Abstract
Background
The drug–drug interactions between pitavastatin and darunavir/ritonavir (DRV/r) as well as pitavastatin and efavirenz (EFV) were examined in an open-label, parallel-arm, pharmacokinetic (PK) study in HIV-uninfected healthy volunteers.
Methods
Subjects received a pitavastatin dose of 2 mg for 4 days, followed by either EFV 600 mg (n = 14) or DRV/r 800/100 mg (n = 14) daily for 10 days, and pitavastatin 2 mg coadministered with EFV 600 mg or DRV/r 800/100 mg for 4 days. Full PK profiles were determined for pitavastatin and its lactone metabolite on days 4 and 18 and for EFV or DRV on days 14 and 18.
Results
In the EFV arm, the geometric mean area under the concentration time curve (AUC0-τ) and Cmax of pitavastatin were 85.3 ng·h·mL−1 and 15.6 ng/mL, respectively, when given alone, versus 76 ng·h·mL−1 and 18.8 ng/mL when coadministered with EFV. The geometric mean ratio for pitavastatin with EFV versus alone was 0.89 [90% confidence interval (CI): 0.73 to 1.09] for AUC0-τ and 1.20 (90% CI: 0.79 to 1.83) for Cmax. In the DRV/r arm, AUC0-τ and Cmax were 62.8 ng·h·mL−1 and 24.0 ng/mL, respectively, when pitavastatin was administered alone, versus 56.9 ng·h·mL−1 and 23.2 ng/mL when coadministered with DRV/r. The geometric mean ratio for pitavastatin with DRV/r versus alone was 0.91 (90% CI: 0.78 to 1.06) for AUC0-τ and 0.93 (90% CI: 0.72 to 1.19) for Cmax.
Conclusions
There were no significant PK interactions between pitavastatin and EFV or DRV/r. No significant safety issues or lipid changes were noted.
Keywords: pitavastatin, efavirenz, darunavir, ritonavir, HMG-CoA reductase inhibitors, statins, drug interactions
INTRODUCTION
HIV-infected individuals are at increased risk of coronary heart disease because of many factors including traditional risk, chronic inflammation caused by the virus itself, and lipid metabolism abnormalities associated with the progression of HIV disease and potential toxicities attributed to certain antiretroviral drugs. The guidelines of the HIV Medical Association (HIVMA) of the Infectious Disease Society of America (IDSA) support managing dyslipidemia in HIV-infected patients similar to the general population.1 Essential tools for the management of dyslipidemia in HIV-infected persons are diet, exercise, smoking cessation, lipid-lowering drugs, and in particular, 3-hydroxyl-3-methylglutaryl-coenzyme A reductase inhibitors (statins). Pitavastatin, the most recently approved statin in the United States, has been shown to have potent effects on low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol in the general population, including people with metabolic syndrome and type 2 diabetes.2 Significant reductions in total cholesterol and LDL-C have also been demonstrated in HIV-infected individuals.3 Although most statins are metabolized primarily through the CYP3A4 isozyme, pitavastatin is metabolized by glucuronidation and offers the potential for reduced drug–drug interactions (DDIs) when coadministered with antiretrovirals. The protease inhibitor (PI), darunavir with ritonavir pharmacokinetic (PK) enhancement (DRV/r) and the nonnucleoside reverse transcriptase inhibitor (NNRTI), efavirenz (EFV) are 2 of the most potent and commonly used antiretrovirals but have well described DDIs when coadministered with many statins.4,5
Ritonavir is a potent inhibitor of CYP3A4 among PIs, which has led to its use as a PK enhancer for other PIs including DRV. The coadministration of ritonavir-enhanced PIs with most statins is a cause for concern in terms of DDIs and can result in elevated plasma concentrations of statins and muscle-associated adverse effects such as myositis and rhabdomyolysis.6 A PK study assessing the potential DDIs of lopinavir/ritonavir in combination with pitavastatin showed that concomitant administration of both drugs was safe and well tolerated.7
EFV is a potent inducer of the CYP3A4 isozyme and interacts with statins that are metabolized by CYP3A4. When coadministered with statins such as simvastatin, atorvastatin, and pravastatin, EFV acts as a potent inducer of statin metabolism and requires an increase in statin dose to achieve lipid goals.5,8
The objectives of this study were to evaluate potential drug interactions between EFV or DRV/r in combination with pitavastatin.
