Abstract
Pancreatic cancer (PC) has been one of the deadliest of all cancers, with almost uniform lethality despite aggressive treatment. Recently, there have been important advances in the molecular, pathological and biological understanding of pancreatic cancer. Even after the emergence of recent new targeted agents and the use of multiple therapeutic combinations, no treatment option is viable in patients with advanced cancer. Developing novel strategies to target progression of PC is of intense interest. A small population of pancreatic cancer stem cells (CSCs) has been found to be resistant to chemotherapy and radiation therapy. CSCs are believed to be responsible for tumor initiation, progression and metastasis. The CSC research has recently achieved much progress in a variety of solid tumors, including pancreatic cancer to some extent. This leads to focus on understanding the role of pancreatic CSCs. The focus on CSCs may offer new targets for prevention and treatment of this deadly cancer. We review the most salient developments in important areas of pancreatic CSCs. Here, we provide a review of current updates and new insights on the role of CSCs in pancreatic tumor progression with special emphasis on DclK1 and Lgr5, signaling pathways altered by CSCs, and the role of CSCs in prevention and treatment of PC.
Keywords: Pancreatic cancer, Cancer stem cells, DclK1, Lgr5, Prevention, Treatment
Core tip: Despite aggressive treatment modalities, pancreatic cancer represents most lethal malignancy with uniform lethality. The pancreatic cancer stem cells (CSCs) have been found to be resistant to chemotherapy and radiation therapy. This review summarizes the important role of CSCs in pancreatic cancer tumor progression with emphasis on DclK1 and Lgr5 CSCs, molecular signaling altered by CSCs and the important role of pancreatic CSCs in prevention and treatment of pancreatic cancer.
INTRODUCTION
Pancreatic cancer (PC) is a deadly disease with least survival compared to any other cancers. PC including pancreatic ductal adenocarcinoma (PDAC) is one of the leading causes of cancer deaths in both genders (men and women) in the United States and the seventh world-wide[1,2]. Despite tremendous scientific effort for over three decades, PC remains almost uniformly lethal devastating disease with < 6% five-year survival. The incidence of PC has been increasing over the past ten years. In 2013, of 46420 people (23530 men and 22890 women) diagnosed with PC, 39590 people (20170 men and 19420 women) were expected to die in the United States[1]. Worldwide, 338000 people were diagnosed with PC in 2012[2]. Lack of early diagnosis and effective interventions are the major factors in the poor prognosis and dismal survival rates[3-5].
Recently, stem cells of pancreatic cancer (CSCs)/ or cancer/tumor initiating cells that often are resistant to treatment have been identified[6-9]. The CSCs occupying a very small part of the entire cancer tissue are the reasons for tumor resistance against conventional chemotherapy and re-growth of untreatable tumors. Although the CSCs exists a small population in the cancer tissues, very recent evidence shows that CSCs contribute to tumor initiation, growth, metastasis, and resistance to therapy[6-10]. Cancer stem cell (CSC) research has achieved much progress in a variety of solid tumors, including pancreatic cancer to some extent[11]. The identification of specific cell surface markers enabled purification of CSCs from different cell lines or clinical tissues/samples, leading to evaluate the nature of CSCs[12-14]. Understanding of pancreatic CSCs may improve existing therapies and deepen insight into the role of CSCs in pancreatic cancer progression[11].
In this mini-review, we will comprehensively focus on pancreatic CSCs with emphasis on DclK1 and Lgr5. Also, we review the signaling pathways altered by CSCs, and the role of CSCs in prevention and treatment of PC (Figure 1).
Figure 1.
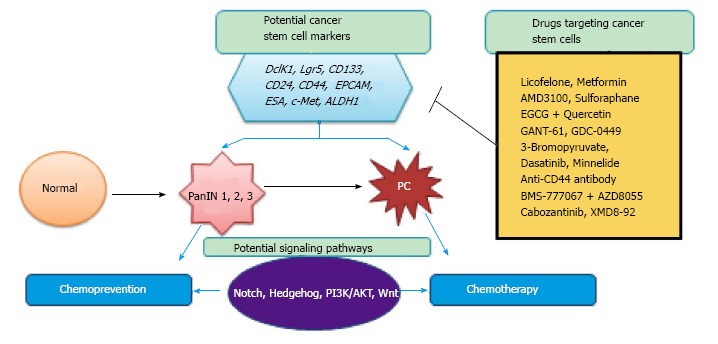
Schematic representation of the role of cancer stem cells markers during pancreatic cancer development, signaling pathways involved and cancer stem cells inhibition by various drugs by chemoprevention and/or chemotherapy. PC: Pancreatic cancer.
ROLE OF CSC IN PC
Little has changed over last three decades for 5-year pancreatic cancer survival despite introduction of new and targeted chemotherapeutic agents[15]. It is primarily due to highly aggressive nature of the PC disease as majority of the patients’ present late stage disease and doesn’t respond to therapy thereby showing chemoresistance[15]. Recently, attention has been focused on pancreatic CSC levels to find better ways to combat PC. Cell membrane markers have made possible the identification of CSCs. Pancreatic CSC populations account for less than 1% of all pancreatic cancer cells[14]. The pancreatic CSCs identified so far are CD133, CD24, CD44, EPCAM, ESA, c-Met, Aldh1, and more recently DclK1 and Lgr5[9-18]. These pancreatic CSC markers are well known based on several xenograft studies. All these markers may mark themselves or co-express to show the CSC properties[19-21]. CD133 pancreatic CSC cells co-express the CXCR4 receptor at the invading margins of human pancreatic ductal tumors[14,19]. Other CSC markers that co-express (CD24+/CD44+/EPCAM and CD133+) show CSC properties making them often resistant to chemo and radiotherapy. However, complete overlap may not be found between these different CSC markers[22].
