Abstract
The field of percutaneous valvular interventions is one of the most exciting and rapidly developing within interventional cardiology. Percutaneous procedures focusing on aortic and mitral valve replacement or interventional treatment as well as techniques of percutaneous pulmonary valve implantation have already reached worldwide clinical acceptance and routine interventional procedure status. Although techniques of percutaneous pulmonary valve implantation have been described just a decade ago, two stent-mounted complementary devices were successfully introduced and more than 3000 of these procedures have been performed worldwide. In contrast, percutaneous treatment of tricuspid valve dysfunction is still evolving on a much earlier level and has so far not reached routine interventional procedure status. Taking into account that an “interdisciplinary challenging”, heterogeneous population of patients previously treated by corrective, semi-corrective or palliative surgical procedures is growing inexorably, there is a rapidly increasing need of treatment options besides redo-surgery. Therefore, the review intends to reflect on clinical expansion of percutaneous pulmonary and tricuspid valve procedures, to update on current devices, to discuss indications and patient selection criteria, to report on clinical results and finally to consider future directions.
Keywords: Congenital heart disease, Right ventricular outflow tract dysfunction, Pulmonary regurgitation, Percutaneous pulmonary valve implantation, Percutaneous tricuspid valve implantation
Core tip: The field of percutaneous valve implantation/repair is rapidly developing within interventional cardiology. Percutaneous procedures focusing on aortic, mitral or pulmonary valve dysfunction have almost reached daily routine. In contrast, percutaneous treatment of tricuspid valve dysfunction is still evolving on a much earlier level. Taking into account that an “interdisciplinary challenging” population of patients previously treated by corrective, semi-corrective or palliative surgery is growing inexorably, there is an increasing need of options besides redo-surgery. This review intends to report on clinical application of pulmonary and tricuspid valve procedures. It updates on current devices, patient selection criteria, results and future directions.
INTRODUCTION
Isolated pulmonary and tricuspid valve dysfunction, whether acquired or in the context of congenital heart disease, can be clinically asymptomatic and be tolerated for a long time[1]. In the western world, acquired primary tricuspid or pulmonary valve diseases are rare conditions and mostly related to rheumatic fever, infective endocarditis or rarities (e.g., carcinoid syndrome).
For those cases with underlying congenital heart disease, dysfunction of these valves is both a primary component of many anatomical conditions and a secondary, but common consequence of several early repair strategies[2].
Increasing knowledge about potential harmful effects of chronic pulmonary artery (PA) regurgitation has made the surgical revision of the right ventricular (RV) outflow tract (RVOT) a frequently performed operation in this population[3]. Typically, most of these patients require several redo-operations during their lifetime to halt the detrimental effects of valvular dysfunction. Since techniques of percutaneous pulmonary valve implantation (PPVI) were first described by Bonhoeffer et al[4] more than a decade ago, the procedure has reached worldwide clinical acceptance and routine interventional procedure status. Several devices have been investigated for purposes of PPVI, but so far only the MELODYTM device (Medtronic, MN, United States) has obtained regulatory approval. Interventional procedures focusing on percutaneous tricuspid valve replacement or interventional treatment of severe tricuspid regurgitation are evolving, but yet remain at a much earlier stage and have so far not reached levels of standard procedures[5]. The authors review on clinical expansion of this revolutionary technology, discuss current indications and patient selection criteria, report on clinical results and finally consider future directions.
PPVI
Background and clinical indications
Over the last decades, advances in cardiac surgery, interventional procedures, intensive care and non-invasive imaging have led to a substantial increase in life expectancy for many patients with congenital heart disease. Therefore, an “interdisciplinary challenging”, heterogeneous population of patients treated by corrective, semi-corrective or palliative surgical procedures, sometimes decades ago, is growing inexorably. For approximately 20% of these patients RVOT dysfunction caused by predominant obstruction, by predominant pulmonary regurgitation or both in combined conditions, becomes clinically evident.
Undeniably, surgical pulmonary valve replacement is the most frequent mode of redo-operation in patients with congenital heart disease[3]. Surgery for RVOT dysfunction can be performed with low morbidity and mortality[3]. However, an important drawback of this treatment is the limited lifespan of used conduits that has been reported to be around ten years[6-9]. As a consequence, the majority of patients have to undergo several open-heart procedures during their life that raise potential individual risks for a diversity of complications. To limit the need for redo-operations delaying surgery for as long as possible is the strategy of choice in any individual patient. If the necessary treatment is delayed beyond a certain point of no return, adverse RV loading conditions might lead to irreversible ventricular dysfunction, reduced exercise capacity and ultimately to an increased risk for sudden cardiac death[10-13]. Decision making on ideal timing of pulmonary valve replacement is still challenging in most cases and represents one of the most controversial issues of cardiologists who take care of children and adults with congenital heart disease[3,14,15]. RV volume thresholds on magnetic resonance imaging (MRI) have been proposed as predictors for outcome after conduit placement[14]. An RV end-systolic volume of 150-170 mL/m2 has been reported to deserve as cut-off point above which normalisation of RV dimensions is unlikely following pulmonary valve replacement[13-17]. Nevertheless, the impact of the timing of pulmonary valve replacement on RV function, exercise performance and patient long-term survival remains undefined[13].
With the evolution of PPVI, an effective and feasible non-surgical technique was introduced. It offers a minimally invasive method which can potentially avoid open-heart surgery for RVOT dysfunction in children and adults by restoring acceptable RV loading conditions.
Since the first description of PPVI in 2000[4], more than 3000 percutaneous pulmonary valves have been implanted worldwide[18]. PPVI is performed to prolong the lifespan of RV-to-PA conduits and thereby delaying redo-operations in children and adults with congenital heart disease. Over the last decade, a marked learning curve in outcome post-PPVI could be demonstrated, with improved safety, efficacy and freedom from redo-surgery or re-intervention for pediatric or adult patients who underwent this procedure[13,19-25].
Current devices
The MELODYTM transcatheter pulmonary valve is designed of a segment of bovine jugular vein with a central valve (Figures 1 and 2) that is sewn inside an expanded platinum-iridium stent. The current carrying Cheatham platinum stent (NuMED CP Stent CP8Z34) is of a length 34 mm, “crimpable” to minimum of 6 mm and re-expandable up to 22 mm. The balloon in balloon (BiB) delivery system (EnsembleTM, Medtronic, MN) is commercially available with different outer balloon diameters of 18, 20 and 22 mm.
Figure 1.
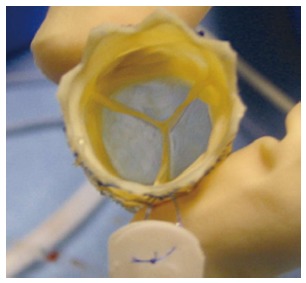
The MELODYTM percutaneous pulmonary valve (Medtronic, MN). The Melody device in “en face” view. Note the blue outflow line to identify the outflow end of the device (courtesy of P.Lurz).
Figure 2.
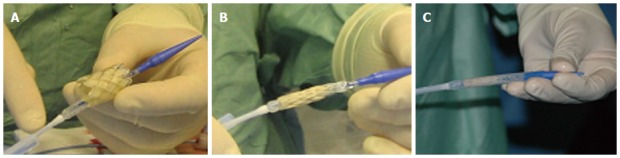
MELODYTM device and its delivery system. A: Uncrimped device on the delivery system with a retractable sheath; B: Crimped device before covering the device to protect it during the delivery; C: Crimped and covered device prepared for delivery (courtesy of Lurz P).