METHODS
This study was a phase I, open-label, parallel 2-arm, drug interaction study to investigate the effects of EFV and DRV/r on the PK of pitavastatin. This study was performed in healthy adult HIV-1 seronegative volunteers to avoid the potential development of drug resistance in HIV-infected persons exposed to DRV/r or EFV monotherapy. The primary objective of the study was to investigate the effect of EFV and DRV/r on the PK of pitavastatin. Secondary objectives were to investigate the effects of pitavastatin on the PK of EFV and DRV/r and to describe the non–steady-state changes in serum LDL-C due to pitavastatin in the presence of EFV or DRV/r.
Subjects
The study population consisted of male and female HIV-seronegative healthy volunteers, 18–60 years of age. Subjects did not have any chronic underlying illnesses and could not be taking chronic medications. Subjects had to weigh within 20% of the normal body weight (body mass index, 18–25) and have a minimum weight of 50 kg. All enrolled volunteers were required to have baseline laboratory values including hemogram and renal function within normal limits and aspartate aminotransferase (AST) and alanine aminotransferase (ALT) up to 1.5× upper limit of normal. Both male and female subjects (except for those documented as not of reproductive potential) had to agree to practice adequate birth control using a combination of 2 accepted birth control methods. Females of childbearing potential had to have a negative pregnancy test within 14 days before study entry, on day of entry, and before each PK visit. Subjects could not have participated in any investigational drug studies within 30 days before study entry. Subjects were enrolled between August 24, 2012 and January 8, 2013. The final study visit for the last subject was completed on February 20, 2013.
Study Design
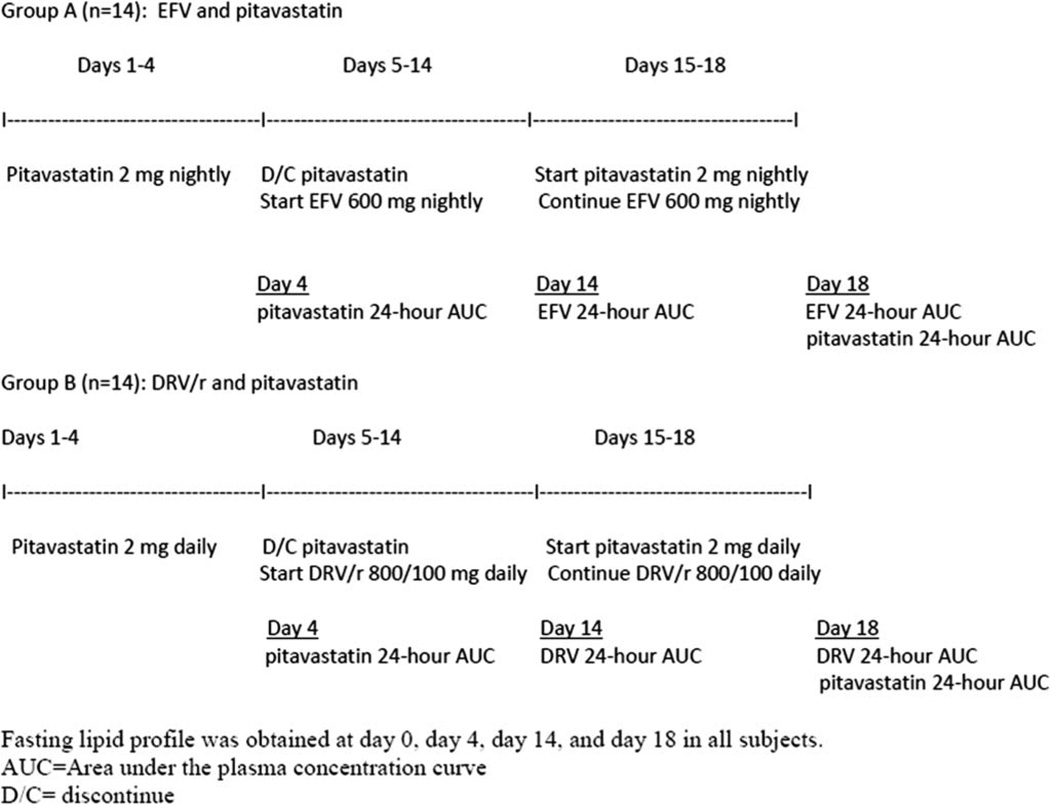
Subjects were recruited to participate in 1 of the 2 parallel study arms. The study design has been previously described,5 and a schematic outline is shown in Figure 1. For the first 3 days, subjects took pitavastatin 2 mg daily. On the fourth day, pitavastatin was administered under direct observation. From day 5 through 13, subjects self-administered 1 tablet of EFV 600 mg (arm A) or 2 tablets of DRV 400 mg and 1 tablet of ritonavir 100 mg (arm B) daily followed by directly observed dosing on day 14. From day 15 through 17, subjects took both pitavastatin 2 mg and EFV 600 mg (arm A) or pitavastatin 2 mg and DRV/r 800/100 mg (arm B) followed by directly observed dosing on day 18. Blood samples were collected before drug administration and at 30 minutes, 1, 2, 3, 4, 6, 8, 12, and 24 hours postdose on days 4, 14, and 18. In arm A, pitavastatin and EFV were administered at night, and PK sampling was scheduled to begin at night, whereas in arm B, drug dosing and PK sampling were completed in the morning. Serum aliquots were stored at −70 °C within 30 minutes of collection.