Most drugs are unable to eradicate these pancreatic CSCs, and this is postulated to be one of the primary reasons for failure of chemotherapy that will lead to tumor relapse and metastasis. The pancreatic CSCs markers CD24, CD44, CD133, CXCR4, ESA and nestin were reported in pancreatic intraepithelial neoplasia and PDAC[23]. Pancreatic CSC populations expressing the cell surface markers CD24, CD44, and ESA[14,19,24] were observed to maintain their cell surface marker phenotype after repeated passages in immunocompromised xenografts mice[12,14,19]. In another study, c-Met positive cells readily formed in vitro spheres/but not the c-Met negative cells[14,25]. Similarly, it was noted that cells expressing combinations of CD44 and c-Met showed increased tumor formation ability and to renew[25]. Kim et al[18] reported that irrespective of CD133 status, the pancreatic cancer cells with high ALDH1 activity were resistant to chemotherapy-induced cell death and are highly tumorigenic[14,18]. The MiaPaCa-2 sphere derived CSC like cells obtained from the xenograft tumors of the mice showed high expression of EpCAM, CD44 and EZH2[14,26]. All of these CSCs have been reviewed elsewhere for pancreatic cancer. However, there are recent studies showing the CSC properties for two important makers DclK1 and Lgr5 in pancreatic cancer.
DclK1 and pancreatic cancer
Dclk1 (Doublecortin-like kinase 1, formerly known as DCAMKL-1 or doublecortin and CaM kinase-like 1) initially was identified as a putative intestinal stem cell marker. Earlier studies have suggested that Dclk1 may mark tumor-initiating cells in a variety of tumor types[27-39]. DclK1 regulates several key oncogenes like c-MYC, KRAS, NOTCH1 and EMT[32,33,39]. Moreover, DclK1 is also reported to be a pancreatic cancer stem cell marker and its expression is found to be upregulated in PDAC[9,39]. In the pancreas and intestines, Dclk1 has also recently been described as an undefined tuft/brush cell marker[36,37]. We have empirically shown DclK1 expression in both murine and human pancreatic intraepithelial neoplasia (PanIN) and PDAC[9]. DCLK1 is also expressed by isolated cells in the pancreatic duct and islets of normal mouse pancreas, and prior studies have suggested that these non-neoplastic Dclk1-expressing pancreatic cells were associated with progenitor-like function[31]. Further, it was shown that DCLK1HI/AcTubHI mouse PanIN cells display specialized morphology, unique patterns of gene expression, and enhanced PanIN sphere-forming ability[6]. The lineage tracing techniques using alternate DNA recombinases (e.g., Dre, Flp) or the development of non-Cre/lox-based methods for Kras activation in the murine pancreas will further the progress in this area. Multiple pathways that contribute to the CSC properties of DCLK1HI/AcTubHI pancreatic cancer cells were identified by using whole genome transcriptome analysis[6]. Studies identified ABL1 and IGF1R to be highly expressed in DCLK1HI/AcTubHI cells, complementing ongoing preclinical and clinical evaluation of ABL1 and IGF1R pathway inhibition as new forms of targeted therapy for pancreatic cancer[38,39]. These studies suggest that both invasive and preinvasive PC may depend on DCLK1 expressing cells with cancer stem cell capabilities. As in the case of intestinal tumorigenesis, targeting this cell population may have therapeutic potential in the treatment and/or chemoprevention of pancreatic cancer. Further, NPsiDCLK1 (nanoparticle-encapsulated siRNA) knockdown of Dclk1 in the mouse xenograft lead to downregulation of important markers of pluripotency like SOX2, NANOG, OCT4 and KLF4 and EMT, and angiogenic factors[40]. These data clearly supports a central regulatory role of Dclk1 in pancreatic tumorigenesis[39]. However, further studies are underway to evaluate the potential of Dclk1 as pancreatic CSC and specifically target DclK1 along or in combination with other CSC markers.
Lgr5 and pancreatic cancer
Lgr5 also known as G-protein coupled receptor 49 (GPR49) or G-protein coupled receptor 67 (GPR67) or Leucine-rich repeat-containing G-protein coupled receptor 5 (LGR5) is the Wnt target gene that marks Wnt driven actively dividing stem cells[41-43]. In the stem cell hierarchy, LGR5 is on a higher level than CD133, however, its expression and function in the tissues of pancreatic cancer is unclear. Recent studies investigated tissue expression of LGR5 and CD133 in resected pancreatic ductal adenocarcinoma who underwent resection and demonstrated cytoplasmic expression of LGR5 in the PC cells[17].
The maturation lineages of proximal peribiliary glands to the distal pancreatic ductal glands start near duodenum where cells express pluripotency markers (SOX2, OCT4 and NANOG), self-replication, proliferation and early hepato-pancreas commitment (Lgr5, PDX1, SOX17, SOX9)[44]. A biological framework for life-long pancreatic organogenesis is constituted by the biliary tree-derived stem cells and their connections to committed progenitors of the pancreas[40]. In the normal pancreas, LGR5 is co-localized with insulin and Nanog in the beta cells clusters and is exclusively expressed in the islets of the Langerhans[45].
Since, Lgr5 is a stem cells marker in various organs and acts as Wnt-agonistic R-spondins receptor, the Lgr5 positive stem cells from intestine, liver and stomach form organoids in three dimensional cultures that resemble the tissues of origin[42,46,47]. In a normal adult pancreas, Lgr5 is not expressed when Wnt signaling is inactive under normal conditions[48]. However, upon the injury, Wnt pathway is activated by partial duct ligation, and Lgr5 is concomitantly expressed in the regenerating ducts[49]. It was observed that pancreatic duct or fragments in the RSPO-1 cultures initiate Lgr5 expression and develop into organoids that can clonally expand containing Lgr5 stem/progenitor cells[49]. The histoanatomical distribution, prevalence, and tumor biological significance of LGR5 in gastrointestinal tract tumors (including pancreatic cancer) was tested on transcriptional and translational level in tissues of 127 patients (malignant and non-malignant). It was noted that the non-neoplastic tissue usually had very few scattered LGR5 positive cells. The corresponding malignant pancreatic cancers showed significantly more LGR5 positive cells at protein and mRNA levels compared with the non-neoplastic tissue[50]. Lgr5 co-expressed with other CSC markers Mushashi-1, CD44 and ADAM17 and the patients with high Lgr5 positive cells had shorter median survival[50]. Future studies are required to understand the role and clarify the prognostic value of Lgr5 in the pancreatic cancer.
