Abstract
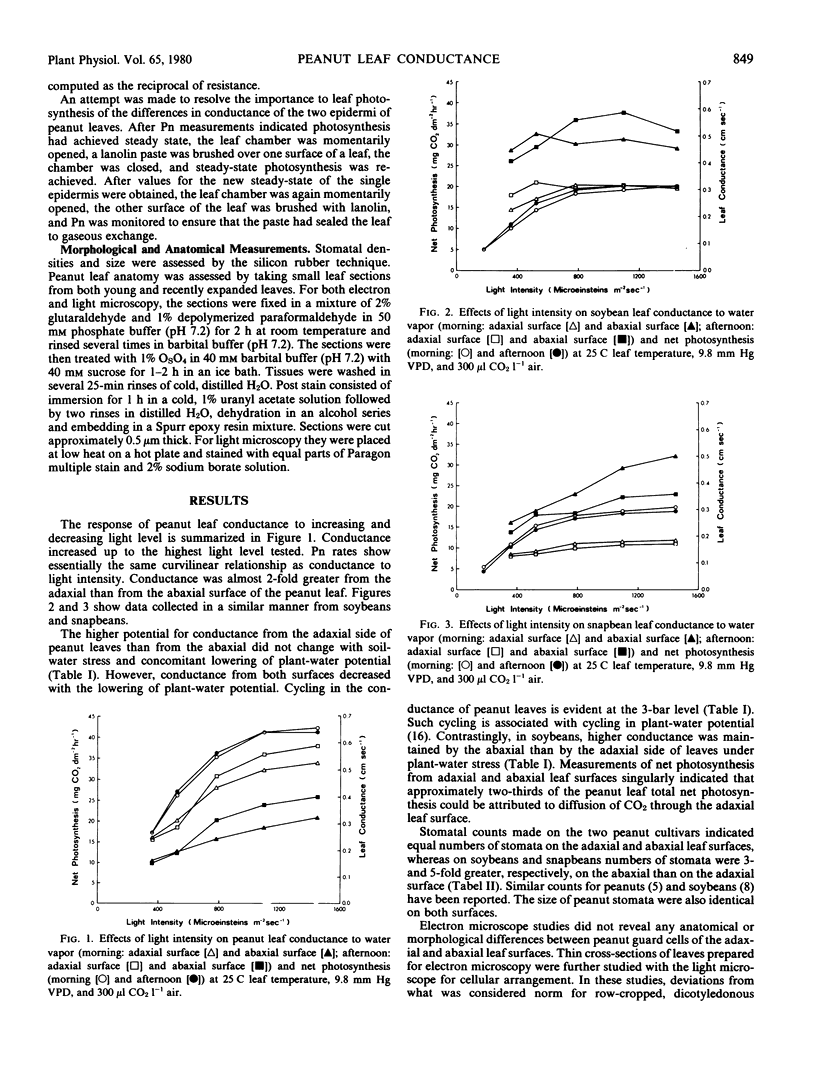
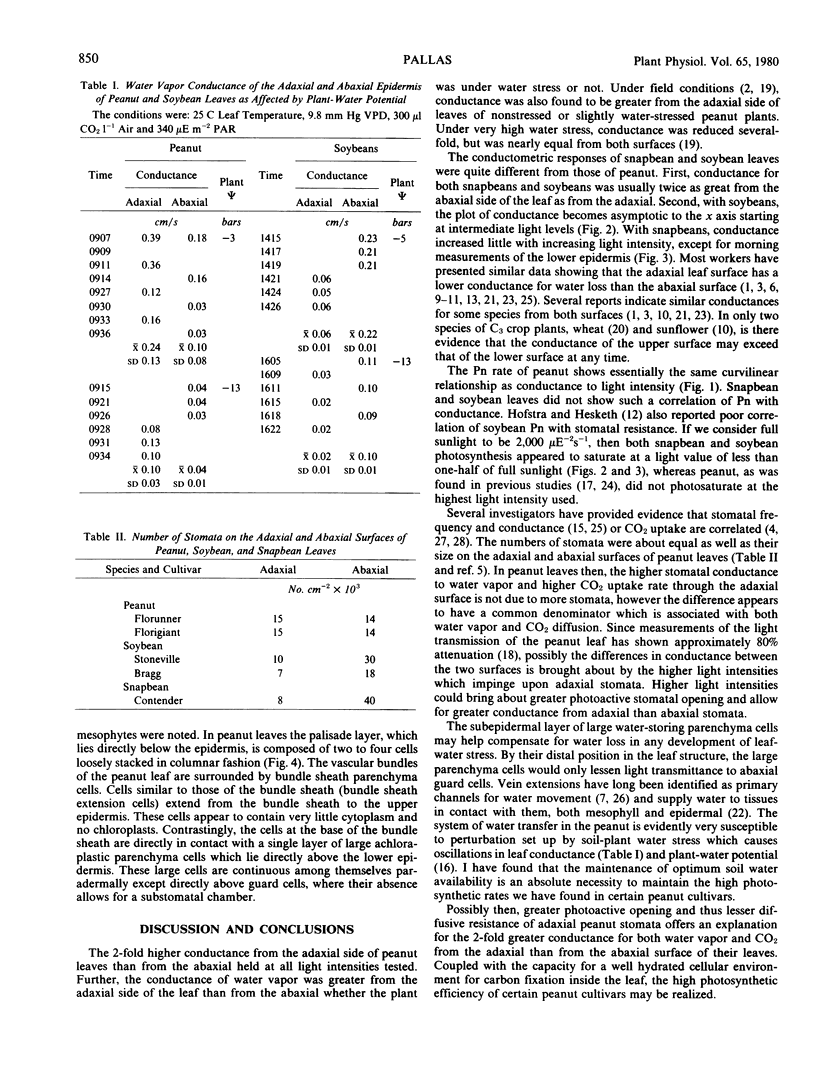
Conductance to gaseous transfer is normally considered to be greater from the abaxial than from the adaxial side of a leaf. Measurements of the conductance to water vapor of peanut leaves (Arachis hypogaea L.) under well watered and stress conditions in a controlled environment, however, indicated a 2-fold higher conductance from the adaxial side of the leaf than from the abaxial. Studies of conductance as light level was varied showed an increase in conductance from either surface with increasing light level, but conductance was always greater from the adaxial surface at any given light level. In contrast, measurements of soybean (Glycine max [L.] Merr.) and snapbean (Phaseolus vulgaris L.) leaf conductance showed an approximate 2-fold greater conductance from the abaxial surface than from the adaxial. Approximately the same number of stomata were present on both peanut leaf surfaces and stomatal size was similar. Electron microscopic examination of peanut leaves did not reveal any major structural differences between stomata on the two surfaces that would account for the differences in conductance. Light microscope studies of leaf sections revealed an extensive network of bundle sheaths with achloraplastic bundle sheath extensions; the lower epidermis was lined with a single layer of large achloraplastic parenchyma cells. Measurements of net photosynthesis made on upper and lower leaf surfaces collectively and individually indicated