32-cell stage (E1.5–E3.5). (A–B) Immunostaining for PARD6B (green in A) and PRKCZ (green in B) shows strong apical membrane-enrichment in the outer cells of control embryos. With Y-27632 treatment, PARD6B and PRKCZ show membrane-enrichment extending to the basolateral region. (C–D) Immunostaining for SCRIB (green in C) and LLGL1 (green in D) shows distinct basolateral membrane-enrichment in the outer cells of control embryos. With Y-27632 treatment, SCRIB and LLGL1 show membrane-enrichment extending to the apical region. (E–F) Staining for actin filaments (green in E) and microtubules (green in F) shows no evident difference between control and Y-27632 treatment. (G) Immunostaining for CDH1 (E-cadherin) shows distinct basolateral membrane-localization in most of the control embryos. With Y-27632 treatment, a significantly higher number of embryos exhibited apical staining of CDH1. Images are optical sections captured by confocal microscopy. Blue, DAPI. Abbreviations: A (apical), B (basal). Scale bar (A–G): 20 µm.
Because establishment of correct apical-basal cell polarity is linked to the integrity of cytoskeleton and cell-cell adhesion (Baum and Georgiou, 2011; McCaffrey and Macara, 2011; St Johnston and Sanson, 2011), we also compared distributions of actin filaments, microtubules, and CDH1 (E-cadherin) between control and Y-27632-treated embryos at 16- to 32-cell stages (Fig. 4E–G). In both groups, actin filaments were localized along the entire cell membrane, including apical and basolateral sides (n=18 and 20 for control and Y-27632-treated, respectively), whereas microtubules were more diffusely distributed in the cytoplasm, with enrichment toward the periphery of the embryos (n=16 and 17 for control and Y-27632-treated, respectively). Thus, for these cytoskeletal components, no clear difference was observed between control and Y-27632-treated embryos. In contrast, subtle but significant alteration in CDH1 distribution was caused by Y-27632 treatment. In control embryos (n=16), CDH1 immunostaining was localized almost exclusively to cell-cell boundaries, and very weak signal was observed along the apical membranes, although a few embryos (3 out 16) exhibited distinct apical staining, which was seen as a discrete peak on the apical end in Plot Profile. However, in Y-27632-treated embryos (n=17), many (14 out 17) displayed distinct staining for CDH1 at the apical membrane (Fig. 4G), and this frequency was statistically higher than in control embryos (p < 0.001; chi-square test). This suggests that ROCK activity is also essential for confinement of CDH1 to basolateral membranes.
Inhibition of ROCK impairs apical accumulation of PCM1 during compaction
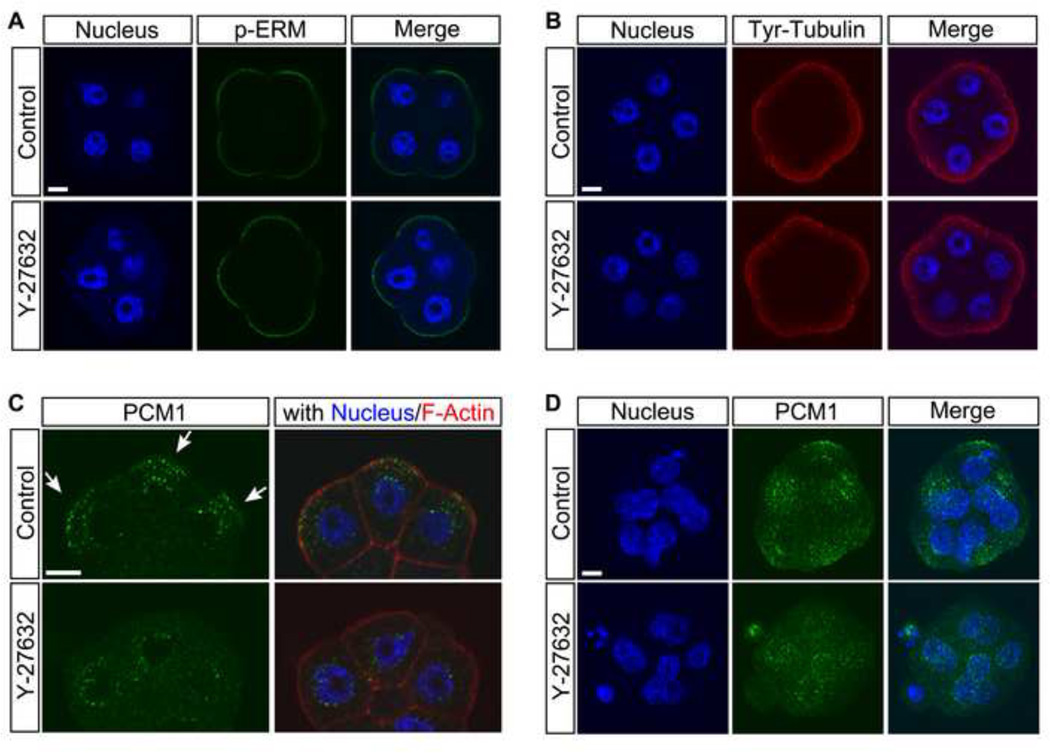
Molecular signs of apical-basal polarization emerge during compaction at the late 8-cell stage. Namely, phosphorylated forms of Ezrin/Radixin/Moesin (p-ERM) are involved in the formation of apical microvilli, and start to localize at the apical membrane during compaction (Dard et al., 2004). Also, the asymmetric reorganization of the microtubule network takes place during compaction, as tyrosinated tubulin becomes enriched at the apical cortex, and puncta of pericentriolar material 1 (PCM1) accumulate near the apical membrane (Houliston and Maro, 1989; Houliston et al., 1987; Tao et al., 2012). Thus, we investigated whether inhibition of ROCK activity also affects these early events of apical-basal polarization.