SIGNALING PATHWAYS ALTERED BY CSCS
Pancreatic cancer cells and pancreatic CSCs share many characteristics of the carcinogenesis process including self-renewal, proliferation and immortality and several signaling pathways. Researchers have identified several CSC specific markers. In a recent study, investigators detected high expression of CD24, CD44, Dclk1, CXCR4, ESA, and Nestin-positive cells in the low-grade PanIN, high-grade PanIN, and PDAC (pancreatic ductal adenocarcinoma) tumors with fewer in normal ducts[9,12,13,51,52]. These studies clearly demonstrate that pancreatic CSCs correlate with the step-wise progression of pancreatic intraepithelial neoplasias (PanINs) (PanIN1-2-3) to PC (Figure 1). During this process of early tumorigenesis after Kras mutations, several of the transcription factors like Oct4 and Nanog gets overexpressed linearly with the PanIN progression to PDAC[53]. Oncogenic Kras mutations in the pancreas activate important signaling pathways such as sonic hedgehog, Wnt, PI3K/AKT, Notch and PTEN, Bmi1 that lead the transformation of the normal pancreas to the malignant PanINs and PDAC[54-59].
Researchers have observed aberrant expression of Hedgehog during the PC initiation followed by progression of the pancreatic precursor PanIN lesions to pancreatic adenocarcinoma[60]. This clearly underscores the importance of hedgehog signaling in a sequential development of PC. Recently, mounting evidence supports hedgehog signaling activation in pancreatic CSCs. Hedgehog pathway plays a significant role by regulating pluripotency maintaining factors like Oct4, Nanog, c-Myc, and Sox2 to the maintenance of stem-ness[61-66]. Apart from hedgehog, increasing evidence suggests that Notch signaling activation was associated with molecular characteristics of CSCs in PC cases[67,68]. This was shown by the studies that overexpression of Notch1 leads to increasing in the formation of pancreatospheres along with expression of the CD44 and EpCAM CSC surface markers. Other studies reported that expression of Oct4, Nanog, and PDX1 in Notch2 positive human pancreatic adenocarcinoma cells[69]. The Oct4, Nanog and PDX1 are also considered as markers of self-renewal of pancreatic CSCs. Also, the expression of ALDH1 was associated with poor overall survival durations in PDAC patients[70].
Newer studies illustrated involvement of PI3K/AKT signaling in pancreatic CSCs proliferation. In this direction, a recent study showed that CSCs like CD133 showed high levels of mTOR signaling in pancreatic adenocarcinoma. This underlines that the PI3K/AKT pathway plays a prominent role in pancreatic CSCs[71] consistent with significant inhibition of CD133 cells by the mTOR inhibitor rapamycin[71]. However, the functions of Wnt signaling in pancreatic CSCs are less and poorly understood. Lgr5 is the Wnt target gene that is being recently explored as pancreatic CSC. Like other important signaling pathways, the Wnt/β-catenin signaling pathway play a critical role in the pancreatic cancer development and progression.
ROLE OF PANCREATIC CSCS IN PREVENTION AND TREATMENT
Advances in the identification and characterization of pancreatic CSCs have created new opportunities for genetic targeting in treatment applications. Pancreatic CSCs are essential drivers for cancer progression and metastasis and are responsible for therapeutic resistance. Targetable cellular markers including CD24, CD44, CD133, ESA, ALDH1, c-Met, EPCAM, nestin, Lgr5 and DclK1 have been employed to characterize pancreatic CSCs[9-14,64]. Studies suggest that the use of the drugs that target PI3/AKT/mTOR, Hedgehog, c-Met and other developmental signaling pathways might deplete the populations of CSCs. Conceivably, multidrug combinations and multitargeting approaches will produce maximal anti-tumor efficacy by depleting resistant CSCs in PC[72]. So far, no clinical studies have been aimed at targeting pancreatic CSCs specifically in PC patients. The pancreatic cancer precursors, PanINs, progress slowly over many years to the development of invasive PC[3-5]. Hence, developing novel strategies to delay or inhibit progression of pancreatic cancer targeting CSCs are warranted. The K-rasG12D genetically engineered mouse (GEM) is an excellent model that shows the development of lesions closely resembling human PanIN’s with progression to PDAC and it has been used successfully for chemoprevention studies[5,8,72-78]. We have shown that licofelone, a dual COX-5-LOX inhibitor, exhibits chemopreventive efficacy against pancreatic cancer in p48Cre/+-LSL-KrasG12D/+ mice in part by inhibiting CSCs[72]. We have also shown that metformin, an anti-diabetic drug significantly prevents progression of pancreatic cancer by targeting in part CSCs[8].
