Abstract
IMPORTANCE
Converging evidence indicates that brain abnormalities in autism spectrum disorder (ASD) involve atypical network connectivity, but it is unclear whether altered connectivity is especially prominent in brain networks that participate in social cognition.
OBJECTIVE
To investigate whether adolescents with ASD show altered functional connectivity in 2 brain networks putatively impaired in ASD and involved in social processing, theory of mind (ToM) and mirror neuron system (MNS).
DESIGN, SETTING, AND PARTICIPANTS
Cross-sectional study using resting-state functional magnetic resonance imaging involving 25 adolescents with ASD between the ages of 11 and 18 years and 25 typically developing adolescents matched for age, handedness, and nonverbal IQ.
MAIN OUTCOMES AND MEASURES
Statistical parametric maps testing the degree of whole-brain functional connectivity and social functioning measures.
RESULTS
Relative to typically developing controls, participants with ASD showed a mixed pattern of both over- and underconnectivity in the ToM network, which was associated with greater social impairment. Increased connectivity in the ASD group was detected primarily between the regions of the MNS and ToM, and was correlated with sociocommunicative measures, suggesting that excessive ToM-MNS cross talk might be associated with social impairment. In a secondary analysis comparing a subset of the 15 participants with ASD with the most severe symptomology and a tightly matched subset of 15 typically developing controls, participants with ASD showed exclusive overconnectivity effects in both ToM and MNS networks, which were also associated with greater social dysfunction.
CONCLUSIONS AND RELEVANCE
Adolescents with ASD showed atypically increased functional connectivity involving the mentalizing and mirror neuron systems, largely reflecting greater cross talk between the 2. This finding is consistent with emerging evidence of reduced network segregation in ASD and challenges the prevailing theory of general long-distance underconnectivity in ASD. This excess ToM-MNS connectivity may reflect immature or aberrant developmental processes in 2 brain networks involved in understanding of others, a domain of impairment in ASD. Further, robust links with sociocommunicative symptoms of ASD implicate atypically increased ToM-MNS connectivity in social deficits observed in ASD.
Humans are an inherently social species. Our survival and success depend on our ability to navigate and thrive in complex social situations. This core ability is commonly impaired in autism spectrum disorder (ASD), a neurodevelopmental disorder affecting as many as 1 in 88 children.1 Despite the highly heterogeneous symptom manifestation, impairments in social functioning, including diminished social responsiveness, difficulty relating to others, and recognizing others’ emotions and intentions, are defining features of ASD.2 These social deficits are considered the most universal and specific characteristics of ASD,3 both defining and distinguishing it from other developmental disorders.4 Yet, the neural mechanisms underlying social impairments remain largely undetermined, despite attracting a great deal of research.
Currently, 2 debatably related prominent theories account for social dysfunction in ASD, theory of mind (ToM) and the mirror neuron system (MNS). The ToM, also known as the mentalizing system, refers to the ability to infer contents of other people’s minds, including their beliefs and intentions. This ability to attribute mental states, or to mentalize, is impaired, or at the least delayed in ASD,5–7 giving rise to the mind-blindness theory of autism.8 The MNS refers to the brain mirror mechanisms that allow us to understand meaning of the actions and emotions of others by internally simulating and replicating them9 (as inferred from the original discovery in macaques of neurons firing during both action execution and observation10). Evidence showing that imitation, a behavioral correlate of the MNS,11 is impaired in ASD12 has given rise to the dominant theory that atypical MNS functioning may be a key to understanding the nature of social deficits in ASD13–15 (although see the article by Dinstein and colleagues16 for alternative views).
Even though both ToM and the MNS are involved in understanding others, a meta-analysis of more than 200 functional magnetic resonance imaging task-based activation studies17 confirmed that, functionally and anatomically, they are 2 distinct systems. While the MNS is an action-understanding system, activated only in the presence of biological motion (eg, when moving body parts such as hands or face are observed), ToM is recruited during a more abstract processing of others’ intentionality, in the absence of any biological motion. Although it is understood that judging others in the real world likely involves both ToM and MNS, the functional distinction between them determined by this meta-analysis has been adapted here. Anatomically, the meta-analysis identified ToM with a frontal-posterior network of brain regions, including the medial prefrontal cortex (mPFC), bilateral temporal-parietal junction(TPJ), and posterior cingulate cortex (PCC)/precuneus, while the human MNS engaged the anterior intraparietal sulcus (aIPS, also referred to as the rostral inferior parietal lobule [IPL]), premotor cortex ([PMC] also referred to as the caudal inferior frontal gyrus [IFG]), and posterior superior temporal sulcus (pSTS).17
While neuroimaging and electrophysiological evidence suggests that ASD is associated with localized abnormalities in certain ToM18,19 and MNS20–23 brain areas, it is also becoming increasingly evident that ASD is characterized by abnormal connectivity throughout the brain,24–27 presumed to stem from altered neurodevelopmental trajectories.28,29 Wide-spread abnormalities in interregional connections in ASD have been predominantly demonstrated with functional connectivity magnetic resonance imaging (fcMRI), assessing functional coordination between spatially distributed brain regions.27 Functional connectivity (FC), inferred from inter-regional cross-correlations of the blood oxygen level–dependent (BOLD) signal, can be detected even at rest, in the absence of an overt cognitive task.30 Importantly, those patterns correspond to brain networks recruited during specific cognitive or mental processes31–34 and are, therefore, thought to reflect intrinsically organized functional networks35 formed by a long history of frequent coactivation associated with functional specialization.36,37 Moreover, resting-state FC patterns are largely consistent with anatomical connectivity38,39 and appear robust and highly reliable across individuals.39–43
The present study investigated whether adolescents with ASD show altered FC in the MNS and ToM, 2 brain networks putatively impaired in ASD and involved in social processing, by using resting-state fcMRI to assess interregional BOLD correlations in these networks. Our aims were 2-fold: to examine the extent of functional specialization, as deduced from the FC, of the ToM and MNS networks in adolescents with ASD (eg, whether the 2 networks are functionally segregated) and to relate FC of these networks involved in understanding others to variation on clinical measures of social impairment. It was hypothesized that individuals with ASD would exhibit aberrant connectivity within and between these networks, compared with matched typically developing (TD) controls, and that those participants with greatest social impairments within the ASD group would show the most atypical connectivity patterns.
