Abstract
Objectives
To examine the contemporary impact of smoking in a multi-ethnic sample, and to explore the respective contributions of inflammation and subclinical atherosclerosis to the cardiovascular consequences of smoking.
Approach and Results
We studied 6,814 participants free of cardiovascular disease (CVD) and coronary heart-disease (CHD) from MESA. Smoking status and cumulative exposure were determined by self-report and confirmed by urinary cotinine. Multivariable Cox-regression was used to estimate the association between smoking parameters and; All-cause CVD; All-cause CHD; and Hard CHD events. We further adjusted for high-sensitivity C-reactive protein (hsCRP) and coronary artery calcium (CAC) in hierarchical Cox-models. We identified 3,218 never-smokers, 2,607 former-smokers, and 971 current-smokers. Median follow-up was 10.2 years. Compared to never-smokers, adjusted Hazard-ratios (HRs) in current-smokers were 1.7 (95% CI, 1.3-2.2) for All-cause CVD, 1.6 (1.1-2.1) for All-cause CHD, and 1.7 (1.2-2.4) for Hard CHD. Similarly, among current-smokers, HRs were higher in the 4th vs 1st quartile of pack-years (e.g. All-cause CHD HR=2.7 [1.1-6.6]). Both CAC>100 and hsCRP≥3mg/L identified higher relative-risk among current-smokers (e.g. All-cause CHD HR of 3.0 [1.5-6.0, compared to CAC=0] and 2.6 [1.4-4.8, compared to hsCRP<2mg/L], respectively). However, CAC was a stronger mediator of events and adversely modified the effect of smoking on events (e.g. p-interaction=0.02 for Hard CHD). Compared to never-smokers, former-smokers (median cessation interval=22 years) had similar adjusted hazard for events.
Conclusion
In this multi-ethnic cohort, current smoking and cumulative exposure remain important modifiable determinants of CVD. Both hsCRP≥3mg/L and particularly CAC>100 identified high-risk smokers who may benefit from more intensive smoking-cessation efforts.
Keywords: Smoking, Inflammation, Coronary Artery Calcium, Cardiovascular Outcomes
Introduction
Smoking remains an important cause of coronary heart disease (CHD) and cardiovascular disease (CVD) 1, 2. However, despite vastly improved understanding, the pathophysiological mechanisms underpinning the association between smoking and CVD have yet to be fully elucidated 3, 4. For example, while the impact of smoking status on CVD is well known 5-8, an adverse association between cumulative smoke exposure (by pack-years) and cardiovascular events has not been consistently demonstrated in modern cohorts 9, 10. Indeed, it has even been questioned whether or not smoking-induced CVD events exhibit a dose-response 11.
In addition, mounting evidence suggests that inflammation and subclinical atherosclerosis are key players in the pathophysiology of smoking-induced CVD. Elevated high sensitivity C-reactive protein (hsCRP), a marker of systemic and vascular inflammation 12, 13, has been demonstrated in smokers 14. Similarly, smoking has been associated with both increased levels of fibrinogen (a marker of inflammation and thrombosis) and coronary artery calcium (CAC) 15-18. Indeed, in an accompanying analysis of subclinical CVD outcomes, we confirmed these findings in a modern multi-ethnic cohort. However, the degree to which smoking-induced aberrations in these novel risk-factors mediate the association between smoking and future cardiovascular events is poorly understood.
Further, in the aftermath of the National Lung Screening Trial 19, 20, chest CT imaging has been recently recommended by the U.S. Preventive Services Task Force for lung cancer screening in targeted smokers 21. Thus, opportunistic acquisition of CAC data at the time of chest CT imaging could yield additional prognostic information regarding CHD and CVD risk in former- and current-smokers undergoing such screening. In this context, it is important to better understand the prognostic implications of different CAC thresholds in a variety of smoking categories (based on both smoking status and quantity of cumulative exposure).
In response to these outstanding uncertainties, we conducted a comprehensive analysis of smoking and prospective cardiovascular events from the Multi-Ethnic Study of Atherosclerosis (MESA). This cohort study provides a unique opportunity to, 1) estimate the respective contributions of both inflammation (measured by hsCRP) and CAC to the adverse cardiovascular consequences of smoking (by performing a mediation analysis) and 2) provide data for monitoring cardiovascular risk among smokers using these novel markers of risk, which are already in widespread clinical use. Finally, this rigorously phenotyped cohort allowed us to confirm the dose-response relationship between smoking and both CHD and CVD events in a contemporary cohort of diverse ethnicity.
Materials and Methods
Materials and Methods for this prospective cohort study are available in the online-only Data Supplement.
Results
At baseline, current-smokers comprised 971 (14%), former-smokers 2607 (39%), and never-smokers 3218 (47%) of the study sample. Current-smokers were younger and more likely to be male. Other baseline differences are shown in Table 1. Among ethnic groups, the highest and lowest prevalence of current-smoking was noted in African Americans (18%) and Chinese Americans (6%), respectively, whereas 13% of Caucasian and 14% of Hispanics reported current-smoking. Despite their younger age, mean (±SD) smoking pack-years were higher in current-smokers (25.6 [±23.6]) as compared to former-smokers (20.2 [±24.9], p<0.001). The mean (±SD) time since cessation in the former-smoker group was 22 (±13) years. Finally, the baseline burden of CAC and inflammation (as measured by hs-CRP) were both higher in current-smokers (Table-1).
Table 1. Characteristics of the MESA Cohort according to baseline Smoking Status.