To date, there are no specific CSC inhibitors tested preclinically using GEMs or clinically in PC patients. Pharmacological or genetic inhibition of JNK leads to loss of stem cell properties in the pancreatic CSCs both in vitro and tumor. Furthermore, loss of Kras in kras mutant pancreatic CSCs led to the loss of stem cell characteristics by downregulation of the JNK pathway[79]. Pancreatic cancers contain 1%-3% of CD133 positive cancer cells, some of which show high expression of CXCR4, a pro-invasive marker[14]. The selective inhibition of CXCR4 signaling in CXCR4+ CSC cells by AMD3100 blocks tumor tissue invasion[13,14], suggesting a potential role of CXCR4 in pancreatic tumor metastasis[14]. Accordingly, it remains possible that there is more than one type of CSC sub-population in pancreatic cancer tissues, which would be consistent with the known heterogeneity of most-human tumors[14,24]. More preclinical animal and human clinical studies are warranted to evaluate the drugs for their specificity in targeting CSCs to inhibit the progression of PC. Combination of epigallocatechin-3 gallate (EGCG) and quercetin, and, Sulforaphane inhibited the self-renewal capacity of pancreatic CSCs via attenuation of the Hedgehog pathway[62-65]. The GANT-61 is a Gli transcription factor inhibitor that inhibits pancreatic CSC viability and induces apoptosis[61]. Together, blockade of hedgehog and mTOR signaling along with standard chemotherapy was found to eliminate pancreatic CSCs[80]. Another hedgehog antagonist Vismodegib (GDC-0449), inhibits pancreatic CSC characteristics in vitro[81]. Using GDC-0449, a human phase I clinical trial is ongoing[82], which preliminarily suggest that GDC-0449 has good safety profile and antitumor activity for some of the locally advanced or metastatic solid tumors. Pancreatic cancer progression was inhibited by sulforaphane, green tea catechins and quercetin by inducing let7a miRNA and inhibiting Kras and ALDH1 activity[82]. The 3-Bromopyruvate, a glycolysis inhibitor blocked CSC signaling in PC cell lines and the spheroids derived from patients[83]. Also, chloroquine (antimalarial agent) decreased CSCs in vitro and in vivo in combination with gemcitabine by inhibiting the hedgehog signaling[84].
An SRC inhibitor, dasatinib in combination with gemcitabine significantly reduced ALDH1 in MiaPaCa parental and gemcitabine resistant cells[85]. A drug under phase 1 trial, Minnelide efficiently decreased CD133 cells in the tumors[86]. Triptolide (plant derived Chinese medicine) is generally used for arthritis and cancer. In clinical trials it inhibited hypoxia-induced transcriptional signaling and EMT and CSC features in cell lines, decreased tumor growth in-vivo, and inhibited CSCs from patient tumors[87].
Vaccine or antibody approaches are recently explored for pancreatic cancer inhibition. In the xenograft models and in-vitro cultures, anti-CD44 monoclonal antibodies decreased growth, metastasis and CSCs including downregulating CSC genes Sox-2, Nanog and Rex1[88]. BMS-777067 (tyrosine kinase inhibitor) in combination with AZD8055 (mTOR inhibitor) successfully inhibited chemoresistant cancer cells and CSCs. However, BMS-777067 alone induces the polyploidy in the CSCs making them insensitive to the therapy[89]. Cabozantinib downregulated c-Met, SOX2 and CD133 in the drug resistant PC cells and CSC spheroids and induced apoptosis[90]. A kinase inhibitor XMD8-92 was shown to down-regulate Dclk1 and its downstream targets including KRAS, ZEB1, ZEB2, c-MYC, NOTCH1, OCT4, SOX2, SNAIL, SLUG, NANOG, KLF4, LIN28, VEGFR1, and VEGFR2 in the tumors of the xenografts[90,91].
IMPLICATIONS AND FUTURE DIRECTIONS
It is now well-established that pancreatic CSCs contribute to aggressive tumor phenotype resistant to radiation and chemotherapy[8,14]. The molecular and pathological characteristics of the pancreatic CSCs are yet to be fully elucidated. Knowledge gained from the GEM models, and, isolation and identification of specific cancer stem cell markers and their characterization in tumor tissues will provide deep insights that will be of value in designing effective strategies for the development of chemoprevention and chemotherapy drugs that reduce tumor aggressiveness by specifically targeting CSCs[8,14]. Although some pathways such as Notch and c-Met signaling are important for pancreatic CSC maintenance, other pathways like Wnt (Lgr5) and pathways related to Dclk1 that are required for pancreatic CSC activity must also be elucidated. Drugs targeting CSCs and their markers might modulate function of these CSCs[8,14]. The validity of this approach was suggested by the finding that metformin, an anti-diabetic drug, displays anti-tumor effects due to the partially targeted elimination of CSCs in pancreatic cancer[8,14]. Additional clinical and preclinical work is required to demonstrate conclusively the therapeutic benefit of metformin[8,14,88], and CSC-targeting drugs in general, for the management of particular cancers[8,14]. As such, CSCs are one of the best targets for cancer treatment. However, we need to improve our understanding of pancreatic CSCs and pancreatic cancer biology to develop optimal treatment (Chemoprevention and Chemotherapy) regimens. Effective treatment of pancreatic cancer could require the continued administration of conventional chemotherapeutic agents in combinations or along with agents that specifically deplete pancreatic CSCs. Pancreatic cancer could be effectively treated if the above conditions are met.
Footnotes
P- Reviewer: Chen YJ, Shi CJ S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
Supported by In part by Kerley Cade Endowed Chair (Chinthalapally V Rao), University of Oklahoma Health Sciences Center; and in part support from the National Cancer Institute, No. 5R03CA181584-02 (Altaf Mohammed).