Methods
Participants
Thirty adolescents with ASD and 26 TD adolescents, between 11 and 18 years of age, were enrolled in the study. After excluding 5 participants with ASD because of excessive head motion ( > 15% of time points) and 1 TD adolescent because of hardware malfunction, the final sample included 25 participants with ASD and 25 TD participants matched for age, handedness, and nonverbal IQ (Table 1; eAppendix 1 and eTable 1 in Supplement). The ASD diagnoses were established using the Autism Diagnostic Interview–Revised (ADI-R),44 the Autism Diagnostic Observation Schedule (ADOS),45 and expert clinical judgment (by one of us, A.J.L.) according to DSM-IV criteria.2 History of autism-related medical conditions (eg, epilepsy, Fragile X syndrome, tuberous sclerosis) served as an exclusionary criterion. Inclusion in the TD group required absence of personal or family history of autism and of personal history of any other neurological or psychiatric conditions. All participants had verbal and nonverbal IQ scores greater than 70, as assessed by the Wechsler Abbreviated Scale of Intelligence.46 In addition to the ADI- and ADOS-derived indices of social behavior available only for participants with ASD, social functioning was also assessed in all participants using the Social Responsiveness Scale (SRS),47 an informant-based rating scale measuring social impairments characteristic of ASD; it was administered to the participants’ parents. Hand preference was assessed with the Edinburgh Handedness Inventory.48 Written and oral informed assent and consent were obtained from all participants and their caregivers in accordance with the institutional review boards of the University of California, San Diego and San Diego State University.
Table 1.
Participant Characteristicsa
| Variable | Mean (SD) [Range]
|
P Value | |
|---|---|---|---|
| ASD (n = 25) | TD (n = 25) | ||
| Sex, M/F | 22/3 | 20/5 | |
| Handedness, R/L | 23/3 | 21/4 | |
| Age, y | 14.8 (1.8) [11.8–17.7] | 14.4 (1.5) [12.1–16.80] | .40 |
| Verbal IQ | 111 (15) [83–145] | 106 (10) [87–126] | .18 |
| Nonverbal IQ | 111 (16) [70–140] | 108 (11) [86–129] | .38 |
| Full-scale IQ | 113 (15) [81–141] | 108 (10) [88–128] | .16 |
| ADOS score | |||
| Communication | 2.9 (1.4) [0–6] | NA | |
| Social interaction | 7.6 (3.2) [1–13] | NA | |
| Repetitive behavior | 2.0 (1.4) [0–5] | NA | |
| ADI-R score | |||
| Social interaction | 16.5 (6.2) [6–25] | NA | |
| Communication | 12.6 (6.2) [2–25] | NA | |
| Repetitive behavior | 6.0 (2.3) [3–11] | NA | |
| SRS, total score | 78.5 (9.8) [58–94] | 41.5 (5.1) [35–52] | <.001 |
Abbreviations: ADI-R, Autism Diagnostic Interview–Revised; ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; NA, not available; SRS, Social Responsiveness Scale; TD, typically developing.
Four of 25 participants with ASD met either the ADOS or the ADI-R cutoff while meeting the clinical diagnostic criteria determined by expert clinical judgment (21 of 25 participants met both ADOS and ADI-R cutoffs). Twelve ASD participants presented with comorbid psychiatric conditions, including attention-deficit hyperactivity disorder (5), obsessive compulsive disorder (2), depression (3), and anxiety (4), with 2 of 12 participants with ASD diagnosed with more than 1 comorbid condition. Ten participants with ASD were reported to be taking psychoactive medications, as detailed in eAppendix 1 and eTable 1 in Supplement.
Magnetic Resonance Imaging Data Acquisition
Imaging data were acquired on a 3-T scanner with an 8-channel head coil (MR750; GE). High-resolution anatomical images were obtained using a standard T1-weighted fast spoiled gradient recalled echo (SPGR) sequence (repetition time = 11.08 milliseconds; echo time = 4.3 milliseconds; flip angle = 45°; field of view = 256 mm; 256 × 256 matrix; 180 slices; and 1 mm3 resolution). Functional T2*-weighted echo-planar images were acquired in one 6 minute, 10 second resting-state scan consisting of 185 whole-brain volumes (repetition time = 2000 milliseconds; echo time = 30 milliseconds; flip angle = 90°; field of view = 220 mm; 64 × 64 matrix; 3.4-mm2 inplane resolution; 3.4-mm slice thickness; and 42 axial slices covering the whole brain). Throughout the scan, participants were instructed to keep their eyes on a white fixation cross displayed in the center of a screen.
Functional Magnetic Resonance Imaging Data Preprocessing
Images were processed primarily using Analysis of Functional NeuroImages (AFNI).49 The first 5 frames were discarded to remove signal equilibration effects, resulting in 180 total whole-brain volumes. Functional data were slice-time and motion-corrected by realigning to the first time point, field-map corrected to remove distortions resulting from magnetic field inhomogeneity, coregistered to the anatomical image using a single transformation matrix, resampled to 3.0-mm isotropic voxels, standardized to the N27 Talairach template,50 and spatially smoothed with an isotropic gaussian filter to an effective full width at half maximum of 6 mm. The resulting images were then bandpass filtered at 0.008 < f < 0.08 Hz to isolate frequencies at which intrinsic network-specific BOLD correlations predominate.30,51
To minimize the confounding effects of head motion on BOLD correlations,52,53 6 scan-to-scan rigid-body motion parameters (3 rotations, 3 translations) estimated from realignment of functional volumes were modeled as nuisance variables and removed with regression, along with the mean white matter and ventricular signals extracted from the masks derived from the Freesurfer automated segmentation of anatomical images into tissue compartments and reduced by 1 voxel in all directions54 (all regressors bandpass filtered at 0.008 < f < 0.08 Hz). Time points with excessive head motion (head displacement > 1.5 mm, computed as the root sum of square of displacement between any 2 time points) and their immediately preceding and following time points were censored from further analyses; blocks of time with fewer than 10 usable consecutive images were also excluded. Based on this criterion, the mean percentage of data censored from all 50 participants was less than 1%. Percentage of censored data did not differ between groups (mean: ASD, 0.71%; TD, 0.67%; t1,48 = 0.06, P = .95). Finally, the root mean square of displacement (RMSD) across the entire time series, calculated for each participant, did not differ between the groups (mean: ASD, 0.133; TD, 0.125; t1,48 = 0.23, P = .82), and was not significantly correlated with age (P = .13) or full-scale IQ (P = .16).