FIGURE 1.
Schematic design.
Compliance with study medications was measured through pill counts and an adherence questionnaire. In addition to PK blood samples, fasting lipid profiles were collected on days 0, 4, 14, and 18 for all subjects. The protocol was approved by the New York University School of Medicine and University at Buffalo Institutional Review Board. Informed consent was obtained from all subjects.
Drug Assays
Day 4 and day 18 samples from subjects on arms A and B were assayed for pitavastatin and its primary metabolite, pitavastatin lactone, at PPD Bionalytical Laboratories (Middleton, WI). Liquid chromatography/tandem mass spectrometry was used to measure the drug concentrations according to the validated PPD Method P1097.01. The lower limit of quantitation values were 0.960 and 1.00 ng/mL for pitavastatin and pitavastatin lactone, respectively. Incurred sample reproducibility was within the accepted standard of ≤20% relative difference between the original and the repeated sample values.
Day 14 and day 18 samples for subjects in arms A and arm B were assayed to quantify concentrations of EFV and DRV in the Translational Pharmacology Research Core Laboratory at University at Buffalo. EFV and DRV were quantified using a previously published simultaneous high-performance liquid chromatography assay with slight modification.9 Briefly, the method involved a liquid–liquid extraction of the plasma sample, followed by separation on a C8 Symmetry column (Waters Associates, Milford, MA) and detection using a Model 996 photodiode array detector (Waters Associates) to assure specificity. The lower limit of quantitation value was 100 ng/mL. During the course of assay application, interassay variation was ≤15%. Verification of accuracy for EFV and DRV concentrations was performed in conjunction with the Clinical Pharmacology Quality Assurance (CPQA) and Quality Control Program.10
Serum total cholesterol, triglycerides, and high-density lipoprotein concentrations were assayed at the Bellevue Hospital Laboratory using standard enzymatic assays. Calculated LDL-C was determined using the Friedewald equation.
Concentration data were used to calculate steady-state plasma PK parameters for pitavastatin, the lactone metabolite of pitavastatin, EFV, and DRV. PK parameters included maximum plasma concentration (Cmax), area under the plasma concentration curve from time 0 to the last measurable concentration (AUC0-τ), and time to achieve maximum plasma concentration (Tmax). Actual sample times were used rather than scheduled sampling times. AUCs were estimated according to the linear trapezoidal rule, without extrapolation beyond the dosing interval.
Statistical Analyses
Statistical analyses were performed using Minitab 16.2.4 software (Minitab Inc., State College, PA). Using a linear mixed-effects model, with treatment as a fixed effect and subject as a random effect, mean log-transformed Cmax and AUC0-τ of pitavastatin alone without EFV or DRV/r (day 4) were compared with those of pitavastatin when coadministered with EFV or DRV/r (day 18) for study arms A and B, respectively.
Similarly, using the same model, mean log-transformed PK parameters for EFV alone and DRV/r alone (day 14) were compared with those of EFV and DRV/r when coadministered with pitavastatin (day 18) for study arms A and B, respectively. The 90% confidence intervals (CIs) for geometric mean ratio of test-to-reference treatments (day 18 vs. day 4 and day 18 vs. day 14) were calculated to evaluate potential for a DDI.