| Never | Former | Current | P-Value* | |
|---|---|---|---|---|
| N=3218, 47% | N=2607, 39% | N=971, 14% | ||
| Male, number (%) | 1,161 (36) | 1,527 (59) | 517 (53) | <0.001 |
| Age | 62.2 (10.5) | 63.4 (9.9) | 58.4 (9.1) | <0.001 |
| Ethnicity | ||||
| White, number (%) | 1,099 (34) | 1,182 (45) | 335 (35) | <0.001 |
| African-American, number (%) | 788 (25) | 728 (28) | 366 (38) | |
| Chinese-American, number (%) | 577 (18) | 173 (7) | 52 (5) | |
| Hispanic, number (%) | 754 (23) | 524 (20) | 218 (22) | |
| Bachelor's degree or higher, number (%) | 1,188 (37) | 974 (37) | 231 (24) | <0.001 |
| Family History of MI, number (%) | 1,220 (38) | 1,090 (42) | 418 (43) | 0.002 |
| Systolic BP (mmHg) | 127.0 (±21.9) | 127.1 (±20.9) | 123.7 (±21.4) | <0.001 |
| History of Hypertension, number (%) | 1,448 (45) | 1,233 (47) | 365 (38) | <0.001 |
| Hypertension Medication, number (%) | 1,208 (38) | 1,024 (39) | 294 (30) | <0.001 |
| History of Diabetes, number (%) | 390 (12) | 347 (13) | 120 (12) | 0.71 |
| Fasting Glucose (mg/dL) | 89 (83-99) | 91 (83-100) | 89 (82-99) | 0.01 |
| Total Cholesterol (mg/dL) | 195.9 (±35.1) | 192.6 (±35.1) | 192.7 (±38.8) | 0.001 |
| LDL-C (mg/dL) | 118.1 (±31.1) | 116.6 (±31.2) | 116.1 (±32.9) | 0.10 |
| HDL-C (mg/dL) | 51.9 (±14.7) | 50.8 (±15.1) | 48.1 (±14.2) | <0.001 |
| Triglycerides (mg/dL) (median) | 111 (79-161) | 109 (75-157) | 118 (84-171) | <0.001 |
| Lipid Medication, number (%) | 513 (16) | 476 (18) | 111 (11) | <0.001 |
| BMI (kg/m²) | 28.1 (±5.5) | 28.7 (±5.5) | 28.1 (±5.3) | <0.001 |
| Heart rate (bpm) | 63.7 (±9.6) | 62.5 (±9.7) | 63.1 (±9.5) | <0.001 |
| Pack-year History, years | ---- | 21.0 (±25.2) | 29.5 (±24.6) | <0.001 |
| hsCRP, mg/L (median) | 1.78 (0.8-4.0) | 1.93 (0.8-4.2) | 2.50 (1.1-4.8) | <0.001 |
| hsCRP ≥3mg/L, number (%) | 1080 (34) | 928 (36) | 432 (45) | <0.001 |
| Fibrinogen, mg/dL | 350 (±74) | 341 (±72) | 352 (±76) | <0.001 |
| CAC=0, number (%) | 1,797 (56) | 1,113 (43) | 494 (51) | <0.001 |
| CAC >100 Agatston Units, number (%) | 615 (19) | 766 (29) | 221 (23) | <0.001 |
| CAC >75th centile†, number (%) | 673 (47) | 706 (47) | 288 (60) | <0.001 |
Values are or proportion (%), mean (±SD), or median (25th-75th),
P values are for differences between groups using one-way ANOVA, Kruskal-Wallis testing, or Chi-square, as appropriate.
MI=Myocardial Infarction, HTN=hypertension, BP=Blood Pressure, mmHg=millimeters of mercury, LDL-C=low density lipoprotein cholesterol, HDL-C=high density lipoprotein cholesterol, BMI=body mass index, BPM=beats per minute, hsCRP=high-sensitivity C-Reactive Protein, CAC=Coronary Artery Calcium
Age and sex-based CAC percentiles are derived from the MESA population and were calculated only in those with CAC>0 at baseline (n=3392)
Crude Incidence Rates according to smoking status and pack-years
Over a median (IQR) follow-up of 10.2 (9.7-10.7) years, 638 All-cause CVD, 449 All-cause CHD, and 284 Hard CHD events were recorded. Incidence rates (per 1,000 person years) for All-cause CVD were 8.8 for never-smokers, 11.5 for former-smokers and 12.5 for current-smokers. Respective incidence rates were 5.9, 8.5 and 8.3 for All-cause CHD and 3.9, 4.6, and 6.1 for Hard CHD (per 1,000 person years). Kaplan-Meier survival curves demonstrated lower cumulative event-free survival in current-smokers compared with former-smokers and never-smokers (Figure-1).
Figure 1. Kaplan-Meier Survival by Smoking Status.
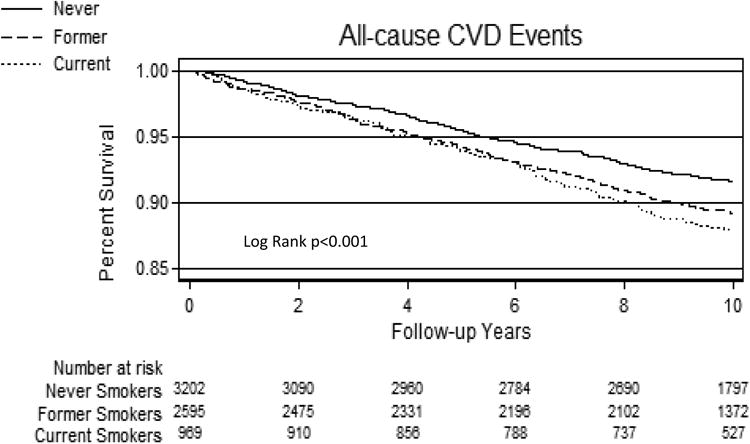
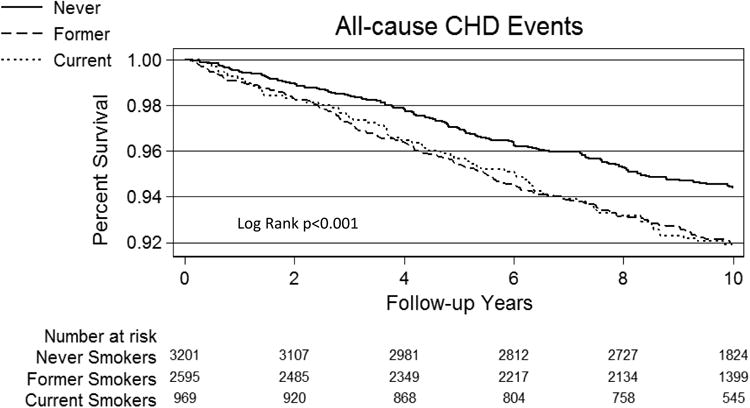
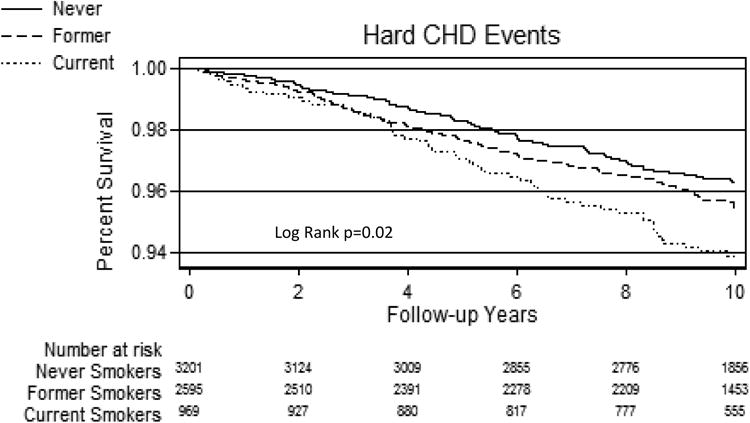
A= All-cause CVD events according to smoking status group
B= All-cause CHD events according to smoking status group
C= Hard CHD events according to smoking status group
CHD=Coronary heart disease events, CVD=Cardiovascular disease events.