ToM and MNS Regions of Interest
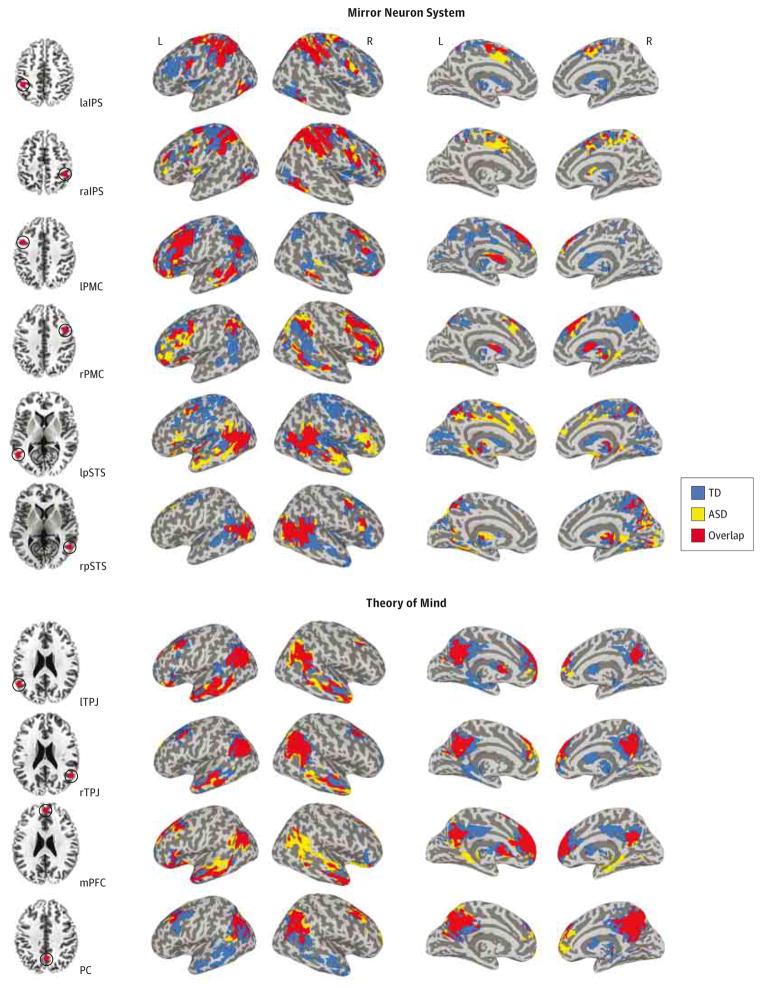
Seeds were placed in regions found to be consistently activated by mentalizing or mirror neuron tasks, as determined by meta-analysis,17 including 4 ToM seeds, such as the mPFC, right and left TPJ, and PCC, and 6 MNS seeds, including the bilateral aIPS, pSTS, and PMC (Figure 1, left panel for seed placements and Talairach coordinates). Seeds were created using the Talairach-Tournoux Stereotaxic Atlas in AFNI as 6 mm-radius spheres, covering 33 voxels in a 3-mm3 space.
Figure 1. Within-Group Functional Connectivity Maps for Mirror Neuron System (MNS) (Top Panel) and Theory of Mind (ToM) (Bottom Panel) Seeds.
Results of the within-group (autism spectrum disorder [ASD], typically developing [TD]; P < .05, corrected) analyses obtained for each MNS and ToM seed (top and bottom panels, respectively) are presented in a conjunction view. Seed regions of interest are presented on the axial slices on the left (red dots reflect the actual size of the spherical regions of interest). Inflated maps were generated using Surface Mapping with Analysis of Functional NeuroImages (SUMA, http://afni.nimh.nih.gov/afni/suma). L indicates left; laIPS, left anterior intraparietal sulcus (Talairach coordinates −40, −40, 45); lpSTS, left posterior superior temporal sulcus (−50, −55, 10); lPMC, left premotor cortex (−40, 5, 40); lTPJ, left temporal-parietal junction (−50, −55, 25); mPFC, medial prefrontal cortex (0, 50, 20); PC, precuneus; PCC, posterior cingulate cortex (0, −60, 40); R, right; raIPS, right anterior intraparietal sulcus (40, −40, 45); rpSTS, right posterior superior temporal sulcus (50, −55, 10); rPMC, right premotor cortex (40, 5, 40); and rTPJ, right temporal-parietal junction (50, −55, 25).
fcMRI Analyses
Following functional magnetic resonance imaging preprocessing and removal of nuisance variables, the average BOLD time course was extracted from each seed and correlated with the time courses of all voxels across the brain (whole-brain voxelwise correlations), for every participant. The resulting correlation coefficients were converted to normally distributed z values (using Fisher r-to-z transformation) and entered into 1– and 2–independent sample(s) t tests to examine within- and between-group FC effects. All statistical maps were corrected for multiple comparisons with a cluster size-corrected threshold of P < .05, using Monte Carlo simulation.55
Summary Connectivity Scores and Correlations With Clinical Measures
To examine relationships between social impairment and FC, within- and between-network connectivity indices were computed by averaging z scores for all within- and between-network region of interest (ROI) pairs, respectively. To minimize multiple comparisons (and associated type I error), 4 a priori selected social-functioning measures were chosen for the correlational analyses with connectivity indices within the ASD cohort, including 3 diagnostic scores (2 ADI-R sociocommunicative components including ADI-R Social and ADI-R Communication, and the ADOS Communication + Social [CS] total score) and 1 parental report sociability score (SRS-Total). The relationship between FC and these 4 measures were examined using Spearman correlations because of the skewed distributions of the clinical measures. Because neither connectivity indices nor social measures were significantly correlated with age (all r’s < 0.22, all P’s > .56), age was excluded from any further analyses.