In compacted 8-cell stage embryos, p-ERM was clearly localized to the apical cortex in both control (n=12) and Y-27632-treated (n=8) embryos (Fig. 5A). Likewise, apical enrichment of tyrosinated tubulin was indistinguishable between control (n=7) and Y-27632-treated (n=8) embryos (Fig. 5B). In contrast, distribution patterns of PCM1 were significantly different between control and Y-27632-treated embryos. In control embryos, PCM1 puncta were accumulated in the area between the apical cortex and nucleus, as seen in confocal optical sections (Fig. 5C, arrows). Apical accumulation of PCM1 puncta was also evident in Z-projected images as clusters that were found near the cortex in most (13 out of 17) embryos (Fig. 5D). However, in Y-27632-treated embryos, apical accumulation of PCM1 was indistinct in confocal optical sections (Fig. 5C, D), and a significantly less number (2 out of 15) of embryos exhibited the apical clustering compared to the control (p < 0.001; chi-square test). These results suggest that ROCK inhibition interferes with a certain, but not all, event of apical-basal polarization at the time of compaction.
Fig. 5.
ROCK inhibition does not interfere with all events of cell polarization at the time of compaction of the 8-cell stage embryo. Embryos were cultured in the absence or presence of Y-27632 from 2- to 8-cell (compacted) stage (E1.5–E2.5). (A–B) Immunostaining for p-ERM (green in A) and tyrosinated tubulin (red in B) shows retention of strong localization in the apical domain in inhibitor-treated embryos that is similar to that in control embryos. (C) Immunostaining for PCM1 (green) shows puncta (arrows) clustered between the apical domain and nucleus in the control embryo. With ROCK inhibition, the apical clustering is lost. Cell boundaries are demarcated by staining for F-actin (red). Images (A–C) are optical sections captured by confocal microscopy. (D) Projections of optical sections in the Z-axis of the embryo immunostained for PCM1 (green). Blue, DAPI. Scale bars: 10 µm.
Inhibition of RHO activity causes cell polarity and Hippo signaling defects similar to ROCK inhibition
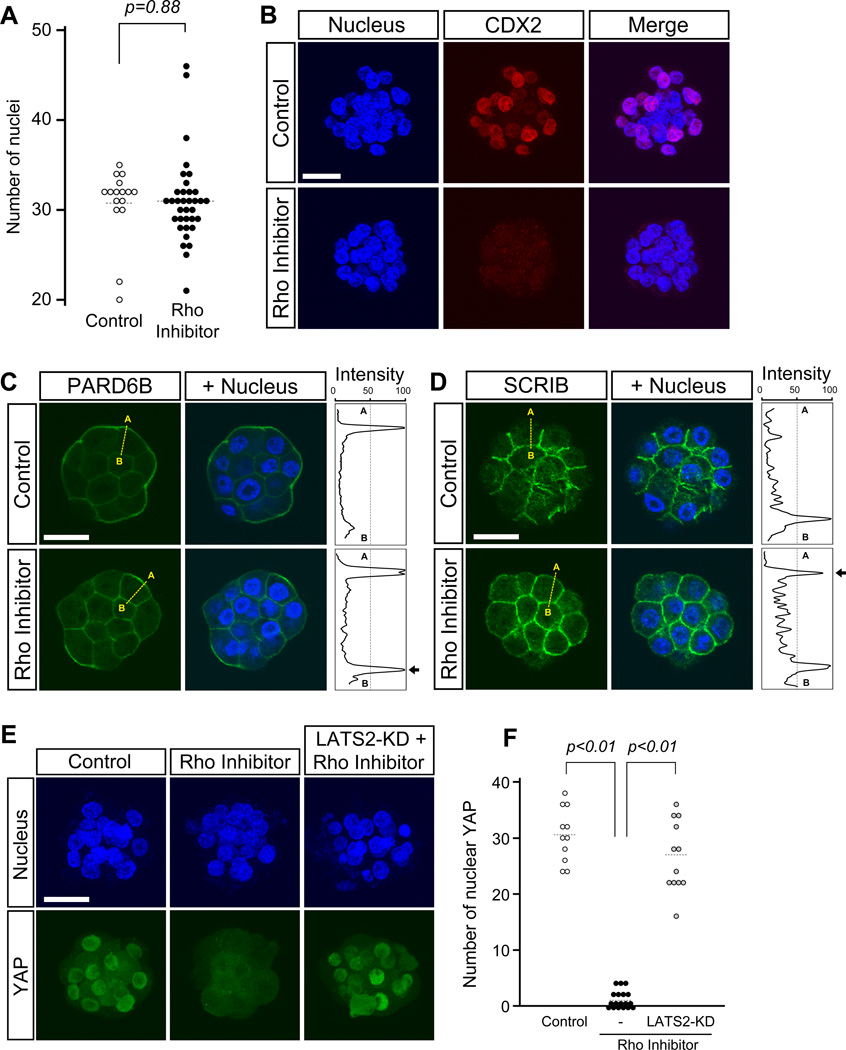
The activity of ROCK is regulated by RHO GTPases in various systems (Amano et al., 2010; Ishizaki et al., 1996; Leung et al., 1996; Matsui et al., 1996). Thus, we examined the role of RHO GTPases in mouse preimplantation development as a potential upstream regulator of ROCK to control apical-basal cell polarization and Hippo signaling. To inhibit the activity of RHO GTPases, we used RHO Inhibitor I, which is Clostridium botulinum C3 transferase conjugated with a cell-penetrating moiety to pass through the plasma membrane and inactivate RHOA, RHOB, and RHOC, but not related GTPases such as CDC42 or RAC1 (Aktories et al., 1989; Han et al., 2001; Just et al., 2001). A previous study has shown that treatment of mouse embryos with C3 transferase, starting before the 8-cell stage, interferes with compaction of blastomeres (Clayton et al., 1999). As shown in the present study, however, inhibition of ROCK activity from the 2-cell stage did not impair compaction. Thus, it is possible that RHO GTPases may regulate compaction in a ROCK-independent manner. Here, to focus on the role of RHO GTPases as an upstream regulator of ROCK with respect to cell polarization and Hippo signaling, we treated embryos with RHO Inhibitor I from the 8-cell stage.