The Edwards SAPIENTM valve (Edwards Lifesciences LLC, Irvine, CA) is radiopaque and made of a trileaflet bovine pericardial valve hand-sewn into a stainless-steel stent (length of 14 or 16 mm) (Figure 3). A sealing cuff covers the proximal part of the stent designed to prevent paravalvular leakage. Currently, the valve is commercially available in 23 and 26 mm diameter sizes and is crimped onto a designated balloon delivery system RetroflexTM III. A 29 mm diameter valve is also available (Edwards SAPIENTM XT). The delivery system requires either 22 Fr (SAPIENTM 23 mm valve) or 24 Fr hydrophilic sheaths (SAPIENTM 26 mm and SAPIENTM XT 29 mm valve) (Figure 3). Promising improvements of design (Edwards eSheathTM) offer even smaller sheath sizes.
Figure 3.
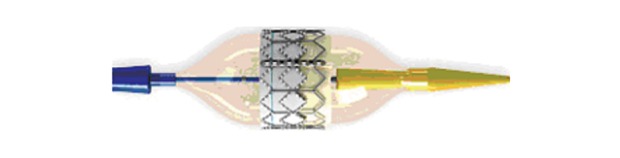
The Edwards SAPIENTM pulmonic transcatheter heart valve (Edwards Lifesciences LLC, Irvine, CA). The SAPIENTM device in lateral view mounted on its delivery system RetroflexTM III (courtesy of Edwards Lifesciences LLC).
Technical details regarding both devices for PPVI are summarized in Table 1.
Table 1.
Devices and delivery systems for percutaneous pulmonary valve implantation
| The MELODYTM transcatheter pulmonary valve | The SAPIENTM pulmonic transcatheter heart valve | |
| Manufactor | Medtronic Inc., MN, United States | Edwards Lifesciences LLC, Irvine, CA, United States |
| Regulatory approval | CE 9/2006 FDA 01/2010 | CE 5/2010 FDA 10/2012 |
| (Tissue) characteristics | Segment of bovine jugular vein with a central valve hand-sewn inside a stent | Trileaflet bovine pericardial valve hand-sewn inside a stent |
| Stent type | Cheatham platinum stent (NuMED CP Stent CP8Z34) Length 34 mm Expandable up to 22 mm | Stainless-steel stent Length of 14 or 16 mm |
| Available sizes | 18, 20, 22 mm (depending on the favoured EnsembleTM delivery (system) | 23, 26, (XT 29a ) mm |
| Delivery system | EnsembleTM (Medtronic, MN) with balloon in balloon (BiB) deployment design | Edwards RetroflexTM III containing a balloon catheter and a deflectable guiding catheter |
| Sheats for implantation | One-piece 22 Fr Teflon sheath | (18 Frb) 22 Fr for 23 mm valves (19 Frb) 24 Fr for 26 mm valves (16 Frb) 24 Fr for 29 mm XT valves |
Technical comparision of the commercially available devices for percutaneous pulmonary valve implantation: the MELODYTM device and the SAPIENTM pulmonic transcatheter heart valve as non-surgical treatment options for RV outflow tract dysfunction (a“Off label-use” in pulmonary position;
Manufacturer’s data given for the Edwards eSheathTM). RV: Right ventricle.
Patient selection criteria
Although sophisticated MR data have been reported, the clinicians’ dilemma of the right timing for treatment of RVOT dysfunction, whether predominantly caused by obstruction or regurgitation has not been solved yet[13]. According to current guidelines for the management of grown-up congenital heart disease[26], patients with RVOT obstruction should be treated if the RV to PA gradient exceeds 60 mmHg or in presence of symptoms due to RVOT obstruction regardless of RVOT gradients. Pulmonary regurgitation can be clinically asymptomatic and be tolerated for a long time[1]. When to intervene is subject to ongoing discussions. It is common sense to base the indication criteria for transcatheter or surgical treatment on a combined assessment of MR-imaging derived RV EDV and systolic function, cardiopulmonary exercise testing and the presence of atrial or ventricular dysrhythmia[13]. According to the 2010 recommendations of the ESC task force PPVI can therefore be indicated, if severe pulmonary regurgitation (as assessed on echocardiography or MR imaging) is accompanied by severe RV dilatation, severe RV dysfunction, clinical symptoms and/or impaired exercise capacity[13,26].
In 2011, the American Heart Association (AHA) stated: “It is reasonable to consider the percutaneous pulmonary valve replacement in patient with RV-to-PA conduits with moderate to severe pulmonary regurgitation or stenosis provided the patient meets inclusion/exclusion criteria for the available valve”. The AHA writing committee recommended this procedure with a Class IIa evidence (Level of evidence: B)[27]. Clinical indications applicable regardless of the device used for valve implantation (MELODYTM and SAPIENTM) are summarized in Table 2.
Table 2.
Clinical and morphological requirements for percutaneous pulmonary valve implantation
| Clinical indications in the context of RV pressure overload/pulmonary stenosis |
| RV systolic pressure > 65% of systemic pressure in symptomatic patients |
| RV systolic pressure > 75% of systemic pressure in asymptomatic patients |
| Clinical indications in the context of RV volume overload/pulmonary regurgitation |
| Severe pulmonary regurgitation on echocardiography or MR imaging and |
| Severe RV dilatation > 150 mL/m2 or the RV to LV end-diastolic ratio of > 1.7 and/or |
| Rapid progressiv RV dilatation and/or |
| Severe RV dysfunction and/or |
| Symptoms and/or |
| Sustained atrial or venticular arrhythmia and/or |
| Impaired exercise capacity [ < 65% compared to norm peak oxygen consumption related to bodyweight (VO2/kg)] |
| Morphological indications |
| Circumferential RV to PA conduit with dimensions ranging from 16 to 22 mm (MelodyTM) |
| Circumferential RV to PA conduit with dimension at surgical implantation of at least 18 mm but no larger than 29 mm (with some degree of conduit narrowing) (SAPIENTM) |
| Exclusion of risk for coronary compression |
RV: Right ventricle; PA: Pulmonary artery.
Although there is no absolute lower age limit, an adequate body size (e.g., weight > 20 kg) is required to accommodate femoral placement of the introducer[21].
Size and shape of the implantation site (“landing zone”) and its anatomical relation to coronary arteries are decisive morphological criteria which have to be appropriate when considering patients as potential candidates for PPVI: In regulatory approved routine use, current MELODYTM devices are not intended for dilatations to diameters of more than 22 mm. Patients with (non-dilated) conduits between the RV and PA of 22 mm and less offer an ideal environment to perform PPVI. In contrast, native or patched RV outflow tracts after surgical repair for Tetralogy of Fallot are often enlarged ( > 22 mm) and therefore do not provide a secure landing zone for MELODYTM valves[14]. In these cases (but not larger than 29 mm) the SAPIENTM valve might be a possible alternative[28-30]. Furthermore, in our experience the RVOT shape (after prior pre-stenting) is of importance: due to its “engineered” nature of sutured pericardial tissue, optimal valved stent function in SAPIENTM procedures is guaranteed by a circular RVOT shape. In PPVI procedures with the MELODYTM valve the RVOT shape itself appears to have less impact on valvular competence.