Similarly, crude All-cause CVD incidence rates (per 1,000 person years) were higher in ever-smokers in the highest quartile of pack-years (for example, 8.1 for the 1st quartile of pack-years versus 18.9 for the 4th, P<0.001). In addition, All-cause CHD incidence rates of 5.3 and 14.7 were recorded in the 1st and 4th quartiles of pack-years, respectively (p<0.001).
Relative associations between smoking status, cumulative exposure, and events
Unadjusted hazard ratios (HR) for All-cause CVD were 1.4 (95% CI, 1.2-1.8) in current-smokers and 1.3 (1.1-1.5) in former-smokers, both compared to never-smokers. This association was stronger for current-smokers after full adjustment for a range of potentially confounding risk factors in Model 1, with a HR for All-cause CVD of 1.7 (95% CI, 1.3-2.2) compared to never-smokers (Table-2). Similar relationships were noted for All-cause CHD (Table-3) and Hard CHD (Supplementary eTable-II). After further addition of pack-years to the variables adjusted for in Model 1, the association between current-smoking status and events was attenuated (HR of 1.36 [0.97-1.90] for All-cause CHD and 1.43 [0.95-2.14] for Hard CHD), but remained significant for All-cause CVD (HR of 1.55 [1.18-2.05]). Neither gender nor race/ethnicity demonstrated interaction with the association between current smoking and events after adjustment in Model 1 (both interaction p-values >0.1). Further, we found no relative association between former-smoking status and any of the three cardiac outcomes in MESA, compared to never-smokers.
Table 2. Hazard Ratios for All-cause CVD, based on Smoking Status and Cumulative Exposure.
| Unadjusted | Model 1 * | Model 2 † | Model 3 ‡ | Model 4 § | Model 5 ‖ | |
|---|---|---|---|---|---|---|
| Smoking Status | ||||||
| Never Smokers | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Former Smokers | 1.31 (1.10, 1.55) | 1.07 (0.89, 1.29) | 1.05 (0.87, 1.26) | 0.98 (0.81, 1.19) | 1.09 (0.91, 1.32) | 0.97 (0.81, 1.18) |
| Current Smokers | 1.43 (1.14, 1.79) | 1.70 (1.32, 2.18) | 1.64 (1.28, 2.11) | 1.48 (1.15, 1.90) | 1.65 (1.28, 2.12) | 1.41 (1.09, 1.82) |
| p for linear trend | <0.001 | <0.001 | 0.001 | 0.02 | 0.001 | 0.04 |
| Packs Years- Former Smokers | ||||||
| 1st Quartile | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 2nd Quartile | 1.37 (0.95, 1.97) | 1.32 (0.90, 1.94) | 1.31 (0.89, 1.93) | 1.23 (0.83, 1.80) | 1.29 (0.88, 1.90) | 1.23 (0.83, 1.81) |
| 3rd Quartile | 1.25 (0.86, 1.81) | 0.99 (0.67, 1.47) | 0.98 (0.66, 1.46) | 0.86 (0.58, 1.28) | 0.99 (0.67, 1.47) | 0.88 (0.59, 1.31) |
| 4th Quartile | 1.99 (1.41, 2.81) | 1.29 (0.89, 1.87) | 1.27 (0.87, 1.84) | 1.18 (0.81, 1.72) | 1.28 (0.88, 1.86) | 1.19 (0.82, 1.74) |
| Pack-years Continuous | 1.01 (1.003, 1.01) | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 1.00 (0.97, 1.86) | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) |
| Packs Years- Current Smokers | ||||||
| 1st Quartile | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 2nd Quartile | 1.42 (0.64, 2.89) | 1.43 (0.68, 2.98) | 1.39 (0.67, 2.91) | 1.30 (0.62, 2.71) | 1.37 (0.65, 2.86) | 1.26 (0.60, 2.63) |
| 3rd Quartile | 2.62 (1.37, 4.99) | 1.87 (0.95, 3.68) | 1.77 (0.89, 3.50) | 1.76 (0.89, 3.48) | 1.76 (0.89, 3.48) | 1.63 (0.82, 3.25) |
| 4th Quartile | 4.01 (2.16, 7.45) | 2.45 (1.25, 4.78) | 2.21 (1.13, 4.32) | 2.41 (1.23, 4.72) | 2.35 (1.20, 4.58) | 2.13 (1.08, 4.18) |
| Pack-years Continuous | 1.02 (1.01, 1.02) | 1.01 (1.003, 1.02) | 1.01 (1.003, 1.02) | 1.01 (1.002, 1.02) | 1.01 (1.003, 1.02) | 1.01 (1.001, 1.02) |
95% confidence intervals are in brackets: statistically significant associations are highlighted in bold text
Model 1: Adjusted for age, sex, race, MESA site, body mass index, heart rate, hypertension status, diabetes status, LDL-Cholesterol, HDL-Cholesterol, triglycerides, cholesterol lowering medication use, family history of Myocardial Infarction, and education level.
Model 2: Model 1+Log (hsCRP);
Model 3: Model 1+ Log (CAC+1);
Model 4: Model 1+ Fibrinogen;
Model 5: Model 1+Log (hsCRP) + Log (CAC+1) +Fibrinogen
Quartiles of pack-years: Former Smokers= 1st<3, 2nd 3-12, 3rd 12-28, 4th>28 pack-years; Current Smokers= 1st<8, 2nd 8-20, 3rd 20-37, 4th>37. Continuous results are HR per unit increase in pack-year. CHD- Coronary heart disease events, CVD=Cardiovascular disease events, CAC=Coronary artery calcium (in Agatston Units), hsCRP=high-sensitivity C-Reactive Protein (in milligrams per liter)
Table 3. Hazard Ratios for All-cause CHD, based on Smoking Status and Cumulative Exposure.