By E3.5, the majority (14 out of 16) of control embryos had already reached the blastocyst stage, whereas none (0 out of 34) of RHO inhibitor-treated embryos formed a blastocyst cavity. Nonetheless, the average numbers of nuclei per embryo were comparable between the control and RHO inhibitor-treated groups (Fig. 6A), indicating that the lack of cavity formation was not due to developmental arrest. Also, expression of CDX2 was strikingly diminished in RHO inhibitor-treated embryos (n=16) compared to control embryos (n=9) (Fig. 6B). Between 16- and 32-cell stages, the apical marker PARD6B was mislocalized to the basolateral membrane (n=10; Fig. 6C), whereas the basolateral marker SCRIB was mislocalized to the apical membrane (n=10; Fig. 6D) in RHO inhibitor-treated embryos. The matching control embryos displayed normal localization patterns of these polarity regulators, i.e., apically enriched PARD6B (n=5) and basolaterally confined SCRIB (n=10). Furthermore, RHO-inhibitor treatment interfered with nuclear accumulation of YAP, which was reversed by injection of LATS2-KD mRNA (Fig. 6E, F). Altogether, inhibition of RHO activity from the 8-cell stage disturbed the localization of polarity regulators and activated Hippo signaling in a manner similar to inhibition of ROCK, supporting the notion that RHO-ROCK signaling plays an essential role in TE formation through regulation of apical-basal cell polarization and Hippo signaling.
Fig. 6.
RHO inhibition phenocopies the Y-27632-induced disruption of cell polarity and activation of Hippo signaling in embryos. Embryos were cultured in the absence or presence of RHO Inhibitor I from 8- to 32-cell stage (E2.5–E3.5). (A) Comparison of total nuclear numbers. There is no statistically significant difference (Student t-test) between control (n=16) and inhibitor-treated (n=35) embryos. Circles represent the number of DAPI-stained nuclei in individual embryos, and horizontal dashed bars represent the mean value for each group. (B) Immunostaining for the TE lineage marker, CDX2 (red), in representative embryos. CDX2 is diminished in the inhibitor-treated embryo. Images are Z-axis projections of optical sections. (C-D) Immunostaining for apical-basal polarity proteins in representative embryos. In the control embryo, PARD6B (green in C) is strongly enriched in the apical domains and weakly enriched in the basolateral domains of outer cells. With inhibitor treatment, PARD6B localization becomes more distinct in the basolateral region. On the other hand, SCRIB (green in D) is enriched in the basolateral domains of outer cells in the control embryo, but also becomes localized in the apical domains in the treated embryo. Images are optical sections. Abbreviations: A (apical), B (basal). (E) Nuclear YAP (green) diminishes in the inhibitor-treated embryo. Overexpression of dominant-negative LATS (LATS2-KD) rescues nuclear localization of YAP in the inhibitor-treated embryo. Images are Z-axis projections of optical sections. (F) Comparison of the number of YAP-positive nuclei. Nuclear localization of YAP is significantly reduced in RHO inhibitor-treated embryos (n=18) compared to untreated control embryos (n=11; Student t-test). Nuclear localization of YAP is restored in inhibitor-treated embryos that have been overexpressed with LATS-KD (n=12; Student t-test). Circles represent the number of YAP-positive nuclei in individual embryos, and horizontal dashed bars represent the mean value for each group. Blue, DAPI. Scale bars: 20 µm.