No formal sample size calculation was performed. A sample size of 14 subjects per study arm was considered adequate to detect a possible interaction between steady-state EFV or DRV/r and pitavastatin. Subjects who did not complete the 3 PK evaluation visits were replaced to achieve the required sample size.
RESULTS
A total of 57 subjects were screened with 16 screen failures. Eighteen subjects enrolled in arm A (EFV) and 1 withdrew after starting pitavastatin before the day 4 PK visit because of scheduling conflicts. Three subjects withdrew from arm A because of adverse events: headache (grade 1) (n = 1) on day 5, rash (grade 1) (n = 1) on day 14, and moderate headache (grade 2) and nausea (grade 2) (n = 1) on day 18. Fourteen subjects completed all visits in arm A. Twenty-three subjects were enrolled in arm B. Seven subjects never returned for visit 1 and withdrew before starting study medication. Two subjects were withdrawn because of adverse effects (grade 2 rash while taking DRV/r only), and 14 subjects completed all visits.
Because of flooding and the subsequent power failure and evacuation of Bellevue Hospital caused by Hurricane Sandy on October 30, 2012, study samples stored in a −80 °C freezer had to be packed in dry ice and shipped to a temporary storage facility (Cryostar, Long Island, NY). At the time of retrieval of the samples, samples belonging to 4 arm B subjects that were to be shipped for pitavastatin analysis were lost. As a result, pitavastatin analysis was performed on the remaining 10 of the 14 subjects that completed arm B. Thirteen of the 14 subjects in arm A and all 14 subjects in arm B reported taking 100% of their prescribed doses, and high adherence was confirmed by pill counts.
The median age in arm A was 43.5 years, with a range from 19 to 57 years. Twenty-nine percent were women, 29% were black, 14% were Asian, and 21% were Hispanic. The median age in arm B was 36.5 years, with a range from 19 to 59 years. Forty-three percent were women, 43% were black, 14% were Asian, and 21% were Hispanic. Median body mass index was 25.8 in arm A and 27.1 in arm B.
Plasma Concentration–Time Curves for Pitavastatin and EFV and Pitavastatin and DRV/r
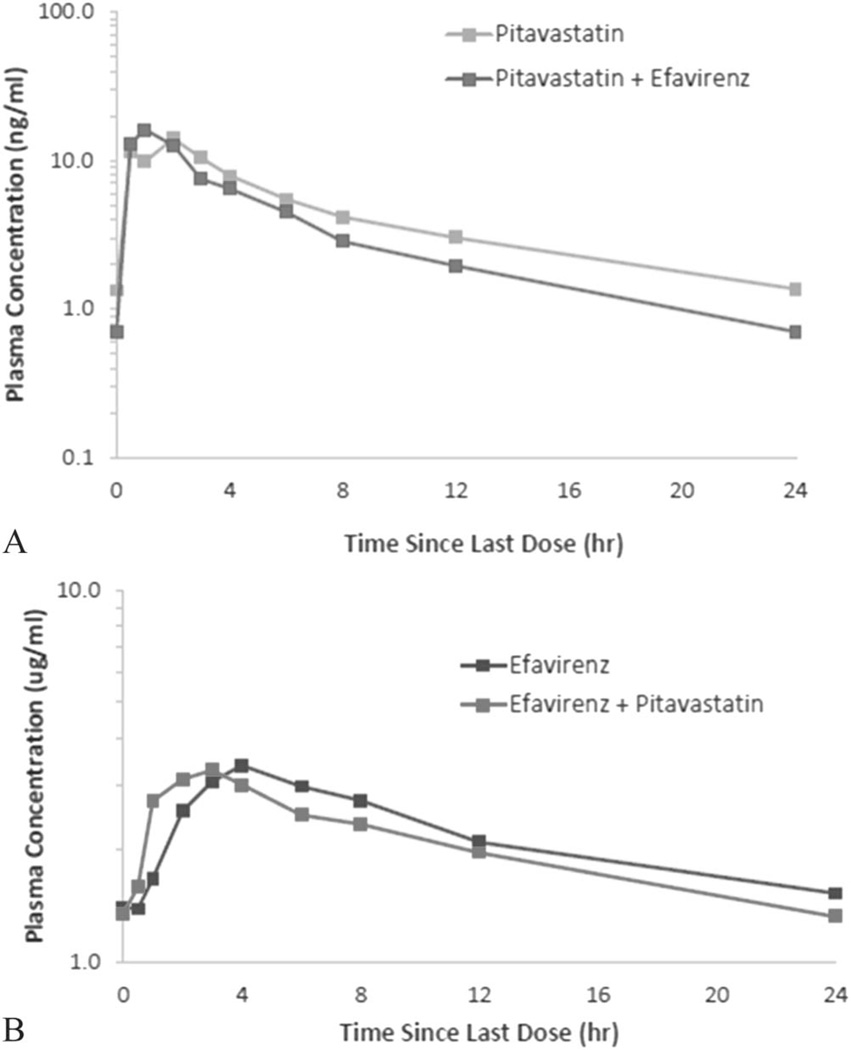
Mean plasma concentrations of pitavastatin alone and pitavastatin coadministered with EFV were similar up to 6 hours after dosing, and thereafter, pitavastatin concentrations at steady state were slightly lower than that with EFV coadministration (Fig. 2A). Mean plasma concentrations of EFV peaked slightly earlier than that with pitavastatin coadministration but remained similar throughout the entire 24-hour PK period (Fig. 2B).