Coronary artery anatomy varies due to a broad spectrum of complex congenital heart defects or after surgical re-insertion into the aorta. In some cases there is relevant proximity of one or more of the relevant coronary artery branches to the main PA. This exposes patients who undergo interventions of the RVOT to the risk for fatal coronary artery obstruction due to expansion of the RVOT[31,32]. Therefore, it is essential to assess the course of proximal coronary arteries in relation to the RVOT prior to PPVI deployment. Some centers prefer MR 3-D whole heart images (Figure 4), but we recommend performing selective coronary angiography and particularly aortic root angiography and simultaneous high-pressure balloon inflation within the landing zone at the time of catheterization in all patients to rule out the risk of coronary compression (Figure 5).
Figure 4.
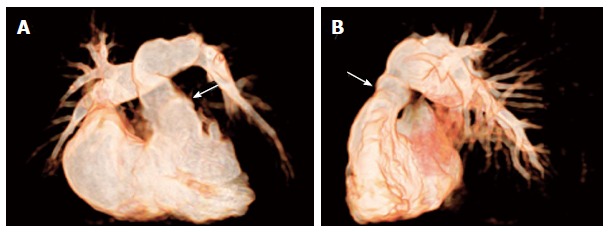
Non-invasive 3D whole heart imaging by magnetic resonance tomography. Non-invasive 3D whole heart imaging by magnetic resonance tomography was performed in a patient with pulmonary atresia with intact ventricular septum after repair by pulmonic homograft implantation (arrows) with RVOT dysfunction prior to PPVI (A) in a.p. and (B) in lat. view. (Courtesy of Wagner R). RVOT: Right ventricular outflow tract; PPVI: Percutaneous pulmonary valve implantation.
Figure 5.
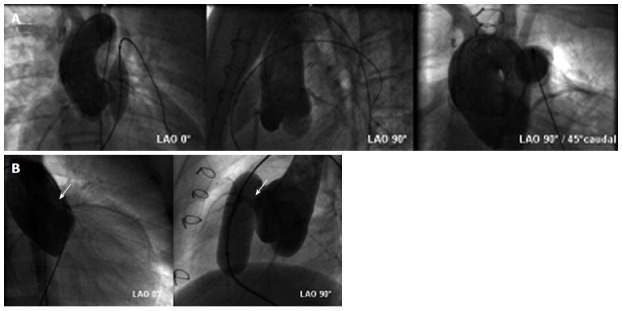
Assessment of risk for coronary compression. A: Aortic root angiogram and simultaneous (high-pressure) balloon inflation within the eligible landing zone is performed to rule out potential for coronary compression (courtesy of Wagner R); B: Aortic root angiogram showing compression of the left anterior descending coronary artery (arrows) during balloon inflation in the conduit. The procedure was therefore abandoned in this patient and no percutaneus pulmonary valve implantation was performed (courtesy of Wagner R).
To facilitate superior immediate haemodynamic results several peri-procedural interventions should be considered: (1) pre-dilatation of the landing zone to resolve relevant stenosis and facilitate positioning of the system; (2) pre-stenting of the RVOT to mark, cover and enhance the “landing zone” and avoid stent fractures; and (3) post-dilatation in any case of residual stenosis more than > 20 mmHg of invasively measured gradient[13].
The optimal timing of pre-dilatation/stenting in relation to definitive PPVI is unknown. Some centers allow stent ingrowth for two to three months. Combined procedures as well as a two-staged procedure are valid options.
Results
Data regarding the haemodynamic outcome post PPVI reported by major reports using MELODYTM [18,19,21,24,33] and using the SAPIENTM device[28,30] are summarized in Table 3.
Table 3.
Haemodynamic outcome immediately post-percutaneous pulmonary valve implantation
| US Melody Valve Trial (n = 124) | London Melody experience (n = 151) | Munich/Berlin Melody experience (n = 102) | Philadelphia Melody experience (n = 104) | Early Edwards experience (n = 7) | Later Edwards experience (n = 36) | |||||||
| Parameter | Pre (median) | Post (median) | Pre (mean) | Post (mean) | Pre (mean) | Post (mean) | Pre (mean) | Post (mean) | Pre (mean) | Post (mean) | Pre (mean) | Post (mean) |
| RV systolic pressure, mmHg | 65 | 41b | 63 | 45b | NA | NA | 72 | 47b | NA | NA | 55 | 42b |
| Peak RV to PA gradient, mmHg | 37 | 12b | 37 | 17b | 37 | 14 | 39 | 11b | NA | NA | 27 | 12b |
| RV to systemic pressure, % | 0.74 | 0.42b | 0.69 | 0.4b | 0.62 | 0.3b | NA | NA | 0.78 | 0.3b | 0.6 | 0.4b |
Invasively measured pressures and gradients pre and post percutaneous pulmonary valve implantation within the largest trials (n > 100) of the MELODYTM[18,19,21,33] and of SAPIENTM implants[28,30] in pulmonary position. In all studies, a profound improvement in RV to systemic pressure ratio in response to PPVI was seen (bP ≤ 0.001) (all parameters expressed by medians). RV: Right ventricular; PA: Pulmonary artery. NA: Not available.
Several mono- and multicenter trials consistently reported a low periprocedural complication rate of six percent using the MELODY TM pulmonic valve[19,33]. Data analysis of the “MELODY TM Registry” data distinguishes between major procedural complications [e.g., homograft rupture, perforation of branch pulmonary arteries, guidewire injury, damage to tricuspid valve, device dislodgement, compression of coronary arterie(s) or obstruction of PA] in 2.7% and 11.9% of minor complications in total of 1003 MELODYM procedures[34].
The “Early Phase 1 International Multicenter Clinical Trial” reporting on SAPIENTM PPVI in 36 patients reported on a successful valve deployment of 97 percent, but seven patients (20.5%) experienced adverse events[30]. The major complication was device dislodgement. In none of the patients, homograft rupture occurred. All of the SAPIENTM patients received pre-stenting (33.3%) or peri-procedural stenting procedures (66.6%).
Coronary compression due to RVOT interventions with bare metal stenting of the RVOT is a well-known and previously described complication[31]. Approximately five to six percent of all patients who are potential candidates for PPVI will have a coronary artery anatomy which bears the risk for coronary obstruction[24]. There are several reports of this potential catastrophic complication[35,36] which is strongly related to early procedural mortality[21]. Ruling out the risk for this complication represents one of the most difficult steps in pre-procedural planning for PPVI. In any case of doubt about the risk of coronary compression, we recommend to abandon the implant in either MELODYTM or SAPIENTM valve procedures (Figure 5).
Follow-up
Short- and medium-term results of PPVI with the MELODYTM and SAPIENTM are thought to be similar, although more data are available for the former. Long-term outcome data for both valved stent types are not yet available.
Overall mortality of PPVI during follow-up procedures was zero to five percent and seems not related to the device itself.
Failure of the device either for the MELODYTM or the SAPIENTM could be related to malfunction of its stent or its sewn valve. Relevant dysfunction of the engrafted valve leads to pulmonary regurgitation which is rare condition that almost only occurs in the context of graft endocarditis[37-40], However, the most common reason for re-operation and re-intervention is re-stenosis of the stent portion of the device. Re-stenosis of the stent can be caused by late recoil or lost of radial strength of the device due to stent fractures. Novel data by Nordmeyer reported of a rate of 11 percent cases of stent fractures[34] representing the most common reason for re-intervention.