Discussion
A previous study showed that pharmacological inhibition of ROCK activity during mouse preimplantation development inhibits blastocyst cavity formation (Kawagishi et al., 2004). However, mechanistic insight into this phenomenon has been absent. For example, which aspects of the embryonic processes or molecular events are affected by ROCK inhibition to cause the cavitation defect has been unknown. In the present study, we showed that inhibition of RHO-ROCK signaling impaired TE characteristics, leading to defective tight junction and diminished CDX2 expression. Nuclear localization of YAP was reduced in RHO-ROCK-inhibited embryos, likely due to LATS-mediated phosphorylation of YAP. Indeed, the phenotype of RHO-ROCK inhibition appears very similar to LATS overexpression with respect to YAP localization and CDX2 expression (Nishioka et al., 2009). Inhibition of RHO-ROCK signaling also disturbed apical-basal cell polarization, as the key components of the polarity regulators were mislocalized. In light of recent studies on Hippo signaling and apical-basal polarization in TE specification (Alarcon, 2010; Cockburn et al., 2013; Hirate et al. 2013; Leung and Zernicka-Goetz, 2013; Lorthongpanich et al., 2013; Nishioka et al., 2009), we propose that RHO-ROCK signaling is a key element of proper lineage formation in preimplantation development by influencing Hippo signaling and cell polarization.
Y-27632-treated embryos exhibited severe defects in cavitation and TE specification, whereas Rock1 and Rock2 zygotic null (Rock1−/−; Rock2−/−) embryos appear to form normal blastocysts with an expanded cavity (Kamijo et al., 2011). A possible reason for such phenotypic difference is that the products of Rock1 and/or Rock2 genes are maternally supplied, as shown in the present study, which may be sufficient to support early preimplantation development in zygotic mutants. However, at this point, we cannot completely rule out the possibility that Y-27632 may interfere with unknown targets other than ROCK, which led to the cavitation failure observed in this study and previously (Kawagishi et al., 2004). Nonetheless, we showed that RHO inhibitor I also produced essentially the same sets of abnormalities, i.e., cavitation failure, reduced CDX2 expression, LATS-mediated inhibition of YAP nuclear localization, and mislocalization of polarity regulators, which strongly suggests that inhibition of RHO-ROCK signaling was responsible for the observed phenotype. Interestingly, some of these abnormalities, specifically reduction in CDX2 expression and YAP nuclear localization, appeared to be more pronounced in RHO-inhibited than in ROCK-inhibited embryos (compare Fig. 6 with Figs. 2, 3). It is possible that RHO inhibitor I is more efficient in blocking RHO-ROCK signaling than Y-27632. Alternatively, RHO GTPases may play an additional role in the regulation of Hippo signaling in a ROCK-independent manner, and thus inhibition of RHO GTPases may exert stronger impact than inhibition of ROCK alone.
Studies using cultured cells have shown that RHO GTPases modulate YAP activity in response to various mechanical and biochemical microenvironments, such as cell attachment to substrate, stiffness of substrate, activation of protease-activated receptors, and ligands to G protein-coupled receptors (GPCR), including serum lipid components (sphingosine-1-phosphate [S1P] and lysophosphatidic acid) and hormones, namely epinephrine and glucagon, that up-regulate intracellular cAMP level (Dupont et al., 2011; Miller et al., 2012; Mo et al., 2012; Yu et al., 2012; Yu et al., 2013; Zhao et al., 2012). Even though the nature of the microenvironments is diverse, RHO GTPases play an essential role in the activation of YAP in all of these cases, which is in line with the situation in preimplantation embryos, as shown in the present study. However, mechanisms that connect RHO GTPases to YAP appear to differ significantly among these cases, particularly with respect to the involvement of ROCK. ROCK acts downstream of RHO GTPases and is required for activation of YAP in the sensing of substrate stiffness (Dupont et al., 2011) and response to S1P (Miller et al., 2012). In contrast, ROCK activity appears to be dispensable in response to protease-activated receptor signaling (Mo et al., 2012) or cell attachment to substrate (Zhao et al., 2012), in which treatment with a pharmacological ROCK inhibitor does not impair YAP activation. Furthermore, involvement of LATS in YAP phosphorylation appears to be different among these cases. Specifically, responses to S1P and activation of protease-activated receptors involve LATS (Mo et al., 2012; Yu et al., 2012), whereas sensation of stiffness does not (Dupont et al., 2011). Thus, there is more than one pathway that connects RHO GTPases to YAP activation depending on the biological events and possibly on cell types. This may possibly be the case in mouse preimplantation development, which might have contributed to apparently stronger impact of RHO inhibitor I than Y-27632, as discussed above.
Recently, we have shown that inhibition of ROCK activity with Y-27632 at later stages of preimplantation development, specifically between E3.5 and E4.5 during cavity expansion, alters ICM morphology without compromising specification of TE, epiblast, and primitive endoderm lineages. These embryos that are treated with Y-27632 at later stages are still capable of implantation, but are impaired in post-implantation development, resulting in significantly higher incidence of fetal loss (Laeno et al., 2013). Thus, the impact of Y-27632-treatment at later stages of preimplantation development is more in line with the post-implantation lethality phenotype of Rock1−/−; Rock2−/− embryos (Kamijo et al., 2011), although further investigations are required to determine when and how exactly these embryos die in utero. Also, future studies to generate compound knockout embryos, in which both maternal and zygotic Rock1 and Rock2 are completely removed, should clarify the role of ROCK in early stages of preimplantation development.