Conflict-of-interest: The authors declare no potential conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 13, 2014
First decision: November 3, 2014
Article in press: December 17, 2014
References
- 1.American Cancer Society. Cancer facts and figures. American Cancer Society (ACS), Atlanta, GA. Available from: http://www.cancer.org/research/cancerfactsstatistics/
- 2.Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet] Lyon, France: International Agency for Research on Cancer; 2013. Available from: http: //globocan.iarc.fr. [Google Scholar]
- 3.Mazur PK, Siveke JT. Genetically engineered mouse models of pancreatic cancer: unravelling tumour biology and progressing translational oncology. Gut. 2012;61:1488–1500. doi: 10.1136/gutjnl-2011-300756. [DOI] [PubMed] [Google Scholar]
- 4.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, Kamiyama M, Hruban RH, Eshleman JR, Nowak MA, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammed A, Janakiram NB, Lightfoot S, Gali H, Vibhudutta A, Rao CV. Early detection and prevention of pancreatic cancer: use of genetically engineered mouse models and advanced imaging technologies. Curr Med Chem. 2012;19:3701–3713. doi: 10.2174/092986712801661095. [DOI] [PubMed] [Google Scholar]
- 6.Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245–256. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohara Y, Oda T, Sugano M, Hashimoto S, Enomoto T, Yamada K, Akashi Y, Miyamoto R, Kobayashi A, Fukunaga K, et al. Histological and prognostic importance of CD44(+) /CD24(+) /EpCAM(+) expression in clinical pancreatic cancer. Cancer Sci. 2013;104:1127–1134. doi: 10.1111/cas.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohammed A, Janakiram NB, Brewer M, Ritchie RL, Marya A, Lightfoot S, Steele VE, Rao CV. Antidiabetic Drug Metformin Prevents Progression of Pancreatic Cancer by Targeting in Part Cancer Stem Cells and mTOR Signaling. Transl Oncol. 2013;6:649–659. doi: 10.1593/tlo.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sureban SM, May R, Lightfoot SA, Hoskins AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R, Mohammed A, Rao CV, et al. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011;71:2328–2338. doi: 10.1158/0008-5472.CAN-10-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Borodyansky L, Yang Y. Genomic instability en route to and from cancer stem cells. Cell Cycle. 2009;8:1000–1002. doi: 10.4161/cc.8.7.8041. [DOI] [PubMed] [Google Scholar]
- 11.Takao S, Ding Q, Matsubara S. Pancreatic cancer stem cells: regulatory networks in the tumor microenvironment and targeted therapy. J Hepatobiliary Pancreat Sci. 2012;19:614–620. doi: 10.1007/s00534-012-0547-1. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 13.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Bao B, Ahmad A, Azmi AS, Ali S, Sarkar FH. Overview of cancer stem cells (CSCs) and mechanisms of their regulation: implications for cancer therapy. Curr Protoc Pharmacol. 2013;Chapter 14:Unit 14.25. doi: 10.1002/0471141755.ph1425s61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habib M, Saif MW. Pancreatic cancer stem cells: their role in pancreatic cancer patient outcomes and what is future? JOP. 2013;14:401–404. doi: 10.6092/1590-8577/1658. [DOI] [PubMed] [Google Scholar]
- 16.Ischenko I, Petrenko O, Hayman MJ. Analysis of the tumor-initiating and metastatic capacity of PDX1-positive cells from the adult pancreas. Proc Natl Acad Sci USA. 2014;111:3466–3471. doi: 10.1073/pnas.1319911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizuno N, Yatabe Y, Hara K, Hijioka S, Imaoka H, Shimizu Y, Ko SB, Yamao K. Cytoplasmic expression of LGR5 in pancreatic adenocarcinoma. Front Physiol. 2013;4:269. doi: 10.3389/fphys.2013.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, Gallick GE. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6:e20636. doi: 10.1371/journal.pone.0020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee CJ, Dosch J, Simeone DM. Pancreatic cancer stem cells. J Clin Oncol. 2008;26:2806–2812. doi: 10.1200/JCO.2008.16.6702. [DOI] [PubMed] [Google Scholar]
- 20.Mizukami T, Kamachi H, Mitsuhashi T, Tsuruga Y, Hatanaka Y, Kamiyama T, Matsuno Y, Taketomi A. Immunohistochemical analysis of cancer stem cell markers in pancreatic adenocarcinoma patients after neoadjuvant chemoradiotherapy. BMC Cancer. 2014;14:687. doi: 10.1186/1471-2407-14-687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu L. Cancer stem cell in the progression and therapy of pancreatic cancer. Front Biosci (Landmark Ed) 2013;18:795–802. doi: 10.2741/4143. [DOI] [PubMed] [Google Scholar]
- 22.Kure S, Matsuda Y, Hagio M, Ueda J, Naito Z, Ishiwata T. Expression of cancer stem cell markers in pancreatic intraepithelial neoplasias and pancreatic ductal adenocarcinomas. Int J Oncol. 2012;41:1314–1324. doi: 10.3892/ijo.2012.1565. [DOI] [PubMed] [Google Scholar]
- 23.Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, Los M. Cancer stem cell markers in common cancers - therapeutic implications. Trends Mol Med. 2008;14:450–460. doi: 10.1016/j.molmed.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218–2227.e5. doi: 10.1053/j.gastro.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, Kong D, Ahmad A, Li Y, Padhye S, et al. Curcumin analogue CDF inhibits pancreatic tumor growth by switching on suppressor microRNAs and attenuating EZH2 expression. Cancer Res. 2012;72:335–345. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MH, Cierpicki T, Derewenda U, Krowarsch D, Feng Y, Devedjiev Y, Dauter Z, Walsh CA, Otlewski J, Bushweller JH, et al. The DCX-domain tandems of doublecortin and doublecortin-like kinase. Nat Struct Biol. 2003;10:324–333. doi: 10.1038/nsb918. [DOI] [PubMed] [Google Scholar]