| Unadjusted | Model 1 * | Model 2 † | Model 3 ‡ | Model 4 § | Model 5 ‖ | |
|---|---|---|---|---|---|---|
| Smoking Status | ||||||
| Never Smokers | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Former Smokers | 1.44 (1.18, 1.77) | 1.14 (0.91, 1.42) | 1.11 (0.89, 1.39) | 1.02 (0.82, 1.28) | 1.16 (0.93, 1.45) | 1.00 (0.80, 1.26) |
| Current Smokers | 1.41 (1.07, 1.86) | 1.55 (1.14, 2.10) | 1.49 (1.09, 2.03) | 1.29 (0.95, 1.76) | 1.49 (1.09, 2.03) | 1.22 (0.89, 1.67) |
| p for linear trend | 0.001 | 0.01 | 0.02 | 0.18 | 0.01 | 0.32 |
| Packs Years- Former Smokers | ||||||
| 1st Quartile | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 2nd Quartile | 1.52 (0.96, 2.38) | 1.51 (0.95, 2.41) | 1.50 (0.94, 2.40) | 1.41 (0.88, 2.26) | 1.47 (0.92, 2.35) | 1.39 (0.87, 2.24) |
| 3rd Quartile | 1.50 (0.96, 2.35) | 1.16 (0.72, 1.87) | 1.14 (0.71, 1.85) | 1.00 (0.62, 1.62) | 1.16 (0.72, 1.87) | 1.01 (0.62, 1.64) |
| 4th Quartile | 2.63 (1.74, 3.99) | 1.69 (1.08, 2.65) | 1.66 (1.06, 2.61) | 1.56 (0.99, 2.43) | 1.70 (1.08, 2.65) | 1.57 (0.99, 2.46) |
| Packs Years Continuous | 1.01 (1.004, 1.01) | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) |
| Packs Years- Current Smokers | ||||||
| 1st Quartile | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| 2nd Quartile | 1.31 (0.49, 3.53) | 1.18 (0.42, 3.29) | 1.11 (0.40, 3.12) | 1.03 (0.37, 2.91) | 1.11 (0.39, 3.11) | 0.97 (0.34, 2.73) |
| 3rd Quartile | 3.78 (1.64, 8.75) | 2.35 (0.98, 5.64) | 2.11 (0.87, 5.11) | 2.13 (0.88, 5.16) | 2.14 (0.88, 5.16) | 1.88 (0.77, 4.58) |
| 4th Quartile | 5.06 (2.23, 11.48) | 2.74 (1.14, 6.62) | 2.39 (0.99, 5.79) | 2.66 (1.09, 6.45) | 2.54 (1.05, 6.17) | 2.23 (0.91, 5.44) |
| Packs Years Continuous | 1.02 (1.01, 1.02) | 1.01 (1.005, 1.02) | 1.01 (1.005, 1.02) | 1.01 (1.003, 1.02) | 1.01 (1.004, 1.02) | 1.01 (1.003, 1.02) |
95% confidence intervals are in brackets: statistically significant associations are highlighted in bold text
Model 1: Adjusted for age, sex, race, MESA site, body mass index, heart rate, hypertension status, diabetes status, LDL-Cholesterol, HDL-Cholesterol, triglycerides, cholesterol lowering medication use, family history of Myocardial Infarction, and education level.
Model 2: Model 1+Log (hsCRP);
Model 3: Model 1+ Log (CAC+1);
Model 4: Model 1+ Fibrinogen;
Model 5: Model 1+Log (hsCRP) + Log (CAC+1) +Fibrinogen
Quartiles of pack-years: Former Smokers= 1st<3, 2nd 3-12, 3rd 12-28, 4th>28 pack-years; Current Smokers= 1st<8, 2nd 8-20, 3rd 20-37, 4th>37.Continuous results are HR per unit increase in pack-year. CHD- Coronary heart disease events, CVD=Cardiovascular disease events, CAC=Coronary artery calcium (in Agatston Units), hsCRP=high-sensitivity C-Reactive Protein (in milligrams per liter)
Regarding cumulative exposure, the multivariable-adjusted HR's in the highest quartile of pack-years in ever-smokers were 1.7 (1.2-2.3) for All-cause CVD, 1.9 (1.3-2.7) for All-cause CHD, and 2.2 (1.4-3.6) for Hard CHD (all compared to the lowest quartile of pack-years, Supplementary eTable-III). However, after stratification by smoking status (either former or current), these dose-response associations were mostly null in former-smokers but stronger in current-smokers (e.g. HR of 2.5 [1.3-4.8] for All-cause CVD, 2.7 [1.1-6.6]] for All-cause CHD, and 2.5 [0.9-6.7] for Hard CHD comparing the 4th versus 1st quartile of pack-years in current-smokers [Tables-2, 3 and Supplementary eTable-II]).The null associations between pack-year quartiles and events among former-smokers persisted even after further adjusting for cessation interval (in years) in the baseline fully adjusted model (Supplementary eTable-IV). In addition to these categorical analyses, cumulative exposure modeled continuously (change in hazard per 1 unit increase in pack-year) was also an independent determinant of events in current-smokers only.
Impact of CAC and hsCRP on the association between smoking and cardiovascular events
When stratified by CAC, the lowest All-cause CVD crude incidence rate was observed in never-smokers with zero CAC (2.9 events per 1,000 person years); whereas current smokers with CAC scores>100 exhibited the highest All-cause CVD incidence rate (27.9 per 1,000 person years, Figure-2). Also of note, current-smokers without CAC exhibited far lower All-cause CVD incidence rates (7.4 per 1,000 person years) than never-smokers with CAC scores>100 (27.5 per 1,000 person years). Similar trends for absolute risk were noted for All-cause and Hard CHD events (Supplementary e-Figures-I and II).
Figure 2. All-cause CVD Incidence Rates (per 1000 person-years) by Smoking Status and Exposure: Stratified by CAC and hsCRP.