Previously, we have demonstrated that knockdown of PARD6B impairs TE lineage formation while enhancing ICM characteristics (Alarcon, 2010). PARD6B-knockdown embryos and RHO-ROCK-inhibited embryos share many phenotypic similarities, such as cavitation failure, defective TJP1 distribution, diminished Cdx2 expression, elevated Nanog expression, reduced YAP nuclear localization, and mislocalization of SCRIB and LLGL1 to the apical domain (Alarcon, 2010; Hirate et al., 2013). These similarities implicate a mechanistic link between RHO-ROCK signaling and PARD6B, or more broadly the Par-3/Par-6/aPKC complex. Indeed, studies using cultured cell lines have shown that these two systems can mutually regulate each other through protein phosphorylation. Specifically, ROCK phosphorylates PAR3 to regulate its association with the complex (Nakayama et al., 2008), whereas aPKC phosphorylates ROCK in a Par-3/Par-6-dependent manner to modulate the integrity of apical junctional complexes (Ishiuchi and Takeichi, 2011). However, there are also noteworthy differences between PARD6B-knockdown and RHO-ROCK-inhibited embryos. First, PARD6B is largely absent in the PARD6B-knockdown embryos, whereas it is still present and distributed to both the apical and basal domains in RHO-ROCK-inhibited embryos. Second, PRKCZ (aPKCzeta) is diffused into the cytoplasm in the former embryos, whereas it is localized to the entire cortex in the latter (Alarcon, 2010). Also, the apical localization of p-ERM is reduced in PARD6B-knockdown embryos (Hirate et al., 2013), whereas it was intact in RHO-ROCK-inhibited embryos. Thus, relationships between RHO-ROCK signaling and PARD6B may be more complex than simple mutual regulation. Nonetheless, RHO-ROCK-inhibited embryos present a unique situation with respect to localization of polarity regulators, which may be useful for further investigations of the mechanisms of apical-basal cell polarization. Specifically, in RHO-ROCK-inhibited embryos, components of the apical (Par-3/Par-6/aPKC) complex and the basal (Scribble/Lgl/Dlg) complex co-existed in the same cortical domains. In many epithelial cell types, distinct apical and basal domains are clearly separated by mutually antagonistic regulations between polarity regulators. For example, the apical component aPKC can phosphorylate basal components PAR1 and LGL to prevent their association with the apical membrane (Hurov et al., 2004; Suzuki et al., 2004; Yamanaka et al., 2003), whereas PAR1 can phosphorylate PAR3 to interfere with its interaction with aPKC (Benton and St Johnston, 2003). It is of particular interest in future studies to examine how the mutual antagonistic mechanisms are affected in RHO-ROCK-inhibited embryos.
The present study as well as the previous studies (Alarcon, 2010; Hirate et al., 2013; Leung and Zernicka-Goetz, 2013) suggest that Hippo signaling is under the control of apical-basal cell polarity in mouse preimplantation embryos. Formation of intact apical-basal polarity inactivates Hippo signaling, which results in YAP nuclear accumulation to turn on TEAD4-mediated transcriptional regulation. If TEAD4 is to be required for epithelialization of TE, as implicated previously (Alarcon, 2010; Nishioka et al., 2008; Yagi et al., 2007), dependence of blastocyst cavitation on the apical-basal cell polarity may be mediated by Hippo signaling. Alternatively, the apical-basal cell polarity may play more direct roles in TE epithelialization independently from Hippo signaling, as the polarity regulators have been shown to control the formation and integrity of epithelial architectures in various systems (Chen and Zhang, 2013; McCaffrey and Macara, 2011). Interestingly, a recent study has shown that Tead4 null zygotes can form a blastocyst cavity when cultured under hypoxic condition (5% oxygen; Kaneko and DePamphilis, 2013). In further studies, it is of particular interest to investigate whether RHO-ROCK-inhibited or PARD6B-knockdown embryos can form a blastocyst cavity under hypoxic condition, which may shed light on how TE epithelialization is regulated by the apical-basal cell polarity and Hippo signaling.
Even though many apical-basal components were mislocalized in ROCK-inhibited embryos, not all polarization events were interfered with, particularly around the time of compaction at the late 8-cell stage. Apical accumulation of PCM1 puncta was inhibited by Y-27632 treatment, whereas apical localizations of p-ERM and tyrosinated tubulin were unaffected. The role of apical PCM1 is currently unknown, although injection of function-interfering anti-PCM1 antibody into fertilized eggs blocks cell cycle progression (Balczon et al., 2002). Interestingly, physical association between PCM1 and PARD6A at the centrosomes and centriolar satellites has been observed in cell lines (Kodani et al., 2010). This raises the possibility that accumulation of PCM1 may be involved in guiding the Par-3/Par-6/aPKC complex to the apical cortex.
Our study implicates that RHO GTPases act through ROCK to activate YAP in a LATS-mediated manner in preimplantation embryos. Nonetheless, it is also possible that the impact of RHO-ROCK signaling on YAP is an indirect consequence of disturbed apical-basal cell polarization, as discussed above. Further investigations on more detailed mechanisms linking RHO-ROCK to YAP in preimplantation embryos should help distinguish between these possibilities.