- 27.Lin PT, Gleeson JG, Corbo JC, Flanagan L, Walsh CA. DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization. J Neurosci. 2000;20:9152–9161. doi: 10.1523/JNEUROSCI.20-24-09152.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.May R, Riehl TE, Hunt C, Sureban SM, Anant S, Houchen CW. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 29.May R, Sureban SM, Hoang N, Riehl TE, Lightfoot SA, Ramanujam R, Wyche JH, Anant S, Houchen CW. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27:2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May R, Sureban SM, Lightfoot SA, Hoskins AB, Brackett DJ, Postier RG, Ramanujam R, Rao CV, Wyche JH, Anant S, et al. Identification of a novel putative pancreatic stem/progenitor cell marker DCAMKL-1 in normal mouse pancreas. Am J Physiol Gastrointest Liver Physiol. 2010;299:G303–G310. doi: 10.1152/ajpgi.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sureban SM, May R, Ramalingam S, Subramaniam D, Natarajan G, Anant S, Houchen CW. Selective blockade of DCAMKL-1 results in tumor growth arrest by a Let-7a MicroRNA-dependent mechanism. Gastroenterology. 2009;137:649–659, 659.e1-2. doi: 10.1053/j.gastro.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sureban SM, May R, Mondalek FG, Qu D, Ponnurangam S, Pantazis P, Anant S, Ramanujam RP, Houchen CW. Nanoparticle-based delivery of siDCAMKL-1 increases microRNA-144 and inhibits colorectal cancer tumor growth via a Notch-1 dependent mechanism. J Nanobiotechnology. 2011;9:40. doi: 10.1186/1477-3155-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali N, Allam H, May R, Sureban SM, Bronze MS, Bader T, Umar S, Anant S, Houchen CW. Hepatitis C virus-induced cancer stem cell-like signatures in cell culture and murine tumor xenografts. J Virol. 2011;85:12292–12303. doi: 10.1128/JVI.05920-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakanishi Y, Seno H, Fukuoka A, Ueo T, Yamaga Y, Maruno T, Nakanishi N, Kanda K, Komekado H, Kawada M, et al. Dclk1 distinguishes between tumor and normal stem cells in the intestine. Nat Genet. 2013;45:98–103. doi: 10.1038/ng.2481. [DOI] [PubMed] [Google Scholar]
- 35.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, et al. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–780. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bjerknes M, Khandanpour C, Möröy T, Fujiyama T, Hoshino M, Klisch TJ, Ding Q, Gan L, Wang J, Martín MG, et al. Origin of the brush cell lineage in the mouse intestinal epithelium. Dev Biol. 2012;362:194–218. doi: 10.1016/j.ydbio.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali Y, Lin Y, Gharibo MM, Gounder MK, Stein MN, Lagattuta TF, Egorin MJ, Rubin EH, Poplin EA. Phase I and pharmacokinetic study of imatinib mesylate (Gleevec) and gemcitabine in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5876–5882. doi: 10.1158/1078-0432.CCR-07-0883. [DOI] [PubMed] [Google Scholar]
- 38.Rieder S, Michalski CW, Friess H, Kleeff J. Insulin-like growth factor signaling as a therapeutic target in pancreatic cancer. Anticancer Agents Med Chem. 2011;11:427–433. doi: 10.2174/187152011795677454. [DOI] [PubMed] [Google Scholar]
- 39.Sureban SM, May R, Qu D, Weygant N, Chandrakesan P, Ali N, Lightfoot SA, Pantazis P, Rao CV, Postier RG, et al. DCLK1 regulates pluripotency and angiogenic factors via microRNA-dependent mechanisms in pancreatic cancer. PLoS One. 2013;8:e73940. doi: 10.1371/journal.pone.0073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 41.Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, Sato T, Stange DE, Begthel H, van den Born M, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 42.Jaks V, Barker N, Kasper M, van Es JH, Snippert HJ, Clevers H, Toftgård R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40:1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Lanzoni G, Carpino G, Cui CB, Dominguez-Bendala J, Wauthier E, Cardinale V, Oikawa T, Pileggi A, Gerber D, et al. Biliary tree stem cells, precursors to pancreatic committed progenitors: evidence for possible life-long pancreatic organogenesis. Stem Cells. 2013;31:1966–1979. doi: 10.1002/stem.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amsterdam A, Raanan C, Schreiber L, Polin N, Givol D. LGR5 and Nanog identify stem cell signature of pancreas beta cells which initiate pancreatic cancer. Biochem Biophys Res Commun. 2013;433:157–162. doi: 10.1016/j.bbrc.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 45.Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 46.Huch M, Dorrell C, Boj SF, van Es JH, Li VS, van de Wetering M, Sato T, Hamer K, Sasaki N, Finegold MJ, et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013;494:247–250. doi: 10.1038/nature11826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasca di Magliano M, Biankin AV, Heiser PW, Cano DA, Gutierrez PJ, Deramaudt T, Segara D, Dawson AC, Kench JG, Henshall SM, et al. Common activation of canonical Wnt signaling in pancreatic adenocarcinoma. PLoS One. 2007;2:e1155. doi: 10.1371/journal.pone.0001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huch M, Bonfanti P, Boj SF, Sato T, Loomans CJ, van de Wetering M, Sojoodi M, Li VS, Schuijers J, Gracanin A, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32:2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon E, Petke D, Böger C, Behrens HM, Warneke V, Ebert M, Röcken C. The spatial distribution of LGR5+ cells correlates with gastric cancer progression. PLoS One. 2012;7:e35486. doi: 10.1371/journal.pone.0035486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dorado J, Lonardo E, Miranda-Lorenzo I, Heeschen C. Pancreatic cancer stem cells: new insights and perspectives. J Gastroenterol. 2011;46:966–973. doi: 10.1007/s00535-011-0422-x. [DOI] [PubMed] [Google Scholar]
- 51.Matsuda Y, Kure S, Ishiwata T. Nestin and other putative cancer stem cell markers in pancreatic cancer. Med Mol Morphol. 2012;45:59–65. doi: 10.1007/s00795-012-0571-x. [DOI] [PubMed] [Google Scholar]