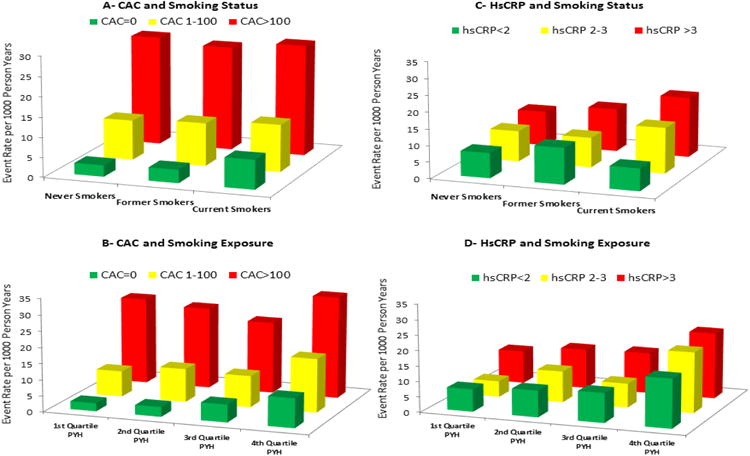
A= All-cause CVD by smoking group, stratified by CAC
B= All-cause CVD by quartiles of smoking exposure in pack-years, stratified by CAC
C= All-cause CVD by smoking group, stratified by hsCRP
D= All-cause CVD by quartiles of smoking exposure in pack-years, stratified by hsCRP
CVD=Cardiovascular disease events, CAC=Coronary artery calcium in Agatston Units, hsCRP=high-sensitivity C-Reactive Protein (in milligrams per deciliter), PYH=Pack-year history in Ever-smokers
Similarly, crude incidence rates among ever-smokers were highest in the 4th Quartile of pack-years within each CAC category. However, ever-smokers with a CAC score=0 in the highest quartile of pack-years had incidence rates of 9.1 for All-cause CVD and 5.6 for All-cause CHD (per 1,000 person years), contrasting with rates of 27.5 for All-cause CVD and 21.1 for All-cause CHD in ever-smokers with CAC>100 from the lowest quartile of pack-years.
In contrast to the monotonic increases in event-rates by CAC strata, crude CHD and CVD event rates were generally similar among each smoking group and cumulative exposure category when stratified by hsCRP (Figure-2 and Supplementary e-Figures I-II).
Table-4 demonstrates adjusted HRs (relative risk) for each of our three main outcomes within each of the smoking status groups, after stratification by CAC and hsCRP. In never- and-former smokers, CAC score categories of 1-100 as well as >100 were associated with greater risk for future events (compared to CAC=0). However, in current-smokers, only the presence of CAC scores>100 were reliably associated with greater relative risk for events. In addition, CAC>100 was consistently associated with greater All-cause CVD and All-cause CHD events within each quartile of pack-years in ever-smokers (Supplementary eTable-V). In addition, when comparing risk between smoking status categories, there was evidence that CAC adversely modified the effect of current smoking on all three events, relative to never-smoking (p-value for interaction 0.04 for All-cause CVD, <0.01 for All-cause CHD, and 0.02 for Hard CHD).
Table 4. All-cause CVD, All-cause CHD, and Hard CHD Hazard Ratios based on CAC and hsCRP, Stratified by Smoking Status*.
| Never Smoker | Former Smoker | Current Smoker | |
|---|---|---|---|
| All-cause CVD | |||
| CAC BURDEN | |||
| CAC=0 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| CAC 1-100 | 2.18 (1.47, 3.24) | 2.17 (1.42, 3.31) | 1.09 (0.62, 1.92) |
| CAC>100 | 4.55 (3.07, 6.75) | 3.84 (2.55, 5.80) | 2.27 (1.31, 3.93) |
| p for linear trend | <0.001 | <0.001 | <0.001 |
| hsCRP LEVELS | |||
| hsCRP<2 mg/L | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| hsCRP 2-3 mg/L | 1.27 (0.84, 1.91) | 0.75 (0.48, 1.17) | 1.63 (0.79, 3.36) |
| hsCRP ≥3 mg/L | 1.47 (1.08, 2.00) | 1.20 (0.89, 1.61) | 2.40 (1.44, 3.98) |
| p for linear trend | 0.01 | 0.21 | <0.001 |
| All-cause CHD | |||
| CAC BURDEN | |||
| CAC=0 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| CAC 1-100 | 4.20 (2.35, 7.53) | 2.32 (1.40, 3.84) | 1.12 (0.54, 2.32) |
| CAC>100 | 11.26 (6.31, 20.09) | 4.40 (2.70, 7.19) | 3.01 (1.52, 5.97) |
| p for linear trend | <0.001 | <0.001 | <0.001 |
| hsCRP LEVELS | |||
| hsCRP<2 mg/L | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| hsCRP 2-3 mg/L | 1.33 (0.81, 2.18) | 0.77 (0.47, 1.28) | 0.79 (0.26, 2.38) |
| hsCRP ≥3 mg/L | 1.47 (1.002, 2.15) | 1.11 (0.78, 1.56) | 2.60 (1.41, 4.78) |
| p for linear trend | 0.09 | 0.78 | <0.001 |
| Hard CHD | |||
| CAC BURDEN | |||
| CAC=0 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| CAC 1-100 | 2.94 (1.52, 5.68) | 2.06 (1.09, 3.90) | 1.06 (0.45, 2.52) |
| CAC>100 | 7.68 (4.02, 14.66) | 2.35 (1.24, 4.44) | 3.75 (1.69, 8.29) |
| p for linear trend | <0.001 | <0.001 | <0.001 |
| hsCRP LEVELS | |||
| hsCRP<2 mg/L | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| hsCRP 2-3 mg/L | 1.46 (0.82, 2.61) | 0.75 (0.39, 1.46) | 0.51 (0.11, 2.28) |
| hsCRP ≥3 mg/L | 1.30 (0.82, 2.08) | 0.86 (0.54, 1.38) | 2.41 (1.20, 4.83) |
| p for linear trend | 0.30 | 0.53 | 0.003 |
95% confidence intervals are in brackets; statistically significant associations are highlighted in bold text.
Model adjusted for age, sex, race, MESA site, body mass index, heart rate, hypertension status, diabetes status, LDL-Cholesterol, HDL-Cholesterol, triglycerides, cholesterol lowering medication use, family history of Myocardial Infarction, and education level.
CHD- Coronary heart disease events, CVD=Cardiovascular disease events, CAC=Coronary artery calcium (in Agatston Units), hsCRP=high-sensitivity C-Reactive Protein (in milligrams per liter)
When the analysis sample was restricted to the 3,404 participants with CAC=0 at baseline, only current-smokers and those reporting the highest cumulative smoking exposure had a relatively increased hazard ratio of both All-cause CVD (1.9 [1.2-3.3] for current-smokers compared to never-smokers; and 3.2 [1.5-6.6] for 4th quartile of pack-years in ever-smokers compared to 1st) and All-cause CHD (2.4 [1.1-5.1] for current-smokers compared to never-smokers; and 3.5 [1.4-8.7] for 4th quartile of pack-years in ever-smokers compared to 1st, Supplementary eTable-VI).