Results
Whole-Brain Connectivity
Results from the whole-brain within-group FC analyses performed for each of the 10 seeds are summarized in Figure 1 (see eTable 2 and 3 in Supplement for detailed descriptions, including peak coordinates). Direct group comparisons (corrected P < . 05) revealed no significant between-group differences in FC for any of the MNS seeds but several significant clusters of differential connectivity for the ToM network, including underconnectivity (TD > ASD) of the bilateral TPJ with the bilateral superior temporal gyri and PCC/precuneus, and overconnectivity (ASD > TD) of the mPFC with the superior parietal lobule (SPL) and middle temporal gyrus, and of PCC/precuneus with the middle frontal gyrus (MFG) and IFG (Figure 2A and Table 2).
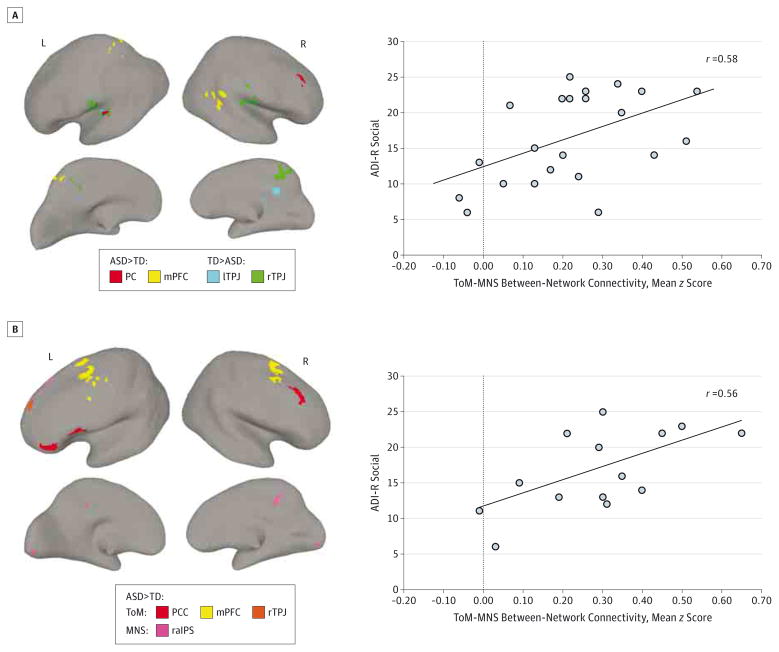
Figure 2. Regions Exhibiting Group Differences (Autism Spectrum Disorder [ASD] vs Typically Developing [TD]) in Functional Connectivity (FC) and the Relationship Between FC and Clinical Severity in the ASD Group.
A, Clusters of significantly different FC (P < .05, corrected) in participants with ASD relative to the TD participants are illustrated for the theory of mind (ToM) seeds. The scatterplot on the right shows the relationship between the ToM–mirror neuron system (MNS) overconnectivity (average z scores for all between-network region of interest pairs) and social symptomatology measured by the Autism Diagnostic Interview–Revised (ADI-R) Social scores (Spearman r25 = 0.58, P = .003). B, Clusters of significantly different FC (P < .05, corrected) in the subset of 15 participants with ASD and Autism Diagnostic Observation Schedule (ADOS) Communication + Social (CS) of 10 or greater and 15 matched TD participants. All depicted ToM and MNS seeds yielded overconnected clusters (ASD > TD). The scatterplot on the right shows the relationship between the ToM-MNS overconnectivity (average z scores for all between-network region of interest pairs) and social symptoms measured by the ADI-R Social scores (Spearman r15 = 0.56, P = .04). Increasing ADI-Social values indicate greater social impairment. lTPJ indicates left temporal-parietal junction; L, left; mPFC, medial prefrontal cortex; PC, precuneus; PCC, posterior cingulate cortex; raIPS, right anterior intraparietal sulcus, R, right; and rTPJ, right temporal-parietal junction.
Table 2.
Regions Exhibiting Group Differences (ASD vs TD) in FC, Separately for MNS and ToM Seedsa
| Seed | Peak Location | Talairach Coordinates | Cluster Volume, μL | t Score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| MNS | ||||||
| laIPS | None | |||||
| raIPS | None | |||||
| lPMC | None | |||||
| rPMC | None | |||||
| lpSTS | None | |||||
| rpSTS | None | |||||
| ToM | ||||||
| lTPJ | L, superior temporal gyrus/pSTS | −50 | −28 | 12 | 918 | 4.64 |
| lTPJ | R PCC | 20 | −34 | 26 | 918 | 3.75 |
| rTPJ | L, superior temporal gyrus/pSTS | −56 | −26 | 12 | 2808 | 4.60 |
| rTPJ | R/L, PCC | 2 | −44 | 50 | 2322 | 4.46 |
| rTPJ | R superior temporal gyrus/pSTS | 46 | −20 | 14 | 1485 | 4.47 |
| mPFC | L, PCC/SPL | −14 | −62 | 50 | 1755 | −4.82 |
| mPFC | R, middle temporal gyrus | 46 | −50 | 20 | 810 | −4.33 |
| PCC | R, middle frontal gyrus, IFG | 38 | 26 | 38 | 999 | −5.17 |
Abbreviations: ASD, autism spectrum disorder; FC, functional connectivity; IFG, inferior frontal gyrus; laIPS, left anterior intraparietal sulcus (−40, −40, 4); L, left; lPMC, left premotor cortex (−40, 5, 40); lpSTS, left posterior superior temporal sulcus (−50, −55, 10); lTPJ, left temporal-parietal junction (−50, −55, 25); MNS, mirror neuron system; mPFC, medial prefrontal cortex (0, 50, 20); PCC, posterior cingulate cortex (0, −60, 40); R, right; raIPS, right anterior intraparietal sulcus (40, −40, 45); rPMC, right premotor cortex (40, 5, 40); rpSTS, right posterior superior temporal sulcus (50, −55, 10); rTPJ, right temporal-parietal junction (50, −55, 25); SPL, superior parietal lobule; TD, typically developing; ToM, theory of mind.