Overall, data are available from the major four short- and medium-term observational studies with a total of over 450 patients with one- to five-year follow-up[19,21,25,33]: freedom from valve dysfunction or re-intervention was approximately 94% at one year follow-up. Patients who did not require re-intervention had consistently mild or none pulmonic valve regurgitation at one-year follow-up. Pulmonary regurgitation decreased from median values of 16% to 27% to one to two percent. Median peak velocity over the RVOT was 1.9 to 2.7 m/s at one-year echocardiographic follow-up. Nordmeyer recently reported preliminary but promising MELODYTM Registry data with a one-year freedom for all case events with 92.5% and 94.2% for PPVI-related events[34].
Data on device function of the SAPIENTM valve are limited and available from smaller short- and medium-term observational studies with a total of less than 100 patients[28,30,41,42]. In the largest series (COMPASSION trial), successful valve deployment was achieved in 33 of 34 attempts[30]. Freedom from re-intervention at six-month follow-up was 97%. Haas et al[41] demonstrated a significant reduction of the RVOT to PA gradient, reduction in RV systolic pressure, increasing of diastolic pulmonary pressure from 6.3 to 14.5 mmHg as a sign of tremendously decreased pulmonary regurgitation with freedom from re-intervention after six months[41].
As for the MELODYTM valve, there are no comparison studies with conventional surgery regarding pulmonary valve replacement by SAPIENTM valves.
Regarding the functional outcome, several studies have shown a marked improvement in NYHA functional class post-PPVI[18,19,33]. The improvement has been maintained consistently for the duration of follow-up, irrespective of the treated lesion (predominant stenosis vs predominant regurgitation)[24].
Parameters of exercise cardiopulmonary function such as peak oxygen consumption related to body weight (VO2/kg), ventilatory efficiency and anaerobic oxygen consumption have been assessed in several studies addressing PPVI[20,43-45]. Only patients with a predominant stenotic lesion showed an improvement in peak VO2/kg. Assuming that significant RVOT obstruction may limit the increase of cardiac output in exercise testing resolving of the obstructive lesion at least partially reverses limited exercise capacity in these patients[45,46]. Recently, we reported on the ability to recovery from exercise as described by VO2 and VCO2 decay after maximal exercise. Recovery form exercise after PPVI improves in both groups (predominant stenosis vs predominant regurgitation). These findings could explain the symptomatic improvement observed in patients with predominant regurgitation despite the lack of increased maximal exercise capacity and might have implications for how we judge procedural success[47].
Right and left ventricular function and calculation of great vessel blood flow analyzed by functional and morphologic MR imaging performed prior to and within one month after PPVI has shown mixed data regarding changes in RV ejection fraction following PPVI, with some studies finding no change[21,33] and others reporting on improvements in the acute or short term[23,43,44,46]. Importantly, PPVI also results in improved left ventricular filling[13,44,48].
Extended indications and future directions
Many patients are not ideal candidates for PPVI procedures due to their small physical size, limited vascular access or, most important, due to the size and shape of the RVOT[49]. The majority of patients suffering from dysfunction of the RVOT have enlargement of the patched RVOT as part of the initial surgical repair strategy[24]. This unmet clinical challenge led to the development of novel approaches to treat RVOT dysfunction using existing interventional pulmonic valve technology. A small case series reveals an approach to implant or post-dilate the MELODYTM valve using 24 mm balloons. This practice does not compromise function of the engrafted valve and may effectively broaden the pool of eligible patients[24,50].
However, RVOT dysfunction with predominant regurgitation and marked dilatation are not be eligible to this approach. Treatment strategies, e.g., MELODYTM valve implantation into the branch pulmonary arteries[51,52] or a “jailing” procedure of the pulmonary bifurcation by implanting a bare metal stent across the main pulmonary into a pulmonary branch have been described as potential options[53].
A hybrid approach combining intra-operative PPVI with simultaneous conduit down-sizing[54] or direct exposure of the RV or RVOT (e.g., after failed percutaneous attempt, “bailout” procedure) have also proven to be feasible[55].
Innovative (experimental) technologies, e.g., the self-expanding Medtronic Native Outflow Tract device[56], infundibular reducer devices[57] or newer low-profile pulmonary valves such as the Colibri Heart Valve (Colibri Heart Valve, LLC, CO, United States) indicate future treatment alternatives and hopefully will offer a non-surgical treatment to a much broader patient population[58].
PERCUTANEOUS TRICUSPID VALVE IMPLANTATION
Background and clinical indications
Primary tricuspid valve disease is rare: the underlying etiology can be of either congenital (Ebstein, tricuspid valve dysplasia) or of acquired nature (e.g., rheumatic, endocarditis or carcinoid disease). RV volume and/or pressure overload, left heart failure or mitral valve dysfunction can result in secondary RV enlargement, geometric distortion and tricuspid annular dilation. These circumstances can promote concomitant tricuspid regurgitation, thus called functional tricuspid regurgitation (80 percent of all cases)[59,60]. Patients with tricuspid regurgitation may be asymptomatic for prolonged periods. Surgical treatment is often reserved for advanced stages of tricuspid disease when dysfunction, particularly in patients with congestive heart failure, has led to symptomatic right heart failure[61]. For that reason, patients undergoing tricuspid repair or replacement procedures tend to be at higher risk with poorer outcome[61]. European and American guidelines on surgical management of valvular heart disease were updated in 2012[62] and confirmed in 2014[60]. The level of indication was raised to Class I and IIa for most situations of functional tricuspid regurgitation[62]. A transcatheter approach for tricuspid valve repair or replacement seems to be desirable and beneficial to his high-risk population but is still a long way ahead[2].
Patient selection criteria (in selected series)
Van Garsse et al[63] reported on the first “Percutaneous Transcatheter Valve-in-Valve Implantation in stenosed Tricuspid Valve Bioprosthesis” in 2011 amongst other case reports with small patient numbers[64-66].
A multicenter series by Roberts et al[67] enrolled 15 patients with failing tricuspid prostheses of whom ten underwent implantation into various failing bioprosthetic valves after careful (echocardiographic) confirmation of a suitable anchor point that allows safe positioning of the stented-valve. Median NYHA class was III and all patients were considered to be “high risk” for conventional surgery for tricuspid prothesis failure. The primary lesion was predominant stenosis (mean gradient > 5 mmHg, mean inflow gradient 12.9 mmHg), although a few had significant regurgitation.
Recently, Cullen et al[5] has reported a single-center series on transvenous Melody “Valve-in-Valve” implantation for bioprosthetic valve dysfunction that enrolled ten “high risk” patients with failing tricuspid prosthesis among others. Patients were considered candidates for the interventional procedure if they had significant bioprosthetic tricuspid valve dysfunction (either stenosis, regurgitation, or both) with co-morbid conditions which would preclude surgery. Median NYHA class was III with seven of the ten tricuspid patients suffering from moderate or worse tricuspid valve regurgitation with a mean inflow gradient 10 ± 4.3 mmHg.
After all, patient selection criteria for percutaneous tricuspid valve replacement are yet based on (very) limited data. Principally, if a percutaneous approach seems to be an option of treatment in clinical practice, the clinical indication for “Valve-in-Valve” implantation should be based on the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS) or the American College of Cardiology/American Heart Association guidelines for tricuspid valve surgery. The percutaneous approach should be reserved particularly for those cases considered to be high-risk cases for conventional surgery[68].