Contrary to CAC, hsCRP levels were not consistently associated with relative increases in cardiovascular events within smoking categories. One important exception was an increased relative hazard for events in current-smokers with hsCRP≥3mg/L [e.g. HR 2.4 [1.4-3.9] for All-cause CVD and 2.6 [1.4-4.8] for All-cause CHD, Table-4]). However, the absolute event rate was consistently higher in the CAC>100 group than in smokers with hsCRP≥3mg/L (Figure-2 and Supplementary e-Figures I-II). In addition, hsCRP did not appear to adversely modify the effect of current-smoking on events compared to never-smoking (interaction p-values all >0.05)
When the analysis sample was restricted to the 1,733 MESA participants with both CAC=0 and hsCRP<2mg/L, current-smoking status (but not former-smoking) continued to be associated with all three events relative to never-smoking, after full adjustment (HR of 2.3 [1.1, 5.1] for All-cause CVD, HR of 3.5 [1.2, 10.6] for All-cause CHD, and HR of 3.4 [1.01, 11.5] for hard-CHD).
Finally, in our mediation analysis, the addition of hsCRP to our fully-adjusted model led to slight attenuation of the hazard for All-cause CVD (HR from 1.70 to 1.64) and for All-cause CHD (HR from 1.55 to 1.49), in current-smokers compared to never-smokers (Table-2 and 3). A more pronounced attenuation of this association was observed after the subsequent addition of CAC (All-cause CVD HR fell from 1.70 to 1.48 and All-cause CHD fell from 1.55 to 1.29). In addition, with the exception of All-cause CVD (residual HR 1.4 [1.1-1.8] for current-smoking), the addition of these 3 mediating variables (hsCRP, Fibrinogen, and CAC) to the fully adjusted models appeared to explain most of the residual impact of current-smoking on cardiac events, particularly for our two CHD events of interest (both p>0.05, Tables-2, 3, and Supplementary eTable-II).
Discussion
In this large ethnically-diverse cohort, we confirm that current smoking remains an important modifiable risk factor for cardiovascular disease in the modern era. In addition, current smokers in the highest quartile of pack-years of cumulative smoke exposure demonstrated increased risk for events compared to those in the lowest quartile, confirming a cumulative dose effect. Importantly, there was no interaction on the association between smoking and cardiac events by race or gender, demonstrating that the impact of smoking on CVD and CHD is generally consistent irrespective of gender and ethnicity 22. Finally, while both CAC>100 and hsCRP≥3 mg/L identify high risk current smokers, CAC is a more consistent risk factor across a number of smoking sub-types (by status and pack-year category) and appears to be a stronger mediator and adverse effect modifier on the putative pathway linking smoking to events.
We believe that these smoking data are among the most rigorous to date, as they are derived from a well characterized modern cohort and facilitated by objective measurement of urinary cotinine (see methods section). From a regulatory science perspective, our results will also inform public health strategies aimed at reducing smoking-related CVD. In addition, the results of this comprehensive analysis confirm and extend prior knowledge regarding smoking-induced CVD. A number of important findings are worth emphasizing.
First, we confirm a cumulative dose-response association between smoking and both CHD and CVD events (irrespective of race or gender); an important result in any analysis of smoking. A now classic report by Hill and Doll was the first to demonstrate a dose-response association between smoking and cardiac events; a finding with important causal implications 23. However, subsequent studies utilizing modern statistical methods (e.g. adjusting for multiple confounders) have yielded conflicting results regarding whether or not a dose-response relationship actually exists for cardiovascular events 9-11, 24. Our results suggest that any doubts about the cumulative effect of active smoking on cardiovascular disease appear unwarranted.
Second, our results also indicate that, after adjustment, former-smokers in populations like the MESA sample do not experience a relative increase in risk of cardiovascular events compared to never-smokers. This lack of an association may also help explain why higher quartiles of cumulative pack-years were also not associated with events among former-smokers in MESA. Prior studies have demonstrated that the risk of cardiac events returns to baseline approximately 5 to 10 years after smoking cessation 25, 26. Since the median time elapsed since smoking cessation in MESA was 22 years, this duration may help explain these findings.
Third, and perhaps most importantly, a major strength of this analysis is the ability to estimate the mediating effects of specific variables thought to be on the causal pathway linking smoking to cardiovascular disease outcomes (such as markers of inflammation, thrombosis, and coronary atherosclerosis). In this MESA cohort, baseline hsCRP appeared to be a relatively weak mediator of cardiac events in smokers, as was Fibrinogen (a more proximate marker of inflammation and thrombosis). We note, however, that in contrast to CAC (which integrates exposures to risk factors over the lifetime 27), a single measurement of hsCRP or Fibrinogen at baseline may not fully capture the longitudinal burden of inflammation in this cohort. Further, when smokers were stratified by hsCRP categories, elevated hsCRP thresholds were not consistently associated with relative increases in cardiovascular events among the smoking sub-types assessed. However, one important exception was in current-smokers with hsCRP levels ≥3mg/L. Indeed, this hs-CRP threshold appears to identify a high risk smoking phenotype with similar relative risk for events to smokers with advanced atherosclerosis by CAC (Table 4).
Nonetheless, our findings implicate subclinical atherosclerosis as a stronger mediator of cardiac events in those who smoke, particularly for CHD events. Our results extend prior findings evaluating the impact of CAC on all-cause mortality in smokers 16, 28. Further, we demonstrate evidence for statistical interaction by CAC, suggesting that CAC may also adversely modify the effect of smoking on cardiovascular events (another finding with causal implications). This finding is intuitive as is well established that CAC correlates closely with the burden of both calcified and non-calcified coronary plaque 29. Thus, a higher CAC score reflects a higher burden of atherosclerosis overall, thereby increasing the absolute risk that smoking-induced effects on atherosclerotic plaque may lead to cardiac events 11.
In keeping with this, we found monotonic elevations in crude CVD and CHD incidence rates among increasing strata CAC within both former and current smoker categories. However, the 1-100 CAC stratum did not identify increased relative risk (compared to the CAC=0 group) among current-smokers in MESA, likely because of the higher absolute risk in current smokers with CAC=0. This phenomenon also explains why the relative hazard for increasing CAC within the current smoking category appears smaller than in the non-smoking group (the higher absolute risk in the reference group of current-smokers with CAC=0 results in a smaller relative risk for increasing CAC in current-smokers compared to non-smokers, despite the fact that absolute risk is higher in smokers with elevated CAC [e.g. figure 2]).”