Numbers in parentheses in Abbreviations refer to Talairach coordinates.
Summary Connectivity Indices and Their Relationship to Clinical Measures
Given this mixed pattern of both weaker and stronger BOLD correlations in the ToM, its connectivity was summarized with 2 separate indices calculated by averaging z scores for significantly underconnected and overconnected clusters, respectively. Because no significant clusters emerged in a direct between-group comparison of the MNS, its mean connectivity was computed by averaging z scores for all MNS ROI pairs. Finally, mean ToM-MNS between-network connectivity was estimated by averaging z scores for all between-network ROI pairs. A correlational matrix of 4 connectivity indices multiplied by 4 social measures yielded a Bonferroni-adjusted P < .05/16 = .003. Significant correlations were detected between ASD social symptoms and the extent of ToM overconnectivity (Table 3); namely, the mean z score for ToM overconnected clusters was correlated with ADI-R Social and ADI-R Communication scores (r = 0.45, P < .05 and r = 0.51, P < .01, respectively), although neither survived Bonferroni correction for multiple comparisons. While no significant FC group differences were detected for the MNS network, its average connectivity was positively correlated with ADI-R Social scores (r = 0.50, P = .01, uncorrected) such that greater MNS connectivity was associated with increase in social symptoms of ASD. Further, the ToM-MNS between-network connectivity was significantly correlated with ADI-R Social scores (r = 0.58, P = .003), indicating that greater ToM-MNS cross talk (atypically increased connectivity and reduced segregation between networks) was associated with more severe social impairment. Importantly, the relationship between abnormal ToM-MNS cross talk and greater social impairment in ASD does not generalize to other between-network patterns of connectivity, as detailed in eAppendix 2 and eTable 5 in Supplement, providing support to the notion that social dysfunction in ASD is specifically associated with inadequate segregation between 2 social networks, ToM and MNS.
Table 3.
Correlations Between Connectivity Indices and Social Symptoms Measures in Participants With Autism Spectrum Disordera
| Variable | ADOS-CS | ADI-R Social | ADI-R Communication | SRS Total |
|---|---|---|---|---|
| ToM overconnectivity (PCC, mPFC) | −0.29 | 0.45b | 0.51c | −0.04 |
| ToM underconnectivity (bilateral TPJ) | −0.14 | 0.22 | 0.05 | −0.42 |
| MNS connectivity | −0.10 | 0.50b | 0.47b | −0.26 |
| ToM-MNS between-network connectivity | −0.07 | 0.58d | 0.57c | 0.05 |
Abbreviations: ADI-R, Autism Diagnostic Interview–Revised; ADOS-CS, Autism Diagnostic Observation Schedule Communication + Social; MNS, mirror neuron system; mPFC, medial prefrontal cortex; PCC, posterior cingulate cortex; SRS, Social Responsiveness Scale; ToM, theory of mind; TPJ, temporal-parietal junction.
All correlation coefficients are calculated with Spearman rank correlations for n = 25.
P < .05 (uncorrected).
P < .01 (uncorrected).
P < .003 (Bonferroni corrected, P < .05).
Post hoc Analysis: Replication and Robustness of Findings in ASD Subset With Most Severe Symptoms
Based on these positive relationships between symptom severity and MNS and ToM-MNS FC, a post hoc FC analysis was performed in a subset of participants with ASD (n = 15) with the highest level of social symptoms as defined by ADOS-CS scores 10 or greater (see eFigure in Supplement for within-group connectivity maps). Direct group comparison of this ASD subsample and 15 TD participants optimally matched on age, motion, and IQ (eTable 4 in Supplement) corroborated earlier results of increased connectivity (ASD > TD) of the mPFC and PCC regions of ToM but also revealed increased, rather than weaker, connectivity (ASD > TD) of the right TPJ region of ToM (Figure 2B, Table 4). Notably, this analysis yielded a significant between-group difference in the MNS network, which was absent in the direct comparison of total samples, with greater connectivity (ASD > TD) between the right aIPS and left superior frontal gyrus and PCC (Figure 2B, Table 4). Finally, consistent with analyses for the entire cohort, positive correlation was detected between greater ToM-MNS between-network connectivity and ADI-Social scores (r = 0.56, P = .04; Figure 2B, right panel), although it did not survive Bonferroni correction for multiple comparisons.
Table 4.
Regions Exhibiting Group Differences in FC in a Subsample of 15 Participants With ASD and ADOS-CS Score 10 or Greater and 15 TD Controls, for MNS and ToM Seedsa
| Seed | Peak Location | Talairach Coordinates | Cluster Volume, μL | t Score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| MNS | ||||||
| laIPS | None | |||||
| raIPS | L, superior frontal gyrus | −16 | 26 | 48 | 1296 | −4.41 |
| raIPS | R/L, PCC | 2 | −38 | 36 | 837 | −3.86 |
| lPMC | None | |||||
| rPMC | None | |||||
| lpSTS | None | |||||
| rpSTS | None | |||||
| ToM | ||||||
| ITPJ | None | |||||
| rTPJ | L, middle frontal gyrus | −16 | 46 | 30 | 756 | −4.30 |
| mPFC | L, middle/superior frontal gyrus | −26 | −10 | 44 | 2808 | −4.12 |
| mPFC | R, superior/middle frontal gyrus | 28 | −8 | 56 | 1944 | −4.70 |
| PCC | L, IFG, p.Tri/p.Op | −52 | 14 | 18 | 1242 | −4.44 |
| PCC | R, middle frontal gyrus | 34 | 22 | 38 | 1269 | −4.66 |
| PCC | L, IFG, p.Tri/p.Orb | −46 | 40 | 2 | 810 | −4.51 |
Abbreviations: ASD, autism spectrum disorder; ADOS-CS, Autism Diagnostic Observation Schedule Communication + Social; FC, functional connectivity; IFG, inferior frontal gyrus; L, left; laIPS, left anterior intraparietal sulcus (−40, −40, 45); lPMC, left premotor cortex (−40, 5, 40); lpSTS, left posterior superior temporal sulcus (−50, −55, 10); lTPJ, left temporal-parietal junction (−50, −55, 25); MNS, mirror neuron system; mPFC, medial prefrontal cortex (0, 50, 20); PCC, posterior cingulate cortex/precuneus (0, −60, 40); raIPS, right anterior intraparietal sulcus (40, −40, 45); R, right; rPMC, right premotor cortex (40, 5, 40); rpSTS, right posterior superior temporal sulcus (50, −55, 10); rTPJ, right temporal-parietal junction (50, −55, 25); TD, typically developing; ToM, theory of mind.