- 52.Wen J, Park JY, Park KH, Chung HW, Bang S, Park SW, Song SY. Oct4 and Nanog expression is associated with early stages of pancreatic carcinogenesis. Pancreas. 2010;39:622–626. doi: 10.1097/MPA.0b013e3181c75f5e. [DOI] [PubMed] [Google Scholar]
- 53.Hill R, Calvopina JH, Kim C, Wang Y, Dawson DW, Donahue TR, Dry S, Wu H. PTEN loss accelerates KrasG12D-induced pancreatic cancer development. Cancer Res. 2010;70:7114–7124. doi: 10.1158/0008-5472.CAN-10-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martínez-Romero C, Rooman I, Skoudy A, Guerra C, Molero X, González A, Iglesias M, Lobato T, Bosch A, Barbacid M, et al. The epigenetic regulators Bmi1 and Ring1B are differentially regulated in pancreatitis and pancreatic ductal adenocarcinoma. J Pathol. 2009;219:205–213. doi: 10.1002/path.2585. [DOI] [PubMed] [Google Scholar]
- 55.Skoudy A, Hernández-Muñoz I, Navarro P. Pancreatic ductal adenocarcinoma and transcription factors: role of c-Myc. J Gastrointest Cancer. 2011;42:76–84. doi: 10.1007/s12029-011-9258-0. [DOI] [PubMed] [Google Scholar]
- 56.Yu J, Ohuchida K, Mizumoto K, Ishikawa N, Ogura Y, Yamada D, Egami T, Fujita H, Ohashi S, Nagai E, et al. Overexpression of c-met in the early stage of pancreatic carcinogenesis; altered expression is not sufficient for progression from chronic pancreatitis to pancreatic cancer. World J Gastroenterol. 2006;12:3878–3882. doi: 10.3748/wjg.v12.i24.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yauch RL, Gould SE, Scales SJ, Tang T, Tian H, Ahn CP, Marshall D, Fu L, Januario T, Kallop D, et al. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 58.Zeng G, Germinaro M, Micsenyi A, Monga NK, Bell A, Sood A, Malhotra V, Sood N, Midda V, Monga DK, et al. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia. 2006;8:279–289. doi: 10.1593/neo.05607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del Castillo C, Yajnik V, et al. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fu J, Rodova M, Roy SK, Sharma J, Singh KP, Srivastava RK, Shankar S. GANT-61 inhibits pancreatic cancer stem cell growth in vitro and in NOD/SCID/IL2R gamma null mice xenograft. Cancer Lett. 2013;330:22–32. doi: 10.1016/j.canlet.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang SN, Fu J, Nall D, Rodova M, Shankar S, Srivastava RK. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int J Cancer. 2012;131:30–40. doi: 10.1002/ijc.26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rodova M, Fu J, Watkins DN, Srivastava RK, Shankar S. Sonic hedgehog signaling inhibition provides opportunities for targeted therapy by sulforaphane in regulating pancreatic cancer stem cell self-renewal. PLoS One. 2012;7:e46083. doi: 10.1371/journal.pone.0046083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li SH, Fu J, Watkins DN, Srivastava RK, Shankar S. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Mol Cell Biochem. 2013;373:217–227. doi: 10.1007/s11010-012-1493-6. [DOI] [PubMed] [Google Scholar]
- 64.Srivastava RK, Tang SN, Zhu W, Meeker D, Shankar S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front Biosci (Elite Ed) 2011;3:515–528. doi: 10.2741/e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang FT, Zhuan-Sun YX, Zhuang YY, Wei SL, Tang J, Chen WB, Zhang SN. Inhibition of hedgehog signaling depresses self-renewal of pancreatic cancer stem cells and reverses chemoresistance. Int J Oncol. 2012;41:1707–1714. doi: 10.3892/ijo.2012.1597. [DOI] [PubMed] [Google Scholar]
- 66.Wang Z, Li Y, Banerjee S, Sarkar FH. Emerging role of Notch in stem cells and cancer. Cancer Lett. 2009;279:8–12. doi: 10.1016/j.canlet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abel EV, Kim EJ, Wu J, Hynes M, Bednar F, Proctor E, Wang L, Dziubinski ML, Simeone DM. The Notch pathway is important in maintaining the cancer stem cell population in pancreatic cancer. PLoS One. 2014;9:e91983. doi: 10.1371/journal.pone.0091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou ZC, Dong QG, Fu DL, Gong YY, Ni QX. Characteristics of Notch2(+) pancreatic cancer stem-like cells and the relationship with centroacinar cells. Cell Biol Int. 2013;37:805–811. doi: 10.1002/cbin.10102. [DOI] [PubMed] [Google Scholar]
- 69.Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, Streppel MM, Rasheed ZA, Hidalgo M, Maitra A, et al. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett. 2013;335:41–51. doi: 10.1016/j.canlet.2013.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mueller MT, Hermann PC, Witthauer J, Rubio-Viqueira B, Leicht SF, Huber S, Ellwart JW, Mustafa M, Bartenstein P, D’Haese JG, et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–1113. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 71.Mohammed A, Janakiram NB, Ely M, Lightfoot S, Steele VE, Rao CV. Licofelone, a novel dual COX-LOX inhibitor prevents progression of PanIN lesions to pancreatic carcinoma by targeting miRNAs and cancer stem cells in p48Cre/ -LSL-KrasG12D/ transgenic mice. In: Proceedings of the 102nd Annual Meeting of the American Association for Cancer Research; 2011 Apr 2-6; Orlando, FL. Philadelphia (PA): AACR. Cancer Res. 2011;71:2839. [Google Scholar]
- 72.Mohammed A, Janakiram NB, Brewer M, Biddick L, Lightfoot S, Steele VE, Rao CV. Targeting COX-LOX and EGFR pathways simultaneously by licofelone and gefitinib lead to complete blockade of progression of PanINs to pancreatic ductal adenocarcinoma. In: Proceedings of the 103rd Annual Meeting of the American Association for Cancer Research; 2012 Mar 31-Apr 4; Chicago, IL. Philadelphia (PA): AACR. Cancer Res. 2012;72:1005. [Google Scholar]