Devices for tricuspid valve implantation
Two percutaneous devices have been described for transcatheter valve implantation in failing bioprosthetic valves so far. These are the Edwards SAPIEN™ and its iterations and the Medtronic MELODY™ valve as described previously (Figure 6).
Figure 6.
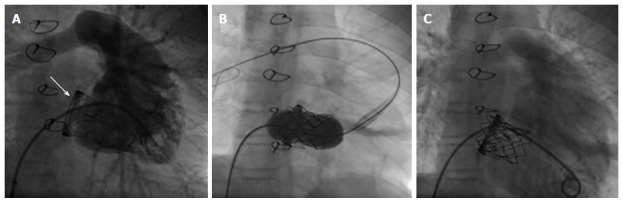
MELODY™ device for “Valve-in-Valve” implantation in tricuspid position. MELODY™ valve implantation procedure in a patient with severe stenosis of a prosthetic biological tricuspid valve (arrow): fluroscopy reveals (A) a RV angiogram in systoly prior to implantation (B) guidewire in the right PA and stable device position after inflating of both balloons of the BiB-delivery system and (C) RV angiogram showing stent position within the biological prosthesis and relative to the ventricle. There is no tricuspid regurgitation. (Courtesy of Wagner R). RV: Right ventricular; PA: Pulmonary artery.
So far, none of them have been approved or certified to be delivered in tricuspid position. Therefore, implantation of these devices in tricuspid position is off-label-use.
Results
In both series procedural success with device deployment was achieved in all of the tricuspid patients[5,67]. There were no early periprocedural complications in the Cullen series. Procedural complications occurred in one of Roberts’ patients (atrioventricular block requiring a pacemaker). Another patient suffered endocarditis eight weeks post-procedural. In both series mean tricuspid gradient decreased significantly (drop to 5.6 ± 2.5 mmHg in Cullen’s patients, drop to 3.9 mmHg in Roberts’ series). The level of regurgitation revealed to be mild or none in all but one case of the Cullen’s series.
Follow-up and outcome
Mean follow-up was nine months in the series by Roberts but with 41 d (range 11 to 209) shorter and more heterogeneous in Cullen’s patients. As reported by Roberts et al[67] functional class improved in 12 of the treated patients. Nine of the patients sustained the good interventional result nine months after implantation with one percutaneous valve-in-valve which had to be replaced. Cullen’s group observed a 30-d readmission rate with three out of ten in the tricuspid patients. And NYHA functional class improvement in nine of the ten treated patients.
Extended indications and future directions
Several groups selected similar patients to demonstrate the feasibility of percutaneous deployment of stent-mounted valves (SAPIEN, SAPIEN™ XT or MELODY™) into the venous system (inferior and/or superior vena cava). The focus is not on the tricuspid regurgitation itself, but rather on its hemodynamic disturbance. These procedures are therefore called “Caval-valve Implantation”[69-72].
Although, a number of animal studies examined the experimental feasibility of percutaneous valve implantation into a native tricuspid valve[2,73,74], Kefer et al[75] recently demonstrated feasibility of SAPIEN™ valve implantation into a “native” tricuspid annulus after failed repair without bioprothesis but mixed tricuspid disease.
SUMMARY AND PERSPECTIVE
The aim of PPVI is to prolong the lifespan of surgically placed conduits. The prolonged conduit lifespan, and hence delayed surgery, should limit the number of needed open chest redo-operations over the patients’ total lifespan in cases of congenital and acquired heart disease that indicated the implantation of pulmonic conduits. This sophisticated strategy potentially improves these patients’ life expectancy. Pulmonary valve replacement with stent-mounted with stent-mounted valves containing xenograft materials represents the derived valves represents the non-surgical treatment of choice in patients with dysfunction of the RVOT. Although indications continue to extend even to patients with “native”, but dysfunctional RV outflow tracts, the diameter of the proposed implantation site limits the feasibility in a relevant number of patients. Even though significant improvement has been achieved in early and late outcomes after PPVI, the risk of stent fractures and graft rupture have not yet been sufficiently explored. Further research is necessary to avoid these complications.
Besides, the extending use of PPVI, the percutaneous approach to tricuspid valve replacement has briefly moved beyond its experimental character. It has been shown to be feasible, but should mainly be reserved for high-risk patients with conditions that preclude surgery.
In conclusion, evolution of the interventional treatment of dysfunctional valves/RVOTs can only be achieved by continuous creative thinking and encouraged teamworking of cardiologists, surgeons, specialists in imaging and bio-medical engineers.
Footnotes
Conflict-of-interest: Wagner R reports no conflict of interest. Daehnert I is a proctor for Melody-PPVI (Medtronic). Lurz P is a consultant to Medtronic and has received fees for serving as a speaker.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 30, 2014
First decision: December 12, 2014
Article in press: February 12, 2015
P- Reviewer: Caceres-Loriga F, Kettering K, Kasai T, Lee TS S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Shimazaki Y, Blackstone EH, Kirklin JW. The natural history of isolated congenital pulmonary valve incompetence: surgical implications. Thorac Cardiovasc Surg. 1984;32:257–259. doi: 10.1055/s-2007-1023399. [DOI] [PubMed] [Google Scholar]
- 2.Coats L BP. In: Topol EJTP, editor. Textbook of Interventional Cardiology, 6th ed. Philadelphia: Elsevier; 2012. pp. 684–693. [Google Scholar]
- 3.Warnes CA. The adult with congenital heart disease: born to be bad? J Am Coll Cardiol. 2005;46:1–8. doi: 10.1016/j.jacc.2005.02.083. [DOI] [PubMed] [Google Scholar]
- 4.Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, Acar P, Le Bidois J, Sidi D, Kachaner J. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405. doi: 10.1016/S0140-6736(00)02844-0. [DOI] [PubMed] [Google Scholar]
- 5.Cullen MW, Cabalka AK, Alli OO, Pislaru SV, Sorajja P, Nkomo VT, Malouf JF, Cetta F, Hagler DJ, Rihal CS. Transvenous, antegrade Melody valve-in-valve implantation for bioprosthetic mitral and tricuspid valve dysfunction: a case series in children and adults. JACC Cardiovasc Interv. 2013;6:598–605. doi: 10.1016/j.jcin.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Tweddell JS, Hoffman GM, Fedderly RT, Ghanayem NS, Kampine JM, Berger S, Mussatto KA, Litwin SB. Patients at risk for low systemic oxygen delivery after the Norwood procedure. Ann Thorac Surg. 2000;69:1893–1899. doi: 10.1016/s0003-4975(00)01349-7. [DOI] [PubMed] [Google Scholar]