Numbers in parentheses in Abbreviations refer to Talairach coordinates
Discussion
We used resting-state fcMRI to investigate FC in 2 brain networks crucial for social processing (ToM and MNS) in adolescents with ASD, relative to TD controls. In contrast to previous findings of predominantly reduced connectivity in ASD detected at rest in other functional networks,56–58 a mixed pattern of both overconnectivity and underconnectivity was observed in the ToM network. Namely, relative to TD participants, adolescents with ASD showed enhanced connectivity between mPFC and the SPL, precuneus and right posterior middle temporal gyrus, as well as between PCC/precuneus and the right middle and inferior frontal gyri. On the other hand, the ASD group showed weaker connectivity between the bilateral TPJ and PCC and superior temporal gyrus, including pSTS.
An unexpected finding was the lack of significant between-group differences in the MNS FC. However, when directly comparing a subset of the participants with ASD with the most severe sociocommunicative symptoms and a matched TD subsample, overconnectivity was detected between the raIPS region of the MNS and PCC, as well as between raIPS and the left superior frontal gyrus. This secondary analysis involving only the participants with ASD with the greatest symptom severity also revealed overconnectivity in 3 ToM seeds, namely between the rTPJ and the left MFG, mPFC and the bilateral superior and MFG, and PCC and the right MFG and left IFG. Remarkably, no underconnectivity effects were observed for this more homogeneous ASD subsample; instead, increased connectivity was detected for both MNS and ToM networks. These findings appear inconsistent with the theory of generally reduced long-distance connectivity in ASD59 or the more specific hypothesis of frontoparietal underconnectivity.26
Critically, close examination of the regional specificity of these findings, observed both in the entire sample and in the subset of participants with greatest symptom severity, revealed that atypical connectivity in ASD occurred between the regions of the MNS and ToM. For instance, in the analysis of the entire sample, the bilateral TPJ region of ToM showed reduced connectivity with the superior temporal gyrus, which included the pSTS region of the MNS. Similarly, clusters found to be overconnected with precuneus—a ToM seed—contained IFG, a canonical MNS region. Likewise, in the secondary subset analysis, clusters that emerged as significantly overconnected in both MNS and ToM networks also contained regions from the other network (Table 4); for instance, the raIPS seed of the MNS was overconnected (ASD > TD) with the PCC region of ToM. This pattern of atypical ToM-MNS cross talk suggests that the 2 social brain systems putatively impaired in ASD8,13–15 are less functionally segregated from one another in adolescents with ASD. This is in contrast with typical development, during which functional brain networks become simultaneously more integrated (within-network connections strengthen) and segregated (between-network connections weaken).36,60,61 Thus, the excess ToM-MNS connectivity observed in ASD may reflect immature or aberrant developmental processes in 2 brain networks involved in understanding others. Notably, this finding of atypical ToM-MNS cross talk is consistent with recently emerging evidence of reduced network segregation in ASD.62–65
Overconnectivity was most pronounced in a subsample of 15 participants with ASD with the highest symptom severity. As one possibility, cross talk between ToM and MNS, which largely accounted for the overconnectivity effects, might reflect a compensatory mechanism involving strengthening of the atypical connections secondary to social deficits. Specifically, the dynamic nature and complexity of social stimuli and social interactions may be overtaxing for inefficient neural networks in ASD; as a result, overconnectivity may be a consequence of an overuse of aberrant social circuits. The observed links between ToM-MNS cross-network connectivity and sociocommunicative symptom severity may support this interpretation. At the very least, these findings suggest that connectivity of and between the ToM and MNS networks plays a role in autistic symptomatology.
The detection of ToM overconnectivity in ASD is particularly noteworthy given the findings indicating reduced activation in the key ToM regions in ASD.18,57,66,67 On the other hand, greater ToM connectivity in ASD might be in line with evidence of reduced specialization of mentalizing brain regions in autism as demonstrated by activation for tasks that do not pertain to ToM.68,69 The ToM network is considered crucial for maneuvering in social contexts, as it supports the understanding of other people’s intentions and beliefs. Thus, our finding of ToM overconnectivity in ASD, especially in participants with greater symptom severity, may indicate a state of heightened activity associated with reduced efficiency and behavioral impairment in this domain.8,70
Our second hypothesis regarding links between atypical patterns of connectivity and social symptom severity was also supported. Robust positive correlations were detected between ToM and MNS overconnectivity and ASD sociocommunicative symptoms, as measured by the ADI-R Social and Communication scales, indicating that those with greater social impairment had more increased connections within and between these networks. In particular, the relationship between increased sociocommunicative symptoms and excessive, rather than reduced, ToM-MNS connectivity is consistent with the notion that social dysfunction is associated with inadequate segregation between the 2 social networks.62
While suggesting links between ToM and MNS connectivity and social impairment in ASD, our findings cannot establish causality. Atypical FC of these networks could reflect neurobiological abnormalities contributing to the emergence of social impairment. However, alternatively, abnormal social development in children with ASD may result in aberrant connectivity. This latter possibility is supported by evidence that network connectivity is affected by learning and experience-driven plasticity.54,71,72 Our findings may also reflect a combination of early causative and secondary, experience-driven effects. Notable in this context was the absence of correlations between connectivity measures and ADOS and SRS scores, both of which represent current abilities, contrasted by sizeable correlations between connectivity and ADI-R scores representing the early history of sociocommunicative impairment. While caution is required, given the nonexperimental nature of these measures, this pattern of findings could suggest that at least some of the atypical ToM and MNS connectivity observed here may reflect neural abnormalities possibly contributing to the early emergence of the disorder.