While this manuscript was under review, a new study was published, reporting that treatment of mouse preimplantation embryos with Y-27632 causes defects in the first cleavage and failure in compaction at the 8-cell stage (Duan et al., 2014). These adverse effects of Y-27632 appear more severe than those shown in the present study, and further investigations are required to resolve such apparent discrepancy. Nonetheless, it is important to point out several differences in experimental procedures. In the study by Duan et al. (2014), a much higher concentration of Y-27632 (100 µM) is used compared to the present study (20 µM). Also, the embryo culture condition in Duan et al. (2014) appears to be suboptimal because only about 50% of embryos have reached the morula/blastocyst stage in some experiments. This is in striking contrast to the condition in the present study, which supported development of nearly 100% of embryos to expanded blastocysts. It is possible that the use of a very high concentration of the inhibitor in conjunction with suboptimal culture condition may have contributed to the more severe developmental defects in Duan et al. (2014).
Supplementary Material
Highlights.
Preimplantation mouse development requires RHO-ROCK activity for TE differentiation
The ICM lineage is enhanced by inhibition of RHO-ROCK activity
RHO-ROCK signaling modulates Hippo pathway to activate YAP in LATS-mediated manner
Polarization of apical and basal cell domains requires RHO-ROCK activity
Acknowledgments
We thank Yusuke Marikawa for technical assistance and critical discussions during the course of our investigations. We thank Hiroshi Sasaki and Yoshikazu Hirate for providing dominant-negative LATS, phosphorylation-defective YAP, and β-globin expression constructs. This study was supported by George F. Straub Trust of the Hawaii Community Foundation 13ADVC-60315 and NIH P20GM103457 (VBA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aktories K, Hall A. Botulinum ADP-ribosyltransferase C3: a new tool to study low molecular weight GTP-binding proteins. Trends Pharmacol. Sci. 1989;10:415–418. doi: 10.1016/0165-6147(89)90191-0. [DOI] [PubMed] [Google Scholar]
- Alarcon VB. Cell polarity regulator PARD6B is essential for trophectoderm formation in the preimplantation mouse embryo. Biol. Reprod. 2010;83:347–358. doi: 10.1095/biolreprod.110.084400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Nakayama M, Kaibuchi K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton. 2010;67:545–554. doi: 10.1002/cm.20472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin E, Dubey BN, Zhang SC, Gremer L, Dvorsky R, Moll JM, Taha MS, Nagel-Steger L, Piekorz RP, Somlyo AV, Ahmadian MR. Rho-kinase: regulation, (dys)function, and inhibition. Biol. Chem. 2013;394:1399–1410. doi: 10.1515/hsz-2013-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avruch J, Zhou D, Fitamant J, Bardeesy N, Mou F, Barrufet LR. Protein kinases of the Hippo pathway: regulation and substrates. Semin. Cell Dev. Biol. 2012;23:770–784. doi: 10.1016/j.semcdb.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balczon R, Simerly C, Takahashi D, Schatten G. Arrest of cell cycle progression during first interphase in murine zygotes microinjected with anti-PCM-1 antibodies. Cell Motil. Cytoskeleton. 2002;52:183–192. doi: 10.1002/cm.10043. [DOI] [PubMed] [Google Scholar]
- Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J. Cell Biol. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. Erratum in: Cell 2004 116, 139. [DOI] [PubMed] [Google Scholar]
- Blij S, Frum T, Akyol A, Fearon E, Ralston A. Maternal Cdx2 is dispensable for mouse development. Development. 2012;139:3969–3972. doi: 10.1242/dev.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113:643–655. doi: 10.1016/s0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang M. The Par3/Par6/aPKC complex and epithelial cell polarity. Exp. Cell Res. 2013;319:1357–1364. doi: 10.1016/j.yexcr.2013.03.021. [DOI] [PubMed] [Google Scholar]
- Clayton L, Hall A, Johnson MH. A role for Rho-like GTPases in the polarisation of mouse eight-cell blastomeres. Dev. Biol. 1999;205:322–331. doi: 10.1006/dbio.1998.9117. [DOI] [PubMed] [Google Scholar]
- Cockburn K, Biechele S, Garner J, Rossant J. The Hippo pathway member Nf2 is required for inner cell mass specification. Curr. Biol. 2013;23:1195–1201. doi: 10.1016/j.cub.2013.05.044. [DOI] [PubMed] [Google Scholar]
- Dard N, Le T, Maro B, Louvet-Vallee S. Inactivation of aPKClambda reveals a context dependent allocation of cell lineages in preimplantation mouse embryos. PLoS One. 2009;4:e7117. doi: 10.1371/journal.pone.0007117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dard N, Louvet-Vallee S, Santa-Maria A, Maro B. Phosphorylation of ezrin on threonine T567 plays a crucial role during compaction in the mouse early embryo. Dev. Biol. 2004;271:87–97. doi: 10.1016/j.ydbio.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Duan X, Chen KL, Zhang Y, Cui XS, Kim NH, Sun SC. ROCK inhibition prevents early mouse embryo development. Histochem. Cell Biol. 2014 doi: 10.1007/s00418-014-1201-6. (in press) [DOI] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, Elvassore N, Piccolo S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Eckert JJ, Fleming TP. Tight junction biogenesis during early development. Biochim. Biophys. Acta. 2008;1778:717–728. doi: 10.1016/j.bbamem.2007.09.031. [DOI] [PubMed] [Google Scholar]