- 7.Powell AJ, Lock JE, Keane JF, Perry SB. Prolongation of RV-PA conduit life span by percutaneous stent implantation. Intermediate-term results. Circulation. 1995;92:3282–3288. doi: 10.1161/01.cir.92.11.3282. [DOI] [PubMed] [Google Scholar]
- 8.Oosterhof T, Meijboom FJ, Vliegen HW, Hazekamp MG, Zwinderman AH, Bouma BJ, van Dijk AP, Mulder BJ. Long-term follow-up of homograft function after pulmonary valve replacement in patients with tetralogy of Fallot. Eur Heart J. 2006;27:1478–1484. doi: 10.1093/eurheartj/ehl033. [DOI] [PubMed] [Google Scholar]
- 9.Corno AF. Valved Conduits Right Ventricle to Pulmonary Artery for Complex Congenital Heart Defects, Current Concepts in General Thoracic Surgery. Dr. Lucio Cagini (Ed.) InTech. Philadelphia: Elsevier; 2012. Available from: http:///cdn.intechopen.com/pdfs-wm/41324.pdf. [Google Scholar]
- 10.Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C, Rosenthal M, Nakazawa M, Moller JH, Gillette PC, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet. 2000;356:975–981. doi: 10.1016/S0140-6736(00)02714-8. [DOI] [PubMed] [Google Scholar]
- 11.Frigiola A, Redington AN, Cullen S, Vogel M. Pulmonary regurgitation is an important determinant of right ventricular contractile dysfunction in patients with surgically repaired tetralogy of Fallot. Circulation. 2004;110:II153–II157. doi: 10.1161/01.CIR.0000138397.60956.c2. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho JS, Shinebourne EA, Busst C, Rigby ML, Redington AN. Exercise capacity after complete repair of tetralogy of Fallot: deleterious effects of residual pulmonary regurgitation. Br Heart J. 1992;67:470–473. doi: 10.1136/hrt.67.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lurz P, Bonhoeffer P, Taylor AM. Percutaneous pulmonary valve implantation: an update. Expert Rev Cardiovasc Ther. 2009;7:823–833. doi: 10.1586/erc.09.57. [DOI] [PubMed] [Google Scholar]
- 14.Lurz P, Gaudin R, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2009:112–117. doi: 10.1053/j.pcsu.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Oosterhof T, van Straten A, Vliegen HW, Meijboom FJ, van Dijk AP, Spijkerboer AM, Bouma BJ, Zwinderman AH, Hazekamp MG, de Roos A, et al. Preoperative thresholds for pulmonary valve replacement in patients with corrected tetralogy of Fallot using cardiovascular magnetic resonance. Circulation. 2007;116:545–551. doi: 10.1161/CIRCULATIONAHA.106.659664. [DOI] [PubMed] [Google Scholar]
- 16.Buechel ER, Dave HH, Kellenberger CJ, Dodge-Khatami A, Pretre R, Berger F, Bauersfeld U. Remodelling of the right ventricle after early pulmonary valve replacement in children with repaired tetralogy of Fallot: assessment by cardiovascular magnetic resonance. Eur Heart J. 2005;26:2721–2727. doi: 10.1093/eurheartj/ehi581. [DOI] [PubMed] [Google Scholar]
- 17.Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95:779–782. doi: 10.1016/j.amjcard.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 18.Gillespie MJ, Rome JJ, Levi DS, Williams RJ, Rhodes JF, Cheatham JP, Hellenbrand WE, Jones TK, Vincent JA, Zahn EM, et al. Melody valve implant within failed bioprosthetic valves in the pulmonary position: a multicenter experience. Circ Cardiovasc Interv. 2012;5:862–870. doi: 10.1161/CIRCINTERVENTIONS.112.972216. [DOI] [PubMed] [Google Scholar]
- 19.Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, Muthurangu V, Lee TY, Parenzan G, Derrick G, et al. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964–1972. doi: 10.1161/CIRCULATIONAHA.107.735779. [DOI] [PubMed] [Google Scholar]
- 20.Lurz P, Giardini A, Taylor AM, Nordmeyer J, Muthurangu V, Odendaal D, Mist B, Khambadkone S, Schievano S, Bonhoeffer P, et al. Effect of altering pathologic right ventricular loading conditions by percutaneous pulmonary valve implantation on exercise capacity. Am J Cardiol. 2010;105:721–726. doi: 10.1016/j.amjcard.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 21.Eicken A, Ewert P, Hager A, Peters B, Fratz S, Kuehne T, Busch R, Hess J, Berger F. Percutaneous pulmonary valve implantation: two-centre experience with more than 100 patients. Eur Heart J. 2011;32:1260–1265. doi: 10.1093/eurheartj/ehq520. [DOI] [PubMed] [Google Scholar]
- 22.Boudjemline Y, Brugada G, Van-Aerschot I, Patel M, Basquin A, Bonnet C, Legendre A, Bonnet D, Iserin L. Outcomes and safety of transcatheter pulmonary valve replacement in patients with large patched right ventricular outflow tracts. Arch Cardiovasc Dis. 2012;105:404–413. doi: 10.1016/j.acvd.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Demkow M, Biernacka EK, Spiewak M, Kowalski M, Siudalska H, Wolski P, Sondergaard L, Miśko J, Hoffman P, Rużyłło W. Percutaneous pulmonary valve implantation preceded by routine prestenting with a bare metal stent. Catheter Cardiovasc Interv. 2011;77:381–389. doi: 10.1002/ccd.22700. [DOI] [PubMed] [Google Scholar]
- 24.Gillespie MJ, McElhinney DB. Transcatheter Pulmonary Valve Replacement: A Current Review. Current Pediatrics Reports. 2013;1:83–91. [Google Scholar]
- 25.Butera G, Milanesi O, Spadoni I, Piazza L, Donti A, Ricci C, Agnoletti G, Pangrazi A, Chessa M, Carminati M. Melody transcatheter pulmonary valve implantation. Results from the registry of the Italian Society of Pediatric Cardiology. Catheter Cardiovasc Interv. 2013;81:310–316. doi: 10.1002/ccd.24518. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Heart J. 2010;31:2915–2957. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 27.Feltes TF, Bacha E, Beekman RH, Cheatham JP, Feinstein JA, Gomes AS, Hijazi ZM, Ing FF, de Moor M, Morrow WR, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2607–2652. doi: 10.1161/CIR.0b013e31821b1f10. [DOI] [PubMed] [Google Scholar]
- 28.Boone RH, Webb JG, Horlick E, Benson L, Cao QL, Nadeem N, Kiess M, Hijazi ZM. Transcatheter pulmonary valve implantation using the Edwards SAPIEN transcatheter heart valve. Catheter Cardiovasc Interv. 2010;75:286–294. doi: 10.1002/ccd.22250. [DOI] [PubMed] [Google Scholar]
- 29.Garay F, Webb J, Hijazi ZM. Percutaneous replacement of pulmonary valve using the Edwards-Cribier percutaneous heart valve: first report in a human patient. Catheter Cardiovasc Interv. 2006;67:659–662. doi: 10.1002/ccd.20753. [DOI] [PubMed] [Google Scholar]
- 30.Kenny D, Hijazi ZM, Kar S, Rhodes J, Mullen M, Makkar R, Shirali G, Fogel M, Fahey J, Heitschmidt MG, et al. Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. J Am Coll Cardiol. 2011;58:2248–2256. doi: 10.1016/j.jacc.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 31.Peng LF, McElhinney DB, Nugent AW, Powell AJ, Marshall AC, Bacha EA, Lock JE. Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a 15-year experience. Circulation. 2006;113:2598–2605. doi: 10.1161/CIRCULATIONAHA.105.607127. [DOI] [PubMed] [Google Scholar]