that two-thirds of the peanut leaf's total net photosynthesis can be attributed to diffusion of CO2 through the adaxial leaf surface. Possibly the high photosynthetic efficiency of peanut cultivars as compared with certain other C3 species is associated with the greater conductance of CO2 through their upper leaf surfaces.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ehrler W. L., van Bavel C. H. Leaf diffusion resistance, illuminance, and transpiration. Plant Physiol. 1968 Feb;43(2):208–214. doi: 10.1104/pp.43.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemasu E. T., Tanner C. B. Stomatal diffusion resistance of snap beans. I. Influence of leaf-water potential. Plant Physiol. 1969 Nov;44(11):1547–1552. doi: 10.1104/pp.44.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemasu E. T., Thurtell G. W., Tanner C. B. Design calibration and field use of a stomatal diffusion porometer. Plant Physiol. 1969 Jun;44(6):881–885. doi: 10.1104/pp.44.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschke K., Zeevaart J. A. Abscisic Acid Content, Transpiration, and Stomatal Conductance As Related to Leaf Age in Plants of Xanthium strumarium L. Plant Physiol. 1976 Aug;58(2):169–174. doi: 10.1104/pp.58.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sionit N., Kramer P. J. Water Potential and Stomatal Resistance of Sunflower and Soybean Subjected to Water Stress during Various Growth Stages. Plant Physiol. 1976 Oct;58(4):537–540. doi: 10.1104/pp.58.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]