In this context, it is important to note that the absence of CAC does not completely exclude mild non-calcified plaque, a common finding in smokers 30. This is important as, while a CAC score=0 typically confers a favorable prognosis 31, we have previously demonstrated that current smokers with CAC=0 have an increased relative hazard for all-cause mortality compared to non-smokers with CAC=0 16. In the present analysis we extend this all-cause mortality finding to CHD and CVD events. Further to this we also found that even current smokers with both CAC=0 and hsCRP<2mg/L have relatively higher risk for events than non-smokers with normal levels of these risk markers. Thus, while CAC>100 and CRP ≥3mg/L both identify higher risk smokers, these markers should not be used to reassure smokers when normal.
Finally, our results have potential implications for the interpretation of cardiac findings in smokers undergoing CT screening for lung cancer 32-34; particularly important in the aftermath of the National Lung Screening Trial 19, 20. Specifically, based on our data, current-smokers with CAC>100 have the highest risk phenotype and would benefit most from aggressive smoking cessation and risk factor management. Future research is now needed to determine if systematic acquisition of CAC data (now possible with very low radiation doses 35) at the time of chest-CT cancer screening can be used to change CVD prevention strategies and yield clinical benefit in smokers (either by triggering smoking cessation or optimization of other cardiac risk factors based on CAC data). Notably, while gating of CT images is preferred for CAC quantification, non-gated assessment of CAC is also reasonable 33.
This study has limitations worth noting; 1) Smoking was modeled as a fixed exposure (measured at baseline), as data on smoking from subsequent visits were limited; 2) the 10-year duration of follow-up may have under-estimated the longer-term risks of smoking 25;3) while we performed cotinine reclassification in a random sample representing approximately half the cohort, it is possible that there was misclassification in those with self-reported smoking variables only, however, we repeated all analyses without cotinine reclassification (using self-reported smoking parameters alone) and all results were highly consistent with those presented above (data not shown);4) we had hsCRP and Fibrinogen measurements at only one time point (baseline) and we did not have other biomarkers of thrombosis risk available;5) knowledge of CAC results may have influenced smoking cessation rates as well as preventive therapies, however presumably biasing the association between CAC and events towards the null; and 6) given the many strong social determinants of smoking, residual confounding may remain a problem. Strengths of this study include the large, community-based sample, adjudicated follow-up for events, and rigorous measurement of cardiovascular risk factors, cotinine, and other variables of interest.
In conclusion, current smoking status and cumulative exposure both remain significant independent risk factors for CVD and CHD events in a contemporary cohort of varied ethnicity. While both CAC>100 and hsCRP≥3 mg/L identify high risk smokers who may benefit from more aggressive smoking cessation efforts, CAC may better stratify absolute and relative risk than hsCRP in smokers overall, appears to be a stronger mediator in the putative causal pathway, and adversely modifies the effect of smoking on cardiovascular events.
Supplementary Material
Significance.
In this modern multiethnic cohort, current-smoking remains an important modifiable risk factor for cardiovascular disease outcomes. This analysis also confirms an independent dose-response association between cumulative tobacco exposure and cardiovascular outcomes in current-smokers. Further, these data identify both CAC>100 and hsCRP≥3mg/L as novel markers of excess relative risk in current-smokers, although CAC is a more powerful marker of absolute and relative risk and is a stronger mediator on the putative causal pathway. The findings of this study support future research to; 1) determine the value of obtaining CAC data at the time of chest CT lung cancer screening in smokers; and 2) evaluate the utility of incorporating these two novel biomarkers into strategies designed to augment smoking cessation efforts.
Acknowledgments
We thank the other investigators, staff, and participants of the MESA study for their valuable contributions.
Funding sources: This analysis was supported by funding from the American Heart Association Tobacco Regulation and Addiction Center (A-TRAC, NIH 1 P50 HL120163-01), a member of the FDA Tobacco Centers of Regulatory Science for Research Relevant to the Family Smoking Prevention and Tobacco Control Act (P50). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration. The MESA study which supplied the data for this analysis was supported by contracts N01-HC-95159 through N01-HC-95167 and N01-HC-95169 from the National Heart, Lung, and Blood Institute. The cotinine measurement was supported by contract R01-HL077612. Dr McEvoy is supported by the Pollin Cardiovascular Prevention Fellowship.
Abbreviations
- MESA
Multiethnic Study of Atherosclerosis
- CHD
Coronary heart disease
- CVD
Cardiovascular disease
- CAC
Coronary artery calcium
- MI
Myocardial Infarction
- HsCRP
high-sensitivity C-Reactive Protein
Footnotes
Supplementary information is provided in an Online Appendix.
Disclosures: Dr Budoff serves on a speakers' bureau for GE Healthcare. The remaining authors have no non-financial interests that may be relevant to the submitted work.
References
- 1.Schroeder SA. New evidence that cigarette smoking remains the most important health hazard. The New England journal of medicine. 2013;368:389–390. doi: 10.1056/NEJMe1213751. [DOI] [PubMed] [Google Scholar]
- 2.The health consequences of smoking-50 years of progress: A report of the surgeon general. Atlanta (GA): 2014. [Google Scholar]
- 3.Barua RS, Ambrose JA. Mechanisms of coronary thrombosis in cigarette smoke exposure. Arteriosclerosis, thrombosis, and vascular biology. 2013;33:1460–1467. doi: 10.1161/ATVBAHA.112.300154. [DOI] [PubMed] [Google Scholar]
- 4.Schane RE, Ling PM, Glantz SA. Health effects of light and intermittent smoking: A review. Circulation. 2010;121:1518–1522. doi: 10.1161/CIRCULATIONAHA.109.904235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond EC, Horn D. Smoking and death rates: Report on forty-four months of follow-up of 187,783 men. 2. Death rates by cause. Journal of the American Medical Association. 1958;166:1294–1308. doi: 10.1001/jama.1958.02990110030007. [DOI] [PubMed] [Google Scholar]
- 6.Doll R, Hill AB. The mortality of doctors in relation to their smoking habits; a preliminary report. Br Med J. 1954;1:1451–1455. doi: 10.1136/bmj.1.4877.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doll R, Hill AB. Mortality of british doctors in relation to smoking: Observations on coronary thrombosis. National Cancer Institute monograph. 1966;19:205–268. [PubMed] [Google Scholar]