- 73.Mohammed A, Brewer M, Ritchie RL, Marya A, Lightfoot S, Janakiram NB, Steele VE, Rao CV. Metformin prevents progression of pancreatic intraepithelial neoplasia to ductal adenocarcinoma by targeting cancer stem cells and mTOR signaling. In: Proceedings of the Annual Meeting of the American Association for Cancer Research 2011 Apr 6-10; Washington, DC. AACR. Cancer Res. 2013;73:2268. doi: 10.1593/tlo.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mohammed A, Qian L, Janakiram NB, Lightfoot S, Steele VE, Rao CV. Atorvastatin delays progression of pancreatic lesions to carcinoma by regulating PI3/AKT signaling in p48Cre/+ LSL-KrasG12D/+ mice. Int J Cancer. 2012;131:1951–1962. doi: 10.1002/ijc.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mohammed A, Janakiram NB, Brewer M, Duff A, Lightfoot S, Brush RS, Anderson RE, Rao CV. Endogenous n-3 polyunsaturated fatty acids delay progression of pancreatic ductal adenocarcinoma in Fat-1-p48(Cre/+)-LSL-Kras(G12D/+) mice. Neoplasia. 2012;14:1249–1259. doi: 10.1593/neo.121508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohammed A, Janakiram NB, Brewer M, Ritchie RL, Marya A, Lightfoot S, Steele VE, Rao CV. Eflornithine (DFMO) prevents progression of pancreatic intraepithelial neoplasia to ductal adenocarcinoma in LSL-KrasG12D/ mice. In: Proceedings of the Eleventh Annual AACR International Conference on Frontiers in Cancer Prevention Research; 2012 Oct 16-19; Anaheim, CA. Philadelphia (PA): AACR. Cancer Prev Res. 2012;5:B115. [Google Scholar]
- 77.Mohammed A, Janakiram NB, Li Q, Madka V, Ely M, Lightfoot S, Crawford H, Steele VE, Rao CV. The epidermal growth factor receptor inhibitor gefitinib prevents the progression of pancreatic lesions to carcinoma in a conditional LSL-KrasG12D/+ transgenic mouse model. Cancer Prev Res (Phila) 2010;3:1417–1426. doi: 10.1158/1940-6207.CAPR-10-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okada M, Shibuya K, Sato A, Seino S, Suzuki S, Seino M, Kitanaka C. Targeting the K-Ras--JNK axis eliminates cancer stem-like cells and prevents pancreatic tumor formation. Oncotarget. 2014;5:5100–5112. doi: 10.18632/oncotarget.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh BN, Fu J, Srivastava RK, Shankar S. Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms. PLoS One. 2011;6:e27306. doi: 10.1371/journal.pone.0027306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.LoRusso PM, Rudin CM, Reddy JC, Tibes R, Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Chang I, Darbonne WC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502–2511. doi: 10.1158/1078-0432.CCR-10-2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Appari M, Babu KR, Kaczorowski A, Gross W, Herr I. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition. Int J Oncol. 2014;45:1391–1400. doi: 10.3892/ijo.2014.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Isayev O, Rausch V, Bauer N, Liu L, Fan P, Zhang Y, Gladkich J, Nwaeburu CC, Mattern J, Mollenhauer M, et al. Inhibition of glucose turnover by 3-bromopyruvate counteracts pancreatic cancer stem cell features and sensitizes cells to gemcitabine. Oncotarget. 2014;5:5177–5189. doi: 10.18632/oncotarget.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Balic A, Sørensen MD, Trabulo SM, Sainz B, Cioffi M, Vieira CR, Miranda-Lorenzo I, Hidalgo M, Kleeff J, Erkan M, et al. Chloroquine targets pancreatic cancer stem cells via inhibition of CXCR4 and hedgehog signaling. Mol Cancer Ther. 2014;13:1758–1771. doi: 10.1158/1535-7163.MCT-13-0948. [DOI] [PubMed] [Google Scholar]
- 84.Duong HQ, Yi YW, Kang HJ, Bae I, Jang YJ, Kwak SJ, Seong YS. Combination of dasatinib and gemcitabine reduces the ALDH1A1 expression and the proliferation of gemcitabine-resistant pancreatic cancer MIA PaCa-2 cells. Int J Oncol. 2014;44:2132–2138. doi: 10.3892/ijo.2014.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Banerjee S, Nomura A, Sangwan V, Chugh R, Dudeja V, Vickers SM, Saluja A. CD133+ tumor initiating cells in a syngenic murine model of pancreatic cancer respond to Minnelide. Clin Cancer Res. 2014;20:2388–2399. doi: 10.1158/1078-0432.CCR-13-2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu L, Salnikov AV, Bauer N, Aleksandrowicz E, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Schemmer P, Werner J, et al. Triptolide reverses hypoxia-induced epithelial-mesenchymal transition and stem-like features in pancreatic cancer by NF-κB downregulation. Int J Cancer. 2014;134:2489–2503. doi: 10.1002/ijc.28583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li L, Hao X, Qin J, Tang W, He F, Smith A, Zhang M, Simeone DM, Qiao XT, Chen ZN, et al. Antibody against CD44s inhibits pancreatic tumor initiation and postradiation recurrence in mice. Gastroenterology. 2014;146:1108–1118. doi: 10.1053/j.gastro.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zeng JY, Sharma S, Zhou YQ, Yao HP, Hu X, Zhang R, Wang MH. Synergistic activities of MET/RON inhibitor BMS-777607 and mTOR inhibitor AZD8055 to polyploid cells derived from pancreatic cancer and cancer stem cells. Mol Cancer Ther. 2014;13:37–48. doi: 10.1158/1535-7163.MCT-13-0242. [DOI] [PubMed] [Google Scholar]
- 89.Hage C, Rausch V, Giese N, Giese T, Schönsiegel F, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Herr I. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013;4:e627. doi: 10.1038/cddis.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sureban SM, May R, Weygant N, Qu D, Chandrakesan P, Bannerman-Menson E, Ali N, Pantazis P, Westphalen CB, Wang TC, et al. XMD8-92 inhibits pancreatic tumor xenograft growth via a DCLK1-dependent mechanism. Cancer Lett. 2014;351:151–161. doi: 10.1016/j.canlet.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 91.Lonardo E, Cioffi M, Sancho P, Sanchez-Ripoll Y, Trabulo SM, Dorado J, Balic A, Hidalgo M, Heeschen C. Metformin targets the metabolic achilles heel of human pancreatic cancer stem cells. PLoS One. 2013;8:e76518. doi: 10.1371/journal.pone.0076518. [DOI] [PMC free article] [PubMed] [Google Scholar]