- 32.Sridharan S, Coats L, Khambadkone S, Taylor AM, Bonhoeffer P. Images in cardiovascular medicine. Transcatheter right ventricular outflow tract intervention: the risk to the coronary circulation. Circulation. 2006;113:e934–e935. doi: 10.1161/CIRCULATIONAHA.105.599514. [DOI] [PubMed] [Google Scholar]
- 33.McElhinney DB, Hellenbrand WE, Zahn EM, Jones TK, Cheatham JP, Lock JE, Vincent JA. Short- and medium-term outcomes after transcatheter pulmonary valve placement in the expanded multicenter US melody valve trial. Circulation. 2010;122:507–516. doi: 10.1161/CIRCULATIONAHA.109.921692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nordmeyer J, Ewert P, Gewillig M, Carminati M, Uebing A, Benson L, Schranz D, Daehnert I, Aljufan M, Kretschmar O. Current results of the melody registry: an international multicenter registry of transcatheter pulmonary valve implantation. JACC. 2014;63:Abstract A480. [Google Scholar]
- 35.Biermann D, Schönebeck J, Rebel M, Weil J, Dodge-Khatami A. Left coronary artery occlusion after percutaneous pulmonary valve implantation. Ann Thorac Surg. 2012;94:e7–e9. doi: 10.1016/j.athoracsur.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 36.Mauri L, Frigiola A, Butera G. Emergency surgery for extrinsic coronary compression after percutaneous pulmonary valve implantation. Cardiol Young. 2013;23:463–465. doi: 10.1017/S1047951112001187. [DOI] [PubMed] [Google Scholar]
- 37.Buber J, Bergersen L, Lock JE, Gauvreau K, Esch JJ, Landzberg MJ, Valente AM, Sandora TJ, Marshall AC. Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: a single-center experience. Circ Cardiovasc Interv. 2013;6:301–310. doi: 10.1161/CIRCINTERVENTIONS.112.000348. [DOI] [PubMed] [Google Scholar]
- 38.McElhinney DB, Benson LN, Eicken A, Kreutzer J, Padera RF, Zahn EM. Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: combined results of 3 prospective North American and European studies. Circ Cardiovasc Interv. 2013;6:292–300. doi: 10.1161/CIRCINTERVENTIONS.112.000087. [DOI] [PubMed] [Google Scholar]
- 39.Cheung G, Vejlstrup N, Ihlemann N, Arnous S, Franzen O, Bundgaard H, Søndergaard L. Infective endocarditis following percutaneous pulmonary valve replacement: diagnostic challenges and application of intra-cardiac echocardiography. Int J Cardiol. 2013;169:425–429. doi: 10.1016/j.ijcard.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Villafañe J, Baker GH, Austin EH, Miller S, Peng L, Beekman R. Melody pulmonary valve bacterial endocarditis: experience in four pediatric patients and a review of the literature. Catheter Cardiovasc Interv. 2014;84:212–218. doi: 10.1002/ccd.25375. [DOI] [PubMed] [Google Scholar]
- 41.Haas NA, Moysich A, Neudorf U, Mortezaeian H, Abdel-Wahab M, Schneider H, De Wolf D, Petit J, Narayanswami S, Laser KT, et al. Percutaneous implantation of the Edwards SAPIEN(™) pulmonic valve: initial results in the first 22 patients. Clin Res Cardiol. 2013;102:119–128. doi: 10.1007/s00392-012-0503-8. [DOI] [PubMed] [Google Scholar]
- 42.Demkow M, Rużyłło W, Biernacka EK, Kalińczuk Ł, Spiewak M, Kowalski M, Sitkowska E, Kuśmierczyk M, Różanski J, Banaś S, et al. Percutaneous Edwards SAPIEN(™) valve implantation for significant pulmonary regurgitation after previous surgical repair with a right ventricular outflow patch. Catheter Cardiovasc Interv. 2014;83:474–481. doi: 10.1002/ccd.25096. [DOI] [PubMed] [Google Scholar]
- 43.Coats L, Khambadkone S, Derrick G, Sridharan S, Schievano S, Mist B, Jones R, Deanfield JE, Pellerin D, Bonhoeffer P, et al. Physiological and clinical consequences of relief of right ventricular outflow tract obstruction late after repair of congenital heart defects. Circulation. 2006;113:2037–2044. doi: 10.1161/CIRCULATIONAHA.105.591438. [DOI] [PubMed] [Google Scholar]
- 44.Coats L, Khambadkone S, Derrick G, Hughes M, Jones R, Mist B, Pellerin D, Marek J, Deanfield JE, Bonhoeffer P, et al. Physiological consequences of percutaneous pulmonary valve implantation: the different behaviour of volume- and pressure-overloaded ventricles. Eur Heart J. 2007;28:1886–1893. doi: 10.1093/eurheartj/ehm181. [DOI] [PubMed] [Google Scholar]
- 45.Lurz P, Nordmeyer J, Giardini A, Khambadkone S, Muthurangu V, Schievano S, Thambo JB, Walker F, Cullen S, Derrick G, et al. Early versus late functional outcome after successful percutaneous pulmonary valve implantation: are the acute effects of altered right ventricular loading all we can expect? J Am Coll Cardiol. 2011;57:724–731. doi: 10.1016/j.jacc.2010.07.056. [DOI] [PubMed] [Google Scholar]
- 46.Lurz P, Muthurangu V, Schuler PK, Giardini A, Schievano S, Nordmeyer J, Khambadkone S, Cappeli C, Derrick G, Bonhoeffer P, et al. Impact of reduction in right ventricular pressure and/or volume overload by percutaneous pulmonary valve implantation on biventricular response to exercise: an exercise stress real-time CMR study. Eur Heart J. 2012;33:2434–2441. doi: 10.1093/eurheartj/ehs200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lurz P, Riede FT, Taylor AM, Wagner R, Nordmeyer J, Khambadkone S, Kinzel P, Derrick G, Schuler G, Bonhoeffer P, et al. Impact of percutaneous pulmonary valve implantation for right ventricular outflow tract dysfunction on exercise recovery kinetics. Int J Cardiol. 2014;177:276–280. doi: 10.1016/j.ijcard.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 48.Lurz P, Nordmeyer J, Coats L, Taylor AM, Bonhoeffer P, Schulze-Neick I. Immediate clinical and haemodynamic benefits of restoration of pulmonary valvar competence in patients with pulmonary hypertension. Heart. 2009;95:646–650. doi: 10.1136/hrt.2008.153379. [DOI] [PubMed] [Google Scholar]
- 49.Kreutzer J. Percutaneous pulmonary valve implantation. Revista Argentina de Cardioangiología Intervencionista. 2013;4:84–91. [Google Scholar]
- 50.Cheatham SL, Holzer RJ, Chisolm JL, Cheatham JP. The Medtronic Melody® transcatheter pulmonary valve implanted at 24-mm diameter--it works. Catheter Cardiovasc Interv. 2013;82:816–823. doi: 10.1002/ccd.24821. [DOI] [PubMed] [Google Scholar]