Among limitations of the present study is the exclusion of low-functioning adolescents with ASD, because of the extreme sensitivity of fcMRI to head motion.52,53 While head motion is clearly also an issue in studying high-functioning children, we used a number of procedures beyond conventional motion correction to minimize the effects of head movement. With this in mind, it cannot be determined whether our findings also apply to the lower end of the autistic spectrum.
Conclusions
In sum, the current results demonstrate atypical connectivity of and between ToM and MNS networks in adolescents with ASD, predominantly reflected in overconnectivity. Moreover, the extent of atypical connectivity was correlated with greater social dysfunction, suggesting that abnormal neural connections involving the mentalizing and mirror neuron systems are related to the social impairments observed in ASD.
Supplementary Material
Acknowledgments
Funding/Support: This work was supported by grants from the National Institutes of Health (grants R01 MH081023 to Dr Müller and K01 MH097972 to Dr Fishman) and the Autism Science Foundation (grant 12-1001 to Dr Fishman). Data acquisition in 7 participants was funded by a Congressionally Directed Medical Research Programs grant (grant AR093335 to Dr Pineda).
Footnotes
Conflict of Interest Disclosures: None reported.
Role of the Sponsor: The National Institutes of Health and the Autism Science Foundation had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Additional Contributions: Our strongest gratitude goes to the children and families who so generously dedicated their time and effort to this research. We also thank Mike Datko, MA, University of California San Diego, and Aarti Nair, MA, San Diego State University, for their help with data acquisition and technical assistance. They received no financial compensation.
Author Contributions: Dr Fishman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Fishman, Müller.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Fishman, Keown, Müller.
Critical revision of the manuscript for important intellectual content: Fishman, Lincoln, Pineda, Müller
Statistical analysis: Fishman, Keown, Lincoln, Müller.
Obtained funding: Müller.
Administrative, technical, or material support: Pineda.
Study supervision: Fishman, Lincoln, Müller.
Contributor Information
Inna Fishman, San Diego State University, San Diego, California.
Christopher L. Keown, San Diego State University, San Diego, California.
Alan J. Lincoln, Alliant International University, San Diego, California.
Jaime A. Pineda, University of California San Diego, La Jolla, California.
Ralph-Axel Müller, San Diego State University, San Diego, California.
References
- 1.Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators; Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders: autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61(3):1–19. [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- 3.Sigman M, Dijamco A, Gratier M, Rozga A. Early detection of core deficits in autism. Ment Retard Dev Disabil Res Rev. 2004;10(4):221–233. doi: 10.1002/mrdd.20046. [DOI] [PubMed] [Google Scholar]
- 4.Tager-Flusberg H. The origins of social impairments in autism spectrum disorder: studies of infants at risk. Neural Netw. 2010;23(8–9):1072–1076. doi: 10.1016/j.neunet.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”? Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- 6.Happé FGE. An advanced test of theory of mind: understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. 1994;24(2):129–154. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- 7.Kaland N, Møller-Nielsen A, Callesen K, Mortensen EL, Gottlieb D, Smith L. A new ‘advanced’ test of theory of mind: evidence from children and adolescents with Asperger syndrome. J Child Psychol Psychiatry. 2002;43(4):517–528. doi: 10.1111/1469-7610.00042. [DOI] [PubMed] [Google Scholar]
- 8.Frith U. Mind blindness and the brain in autism. Neuron. 2001;32(6):969–979. doi: 10.1016/s0896-6273(01)00552-9. [DOI] [PubMed] [Google Scholar]
- 9.Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8(9):396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996;3(2):131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 11.Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286(5449):2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- 12.Williams JHG, Whiten A, Singh T. A systematic review of action imitation in autistic spectrum disorder. J Autism Dev Disord. 2004;34(3):285–299. doi: 10.1023/b:jadd.0000029551.56735.3a. [DOI] [PubMed] [Google Scholar]
- 13.Oberman LM, Ramachandran VS. The simulating social mind: the role of the mirror neuron system and simulation in the social and communicative deficits of autism spectrum disorders. Psychol Bull. 2007;133(2):310–327. doi: 10.1037/0033-2909.133.2.310. [DOI] [PubMed] [Google Scholar]
- 14.Rizzolatti G, Fabbri-Destro M. Mirror neurons: from discovery to autism. Exp Brain Res. 2010;200 (3–4):223–237. doi: 10.1007/s00221-009-2002-3. [DOI] [PubMed] [Google Scholar]
- 15.Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25(4):287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
- 16.Dinstein I, Thomas C, Humphreys K, Minshew N, Behrmann M, Heeger DJ. Normal movement selectivity in autism. Neuron. 2010;66(3):461–469. doi: 10.1016/j.neuron.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48 (3):564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Castelli F, Frith C, Happé F, Frith U. Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes. Brain. 2002;125(pt 8):1839–1849. doi: 10.1093/brain/awf189. [DOI] [PubMed] [Google Scholar]
- 19.Spengler S, Bird G, Brass M. Hyperimitation of actions is related to reduced understanding of others’ minds in autism spectrum conditions. Biol Psychiatry. 2010;68(12):1148–1155. doi: 10.1016/j.biopsych.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Dapretto M, Davies MS, Pfeifer JH, et al. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9(1):28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2007;28(5):441–449. doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Brain Res Cogn Brain Res. 2005;24(2):190–198. doi: 10.1016/j.cogbrainres.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Williams JH, Waiter GD, Gilchrist A, Perrett DI, Murray AD, Whiten A. Neural mechanisms of imitation and ‘mirror neuron’ functioning in autistic spectrum disorder. Neuropsychologia. 2006;44(4):610–621. doi: 10.1016/j.neuropsychologia.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Belmonte MK, Cook EH, Jr, Anderson GM, et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol Psychiatry. 2004;9(7):646–663. doi: 10.1038/sj.mp.4001499. [DOI] [PubMed] [Google Scholar]