- 8.Pearl R. Tobacco smoking and longevity. Science. 1938;87:216–217. doi: 10.1126/science.87.2253.216. [DOI] [PubMed] [Google Scholar]
- 9.Freund KM, Belanger AJ, D'Agostino RB, Kannel WB. The health risks of smoking. The framingham study: 34 years of follow-up. Annals of epidemiology. 1993;3:417–424. doi: 10.1016/1047-2797(93)90070-k. [DOI] [PubMed] [Google Scholar]
- 10.Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh artery study. European heart journal. 1999;20:344–353. doi: 10.1053/euhj.1998.1194. [DOI] [PubMed] [Google Scholar]
- 11.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: An update. Journal of the American College of Cardiology. 2004;43:1731–1737. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 12.Libby P. Inflammation in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emerging Risk Factors C, Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: An individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das I. Raised c-reactive protein levels in serum from smokers. Clinica chimica acta; international journal of clinical chemistry. 1985;153:9–13. doi: 10.1016/0009-8981(85)90133-0. [DOI] [PubMed] [Google Scholar]
- 15.Sharrett AR, Ding J, Criqui MH, Saad MF, Liu K, Polak JF, Folsom AR, Tsai MY, Burke GL, Szklo M. Smoking, diabetes, and blood cholesterol differ in their associations with subclinical atherosclerosis: The multiethnic study of atherosclerosis (mesa) Atherosclerosis. 2006;186:441–447. doi: 10.1016/j.atherosclerosis.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 16.McEvoy JW, Blaha MJ, Rivera JJ, Budoff MJ, Khan AN, Shaw LJ, Berman DS, Raggi P, Min JK, Rumberger JA, Callister TQ, Blumenthal RS, Nasir K. Mortality rates in smokers and nonsmokers in the presence or absence of coronary artery calcification. JACC Cardiovascular imaging. 2012;5:1037–1045. doi: 10.1016/j.jcmg.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: Results from the multi-ethnic study of atherosclerosis (mesa) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen T, Frestad D, Kober L, Pedersen JH, Thomsen LH, Dirksen A, Kofoed KF. Development and progression of coronary artery calcification in long-term smokers: Adverse effects of continued smoking. Journal of the American College of Cardiology. 2013;62:255–257. doi: 10.1016/j.jacc.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Kovalchik SA, Tammemagi M, Berg CD, Caporaso NE, Riley TL, Korch M, Silvestri GA, Chaturvedi AK, Katki HA. Targeting of low-dose ct screening according to the risk of lung-cancer death. The New England journal of medicine. 2013;369:245–254. doi: 10.1056/NEJMoa1301851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mets OM, de Jong PA, Prokop M. Computed tomographic screening for lung cancer: An opportunity to evaluate other diseases. JAMA : the journal of the American Medical Association. 2012;308:1433–1434. doi: 10.1001/jama.2012.12656. [DOI] [PubMed] [Google Scholar]
- 21.Moyer VA Force USPST. Screening for lung cancer: U.S. Preventive services task force recommendation statement. Annals of internal medicine. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 22.Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: A systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297–1305. doi: 10.1016/S0140-6736(11)60781-2. [DOI] [PubMed] [Google Scholar]
- 23.Doll R, Peto R, Boreham J, Sutherland I. Mortality in relation to smoking: 50 years' observations on male british doctors. Bmj. 2004;328:1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuller LH, Ockene JK, Meilahn E, Wentworth DN, Svendsen KH, Neaton JD. Cigarette smoking and mortality. Mrfit research group. Preventive medicine. 1991;20:638–654. doi: 10.1016/0091-7435(91)90060-h. [DOI] [PubMed] [Google Scholar]
- 25.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years' observations on male british doctors. Bmj. 1994;309:901–911. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jha P, Ramasundarahettige C, Landsman V, Rostron B, Thun M, Anderson RN, McAfee T, Peto R. 21st-century hazards of smoking and benefits of cessation in the united states. The New England journal of medicine. 2013;368:341–350. doi: 10.1056/NEJMsa1211128. [DOI] [PubMed] [Google Scholar]
- 27.Erbel R, Budoff M. Improvement of cardiovascular risk prediction using coronary imaging: Subclinical atherosclerosis: The memory of lifetime risk factor exposure. European heart journal. 2012;33:1201–1213. doi: 10.1093/eurheartj/ehs076. [DOI] [PubMed] [Google Scholar]
- 28.Shaw LJ, Raggi P, Callister TQ, Berman DS. Prognostic value of coronary artery calcium screening in asymptomatic smokers and non-smokers. European heart journal. 2006;27:968–975. doi: 10.1093/eurheartj/ehi750. [DOI] [PubMed] [Google Scholar]
- 29.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 30.Barbash GI, Reiner J, White HD, Wilcox RG, Armstrong PW, Sadowski Z, Morris D, Aylward P, Woodlief LH, Topol EJ. Evaluation of paradoxic beneficial effects of smoking in patients receiving thrombolytic therapy for acute myocardial infarction: Mechanism of the “smoker's paradox” from the gusto-i trial, with angiographic insights. Global utilization of streptokinase and tissue-plasminogen activator for occluded coronary arteries. Journal of the American College of Cardiology. 1995;26:1222–1229. doi: 10.1016/0735-1097(95)00299-5. [DOI] [PubMed] [Google Scholar]
- 31.Blaha M, Budoff MJ, Shaw LJ, Khosa F, Rumberger JA, Berman D, Callister T, Raggi P, Blumenthal RS, Nasir K. Absence of coronary artery calcification and all-cause mortality. JACC Cardiovascular imaging. 2009;2:692–700. doi: 10.1016/j.jcmg.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 32.National Lung Screening Trial Research T. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom RM, Gareen IF, Gatsonis C, Marcus PM, Sicks JD. Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie X, Zhao Y, de Bock GH, de Jong PA, Mali WP, Oudkerk M, Vliegenthart R. Validation and prognosis of coronary artery calcium scoring in nontriggered thoracic computed tomography: Systematic review and meta-analysis. Circulation Cardiovascular imaging. 2013;6:514–521. doi: 10.1161/CIRCIMAGING.113.000092. [DOI] [PubMed] [Google Scholar]
- 34.Jairam PM, de Jong PA, Mali WP, Isgum I, de Koning HJ, van der Aalst C, Oudkerk M, Vliegenthart R, van der Graaf Y. Impact of cardiovascular calcifications on the detrimental effect of continued smoking on cardiovascular risk in male lung cancer screening participants. PloS one. 2013;8:e66484. doi: 10.1371/journal.pone.0066484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marwan M, Mettin C, Pflederer T, Seltmann M, Schuhback A, Muschiol G, Ropers D, Daniel WG, Achenbach S. Very low-dose coronary artery calcium scanning with high-pitch spiral acquisition mode: Comparison between 120-kv and 100-kv tube voltage protocols. Journal of cardiovascular computed tomography. 2013;7:32–38. doi: 10.1016/j.jcct.2012.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