- 51.Robb JD, Harris MA, Minakawa M, Rodriguez E, Koomalsingh KJ, Shuto T, Shin DC, Dori Y, Glatz AC, Rome JJ, et al. Melody valve implantation into the branch pulmonary arteries for treatment of pulmonary insufficiency in an ovine model of right ventricular outflow tract dysfunction following tetralogy of Fallot repair. Circ Cardiovasc Interv. 2011;4:80–87. doi: 10.1161/CIRCINTERVENTIONS.110.959502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gillespie MJ, Dori Y, Harris MA, Sathanandam S, Glatz AC, Rome JJ. Bilateral branch pulmonary artery melody valve implantation for treatment of complex right ventricular outflow tract dysfunction in a high-risk patient. Circ Cardiovasc Interv. 2011;4:e21–e23. doi: 10.1161/CIRCINTERVENTIONS.111.962373. [DOI] [PubMed] [Google Scholar]
- 53.Boudjemline Y, Legendre A, Ladouceur M, Boughenou MF, Patel M, Bonnet D, Iserin L. Branch pulmonary artery jailing with a bare metal stent to anchor a transcatheter pulmonary valve in patients with patched large right ventricular outflow tract. Circ Cardiovasc Interv. 2012;5:e22–e25. doi: 10.1161/CIRCINTERVENTIONS.112.968610. [DOI] [PubMed] [Google Scholar]
- 54.Bacha EA, Marshall AC, McElhinney DB, del Nido PJ. Expanding the hybrid concept in congenital heart surgery. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2007:146–150. doi: 10.1053/j.pcsu.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 55.Cubeddu RJ, Hijazi ZM. Bailout perventricular pulmonary valve implantation following failed percutaneous attempt using the Edwards Sapien transcatheter heart valve. Catheter Cardiovasc Interv. 2011;77:276–280. doi: 10.1002/ccd.22653. [DOI] [PubMed] [Google Scholar]
- 56.Schievano S, Taylor AM, Capelli C, Coats L, Walker F, Lurz P, Nordmeyer J, Wright S, Khambadkone S, Tsang V, et al. First-in-man implantation of a novel percutaneous valve: a new approach to medical device development. EuroIntervention. 2010;5:745–750. doi: 10.4244/eijv5i6a122. [DOI] [PubMed] [Google Scholar]
- 57.Boudjemline Y, Agnoletti G, Bonnet D, Sidi D, Bonhoeffer P. Percutaneous pulmonary valve replacement in a large right ventricular outflow tract: an experimental study. J Am Coll Cardiol. 2004;43:1082–1087. doi: 10.1016/j.jacc.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 58.Kenny D, Hijazi ZM. The evolution of transcatheter pulmonary valve replacement. Expert Rev Cardiovasc Ther. 2013;11:795–797. doi: 10.1586/14779072.2013.811970. [DOI] [PubMed] [Google Scholar]
- 59.Rogers JH, Bolling SF. Surgical approach to functional tricuspid regurgitation: should we be more aggressive? Curr Opin Cardiol. 2014;29:133–139. doi: 10.1097/HCO.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 60.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 61.Kilic A, Saha-Chaudhuri P, Rankin JS, Conte JV. Trends and outcomes of tricuspid valve surgery in North America: an analysis of more than 50,000 patients from the Society of Thoracic Surgeons database. Ann Thorac Surg. 2013;96:1546–1552; discussion 1552. doi: 10.1016/j.athoracsur.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 62.Vahanian A, Iung B. The new ESC/EACTS guidelines on the management of valvular heart disease. Arch Cardiovasc Dis. 2012;105:465–467. doi: 10.1016/j.acvd.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Van Garsse LA, Ter Bekke RM, van Ommen VG. Percutaneous transcatheter valve-in-valve implantation in stenosed tricuspid valve bioprosthesis. Circulation. 2011;123:e219–e221. doi: 10.1161/CIRCULATIONAHA.110.972836. [DOI] [PubMed] [Google Scholar]
- 64.Tanous D, Nadeem SN, Mason X, Colman JM, Benson LN, Horlick EM. Creation of a functional tricuspid valve: novel use of percutaneously implanted valve in right atrial to right ventricular conduit in a patient with tricuspid atresia. Int J Cardiol. 2010;144:e8–10. doi: 10.1016/j.ijcard.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 65.Riede FT, Dähnert I. Implantation of a Melody valve in tricuspid position. Catheter Cardiovasc Interv. 2012;80:474–476. doi: 10.1002/ccd.23404. [DOI] [PubMed] [Google Scholar]
- 66.Petit CJ, Justino H, Ing FF. Melody valve implantation in the pulmonary and tricuspid position. Catheter Cardiovasc Interv. 2013;82:E944–E946. doi: 10.1002/ccd.24764. [DOI] [PubMed] [Google Scholar]
- 67.Roberts PA, Boudjemline Y, Cheatham JP, Eicken A, Ewert P, McElhinney DB, Hill SL, Berger F, Khan D, Schranz D, et al. Percutaneous tricuspid valve replacement in congenital and acquired heart disease. J Am Coll Cardiol. 2011;58:117–122. doi: 10.1016/j.jacc.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 68.Milburn K, Bapat V, Thomas M. Valve-in-valve implantations: is this the new standard for degenerated bioprostheses? Review of the literature. Clin Res Cardiol. 2014;103:417–429. doi: 10.1007/s00392-013-0653-3. [DOI] [PubMed] [Google Scholar]
- 69.Laule M, Stangl V, Sanad W, Lembcke A, Baumann G, Stangl K. Percutaneous transfemoral management of severe secondary tricuspid regurgitation with Edwards Sapien XT bioprosthesis: first-in-man experience. J Am Coll Cardiol. 2013;61:1929–1931. doi: 10.1016/j.jacc.2013.01.070. [DOI] [PubMed] [Google Scholar]
- 70.Lauten A, Hamadanchi A, Doenst T, Figulla HR. Caval valve implantation for treatment of tricuspid regurgitation: post-mortem evaluation after mid-term follow-up. Eur Heart J. 2014;35:1651. doi: 10.1093/eurheartj/eht471. [DOI] [PubMed] [Google Scholar]
- 71.Lauten A, Laube A, Schubert H, Bischoff S, Nietzsche S, Horstkötter K, Poudel-Bochmann B, Franz M, Lichtenberg A, Figulla HR, et al. Transcatheter treatment of tricuspid regurgitation by caval valve implantation--experimental evaluation of decellularized tissue valves in central venous position. Catheter Cardiovasc Interv. 2015;85:150–160. doi: 10.1002/ccd.25380. [DOI] [PubMed] [Google Scholar]
- 72.Lauten A, Doenst T, Hamadanchi A, Franz M, Figulla HR. Percutaneous bicaval valve implantation for transcatheter treatment of tricuspid regurgitation: clinical observations and 12-month follow-up. Circ Cardiovasc Interv. 2014;7:268–272. doi: 10.1161/CIRCINTERVENTIONS.113.001033. [DOI] [PubMed] [Google Scholar]
- 73.Boudjemline Y, Agnoletti G, Bonnet D, Behr L, Borenstein N, Sidi D, Bonhoeffer P. Steps toward the percutaneous replacement of atrioventricular valves an experimental study. J Am Coll Cardiol. 2005;46:360–365. doi: 10.1016/j.jacc.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 74.Bai Y, Zong GJ, Wang HR, Jiang HB, Wang H, Wu H, Zhao XX, Qin YW. An integrated pericardial valved stent special for percutaneous tricuspid implantation: an animal feasibility study. J Surg Res. 2010;160:215–221. doi: 10.1016/j.jss.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 75.Kefer J, Sluysmans T, Vanoverschelde JL. Transcatheter Sapien valve implantation in a native tricuspid valve after failed surgical repair. Catheter Cardiovasc Interv. 2014;83:841–845. doi: 10.1002/ccd.25330. [DOI] [PubMed] [Google Scholar]