- 25.Müller RA. The study of autism as a distributed disorder. Ment Retard Dev Disabil Res Rev. 2007;13 (1):85–95. doi: 10.1002/mrdd.20141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schipul SE, Keller TA, Just MA. Inter-regional brain communication and its disturbance in autism. Front Syst Neurosci. 2011;5:10. doi: 10.3389/fnsys.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vissers ME, Cohen MX, Geurts HM. Brain connectivity and high functioning autism: a promising path of research that needs refined models, methodological convergence, and stronger behavioral links. Neurosci Biobehav Rev. 2012;36 (1):604–625. doi: 10.1016/j.neubiorev.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31 (3):137–145. doi: 10.1016/j.tins.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Courchesne E, Pierce K, Schumann CM, et al. Mapping early brain development in autism. Neuron. 2007;56(2):399–413. doi: 10.1016/j.neuron.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 30.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 31.Allen EA, Erhardt EB, Damaraju E, et al. A baseline for the multivariate comparison of resting-state networks. Front Syst Neurosci. 2011;5:2. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmons WK, Martin A. Spontaneous resting-state bold fluctuations map domain-specific neurocircuitry. Soc Cogn Affect Neurosci. 2012;7(4):467–475. doi: 10.1093/scan/nsr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fair DA, Cohen AL, Power JD, et al. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5(5):e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fair DA, Dosenbach NUF, Church JA, et al. Development of distinct control networks through segregation and integration. Proc Natl Acad Sci U S A. 2007;104(33):13507–13512. doi: 10.1073/pnas.0705843104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Honey CJ, Sporns O, Cammoun L, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106(6):2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Damoiseaux JS, Rombouts SA, Barkhof F, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shehzad Z, Kelly AM, Reiss PT, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19(10):2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Dijk KRA, Hedden T, Venkataraman A, et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo XN, Di Martino A, Kelly C, et al. The oscillating brain: complex and reliable. Neuroimage. 2010;49(2):1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutter M, LeCouteur A, Lord C. Autism Diagnostic Interview–Revised (ADI-R) Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- 45.Lord C, Risi S, Lambrecht L, et al. The Autism Diagnostic Observation Schedule-Generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 46.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 47.Constantino JN, Gruber CP. Social Responsiveness Scale (SRS) Manual. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- 48.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 49.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 50.Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain. Stuttgart, Germany: Georg Thieme Verlag/Thieme Medical Publishers; 1988. [Google Scholar]
- 51.Cordes D, Haughton VM, Arfanakis K, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR Am J Neuroradiol. 2001;22(7):1326–1333. [PMC free article] [PubMed] [Google Scholar]
- 52.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevens WD, Buckner RL, Schacter DL. Correlated low-frequency BOLD fluctuations in the resting human brain are modulated by recent experience in category-preferential visual regions. Cereb Cortex. 2010;20(8):1997–2006. doi: 10.1093/cercor/bhp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 56.Assaf M, Jagannathan K, Calhoun VD, et al. Abnormal functional connectivity of default mode sub-networks in autism spectrum disorder patients. Neuroimage. 2010;53(1):247–256. doi: 10.1016/j.neuroimage.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kennedy DP, Courchesne E. Functional abnormalities of the default network during self- and other-reflection in autism. Soc Cogn Affect Neurosci. 2008;3(2):177–190. doi: 10.1093/scan/nsn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weng SJ, Wiggins JL, Peltier SJ, et al. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain Res. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Minshew NJ, Williams DL. The new neurobiology of autism: cortex, connectivity, and neuronal organization. Arch Neurol. 2007;64(7):945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dosenbach NU, Nardos B, Cohen AL, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7(7):e1000157. doi: 10.1371/journal.pbio.1000157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shih P, Keehn B, Oram JK, Leyden KM, Keown CL, Müller RA. Functional differentiation of posterior superior temporal sulcus in autism: a functional connectivity MRI study. Biol Psychiatry. 2011;70(3):270–277. doi: 10.1016/j.biopsych.2011.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rudie JD, Shehzad Z, Hernandez LM, et al. Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb Cortex. 2012;22(5):1025–1037. doi: 10.1093/cercor/bhr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rudie JD, Brown JA, Beck-Pancer D, et al. Altered functional and structural brain network organization in autism. Neuroimage Clin. 2012;2:79–94. doi: 10.1016/j.nicl.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nebel MB, Joel SE, Muschelli J, et al. Disruption of functional organization within the primary motor cortex in children with autism. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of theory of mind regions in autism during mental state attribution. Soc Neurosci. 2009;4(2):135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lombardo MV, Chakrabarti B, Bullmore ET, Baron-Cohen S MRC AIMS Consortium. Specialization of right temporo-parietal junction for mentalizing and its relation to social impairments in autism. Neuroimage. 2011;56(3):1832–1838. doi: 10.1016/j.neuroimage.2011.02.067. [DOI] [PubMed] [Google Scholar]
- 68.Wang AT, Lee SS, Sigman M, Dapretto M. Reading affect in the face and voice: neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders. Arch Gen Psychiatry. 2007;64(6):698–708. doi: 10.1001/archpsyc.64.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: an fMRI study. Soc Neurosci. 2008;3(2):97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- 70.Tager-Flusberg H. Evaluating the theory-of-mind hypothesis of autism. Curr Dir Psychol Sci. 2007;16(6):311–315. doi: 10.1111/j.1467-8721.2007.00527.x. [DOI] [Google Scholar]
- 71.Hasson U, Nusbaum HC, Small SL. Task-dependent organization of brain regions active during rest. Proc Natl Acad Sci U S A. 2009;106(26):10841–10846. doi: 10.1073/pnas.0903253106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106(41):17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.