- Fujimori T. Preimplantation development of mouse: a view from cellular behavior. Dev. Growth Differ. 2010;52:253–262. doi: 10.1111/j.1440-169X.2010.01172.x. [DOI] [PubMed] [Google Scholar]
- Genevet A, Tapon N. The Hippo pathway and apico-basal cell polarity. Biochem. J. 2011;436:213–224. doi: 10.1042/BJ20110217. [DOI] [PubMed] [Google Scholar]
- Han S, Arvai AS, Clancy SB, Tainer JA. Crystal structure and novel recognition motif of rho ADP-ribosylating C3 exoenzyme from Clostridium botulinum: structural insights for recognition specificity and catalysis. J. Mol. Biol. 2001;305:95–107. doi: 10.1006/jmbi.2000.4292. [DOI] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 2008;283:5496–5509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- Hiiragi T, Alarcon VB, Fujimori T, Louvet-Vallee S, Maleszewski M, Marikawa Y, Maro B, Solter D. Where do we stand now? Mouse early embryo patterning meeting in Freiburg, Germany (2005) Int. J. Dev. Biol. 2006;50:581–587. doi: 10.1387/ijdb.062181th. [DOI] [PubMed] [Google Scholar]
- Hirate Y, Cockburn K, Rossant J, Sasaki H. Tead4 is constitutively nuclear, while nuclear vs. cytoplasmic Yap distribution is regulated in preimplantation mouse embryos. Proc. Natl. Acad. Sci. USA. 2012;109:E3389–E3390. doi: 10.1073/pnas.1211810109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirate Y, Hirahara S, Inoue K, Suzuki A, Alarcon VB, Akimoto K, Hirai T, Hara T, Adachi M, Chida K, Ohno S, Marikawa Y, Nakao K, Shimono A, Sasaki H. Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Curr. Biol. 2013;23:1181–1194. doi: 10.1016/j.cub.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Home P, Ray S, Dutta D, Bronshteyn I, Larson M, Paul S. GATA3 is selectively expressed in the trophectoderm of peri-implantation embryo and directly regulates Cdx2 gene expression. J. Biol. Chem. 2009;284:28729–28737. doi: 10.1074/jbc.M109.016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Guan KL. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin. Cell Dev. Biol. 2012;23:785–793. doi: 10.1016/j.semcdb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houliston E, Maro B. Posttranslational modification of distinct microtubule subpopulations during cell polarization and differentiation in the mouse preimplantation embryo. J. Cell Biol. 1989;108:543–551. doi: 10.1083/jcb.108.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houliston E, Pickering SJ, Maro B. Redistribution of microtubules and pericentriolar material during the development of polarity in mouse blastomeres. J. Cell Biol. 1987;104:1299–1308. doi: 10.1083/jcb.104.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr. Biol. 2004;14:736–741. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Ishiuchi T, Takeichi M. Willin and Par3 cooperatively regulate epithelial apical constriction through aPKC-mediated ROCK phosphorylation. Nat. Cell Biol. 2011;13:860–866. doi: 10.1038/ncb2274. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakizuka A, Morii N, Narumiya S. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just I, Hofmann F, Genth H, Gerhard R. Bacterial protein toxins inhibiting lowmolecular-mass GTP-binding proteins. Int. J. Med. Microbiol. 2001;291:243–250. doi: 10.1078/1438-4221-00127. [DOI] [PubMed] [Google Scholar]
- Kamijo H, Matsumura Y, Thumkeo D, Koike S, Masu M, Shimizu Y, Ishizaki T, Narumiya S. Impaired vascular remodeling in the yolk sac of embryos deficient in ROCK-I and ROCK-II. Genes to Cells. 2011;16:1012–1021. doi: 10.1111/j.1365-2443.2011.01546.x. [DOI] [PubMed] [Google Scholar]
- Kaneko KJ, DePamphilis ML. TEAD4 establishes the energy homeostasis essential for blastocoel formation. Development. 2013;140:3680–3690. doi: 10.1242/dev.093799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawagishi R, Tahara M, Sawada K, Ikebuchi Y, Morishige K, Sakata M, Tasaka K, Murata Y. Rho-kinase is involved in mouse blastocyst cavity formation. Biochem. Biophys. Res. Commun. 2004;319:643–648. doi: 10.1016/j.bbrc.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Kodani A, Tonthat V, Wu B, Sutterlin C. Par6 alpha interacts with the dynactin subunit p150 Glued and is a critical regulator of centrosomal protein recruitment. Mol. Biol. Cell. 2010;21:3376–3385. doi: 10.1091/mbc.E10-05-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeno AM, Tamashiro DA, Alarcon VB. Rho-associated kinase activity is required for proper morphogenesis of the inner cell mass in the mouse blastocyst. Biol. Reprod. 2013;89:122. doi: 10.1095/biolreprod.113.109470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CY, Zernicka-Goetz M. Angiomotin prevents pluripotent lineage differentiation in mouse embryos via Hippo pathway-dependent and -independent mechanisms. Nat. Commun. 2013;4:2251. doi: 10.1038/ncomms3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol. Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorthongpanich C, Messerschmidt DM, Chan SW, Hong W, Knowles BB, Solter D. Temporal reduction of LATS kinases in the early preimplantation embryo prevents ICM lineage differentiation. Genes Dev. 2013;27:1441–1446. doi: 10.1101/gad.219618.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikawa Y, Alarcon VB. Creation of trophectoderm, the first epithelium, in mouse preimplantation development. Results Probl. Cell Differ. 2012;55:165–184. doi: 10.1007/978-3-642-30406-4_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- McCaffrey LM, Macara IG. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011;21:727–735. doi: 10.1016/j.tcb.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Miller E, Yang J, DeRan M, Wu C, Su AI, Bonamy GM, Liu J, Peters EC, Wu X. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem. Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113:631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Gertsenstein M, Vintersten K, Behringer R. 3rd ed. New York: Cold Spring Harbor Laboratory Press; 2003. Manipulating the Mouse Embryo: A Laboratory Manual; pp. 192–200. [Google Scholar]
- Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, Amano M, Kaibuchi K. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev. Cell. 2008;14:205–215. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, Makita R, Kurihara H, Morin-Kensicki EM, Nojima H, Rossant J, Nakao K, Niwa H, Sasaki H. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev. Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Nishioka N, Yamamoto S, Kiyonari H, Sato H, Sawada A, Ota M, Nakao K, Sasaki H. Tead4 is required for specification of trophectoderm in pre-implantation mouse embryos. Mech. Dev. 2008;125:270–283. doi: 10.1016/j.mod.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Nishioka T, Nakayama M, Amano M, Kaibuchi K. Proteomic screening for Rho-kinase substrates by combining kinase and phosphatase inhibitors with 14-3-3 ζ affinity chromatography. Cell Struct. Funct. 2012;37:39–48. doi: 10.1247/csf.11044. [DOI] [PubMed] [Google Scholar]
- Parsons LM, Grzeschik NA, Allott ML, Richardson HE. Lgl/aPKC and Crb regulate the Salvador/Warts/Hippo pathway. Fly (Austin) 2010;4:288–293. doi: 10.4161/fly.4.4.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plusa B, Frankenberg S, Chalmers A, Hadjantonakis AK, Moore CA, Papalopulu N, Papaioannou VE, Glover DM, Zernicka-Goetz M. Downregulation of Par3 and aPKC function directs cells towards the ICM in the preimplantation mouse embryo. J. Cell Sci. 2005;118:505–515. doi: 10.1242/jcs.01666. [DOI] [PubMed] [Google Scholar]
- Sasaki H. Mechanisms of trophectoderm fate specification in preimplantation mouse development. Dev. Growth Differ. 2010;52:263–273. doi: 10.1111/j.1440-169X.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- St. Johnston D, Sanson B. Epithelial polarity and morphogenesis. Curr. Opin. Cell Biol. 2011;23:540–546. doi: 10.1016/j.ceb.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Stephenson RO, Rossant J, Tam PP. Intercellular interactions, position, and polarity in establishing blastocyst cell lineages and embryonic axes. Cold Spring Harb. Perspect. Biol. 2012;4:1–15. doi: 10.1101/cshperspect.a008235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132:2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- Suwinska A, Czolowska R, Ozdzenski W, Tarkowski AK. Blastomeres of the mouse embryo lose totipotency after the fifth cleavage division: expression of Cdx2 and Oct4 and developmental potential of inner and outer blastomeres of 16- and 32-cell embryos. Dev. Biol. 2008;322:133–144. doi: 10.1016/j.ydbio.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, Kishikawa M, Hirose H, Amano Y, Izumi N, Miwa Y, Ohno S. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr. Biol. 2004;14:1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Takaoka K, Hamada H. Cell fate decisions and axis determination in the early mouse embryo. Development. 2012;139:3–14. doi: 10.1242/dev.060095. [DOI] [PubMed] [Google Scholar]
- Tao H, Inoue K, Kiyonari H, Bassuk AG, Axelrod JD, Sasaki H, Aizawa S, Ueno N. Nuclear localization of Prickle2 is required to establish cell polarity during early mouse embryogenesis. Dev. Biol. 2012;364:138–148. doi: 10.1016/j.ydbio.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ, Pichaud F, Roper K. Sticking together the Crumbs - an unexpected function for an old friend. Nat. Rev. Mol. Cell Biol. 2013;14:307–314. doi: 10.1038/nrm3568. [DOI] [PubMed] [Google Scholar]
- Thumkeo D, Watanabe S, Narumiya S. Physiological roles of Rho and Rho effectors in mammals. Eur. J. Cell Biol. 2013;92:303–315. doi: 10.1016/j.ejcb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Vinot S, Le T, Ohno S, Pawson T, Maro B, Louvet-Vallee S. Asymmetric distribution of PAR proteins in the mouse embryo begins at the 8-cell stage during compaction. Dev. Biol. 2005;282:307–319. doi: 10.1016/j.ydbio.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML, Buonanno A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134:3827–3836. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, Iwamatsu A, Shinohara A, Ohno S. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr. Biol. 2003;13:734–743. doi: 10.1016/s0960-9822(03)00244-6. [DOI] [PubMed] [Google Scholar]
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Genes Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, Fu XD, Mills GB, Guan KL. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernicka-Goetz M. Development: do mouse embryos play dice? Curr. Biol. 2013;23:R15–R17. doi: 10.1016/j.cub.2012.10.032. [DOI] [PubMed] [Google Scholar]
- Zernicka-Goetz M, Morris SA, Bruce AW. Making a firm decision: multifaceted regulation of cell fate in the early mouse embryo. Nat. Rev. Genet. 2009;10:467–477. doi: 10.1038/nrg2564. 2009. [DOI] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai ZC, Guan KL. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.