FIGURE 2.
A, Pitavastatin mean plasma concentration in the presence and absence of EFV (N = 14). B, EFV mean plasma concentration in the presence and absence of pitavastatin (N = 14).
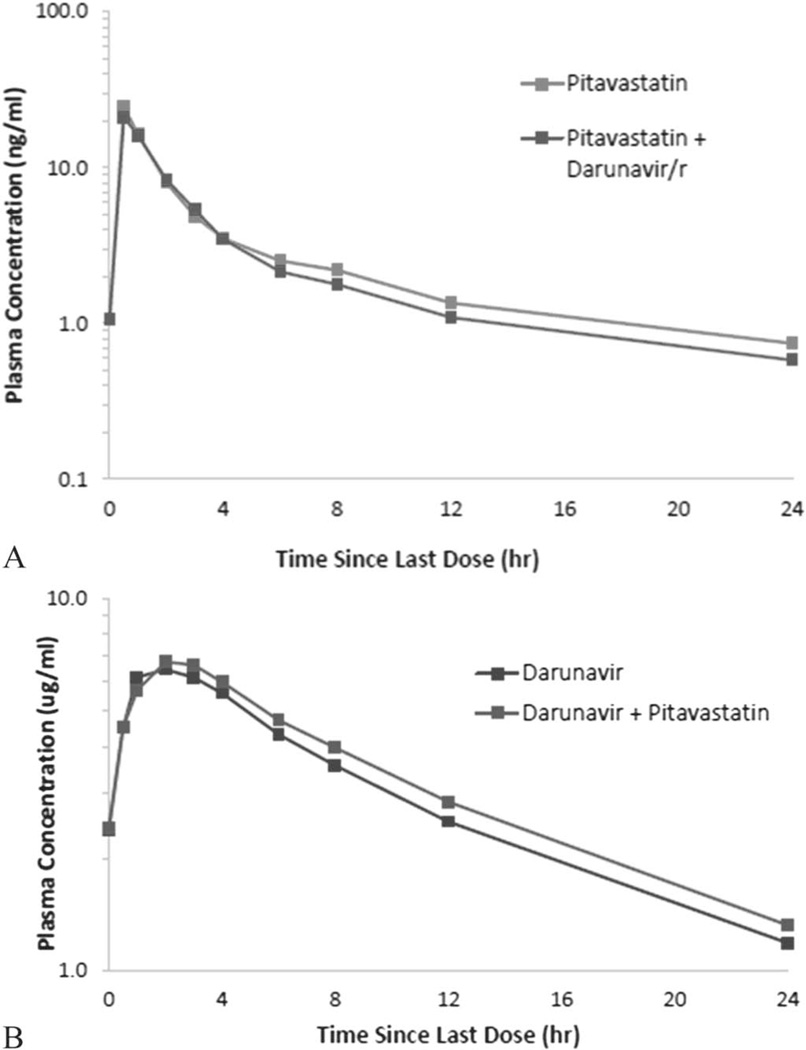
Mean plasma concentrations of pitavastatin were similar between pitavastatin alone and coadministered with DRV/r throughout the 24-hour PK period (Fig. 3A). Mean plasma DRV concentrations also remained similar when DRV/r was administered alone and when it was coadministered with pitavastatin (Fig. 3B).
FIGURE 3.
A, Pitavastatin mean plasma concentration in the presence and absence of DRV/r (N = 10). B, DRV mean plasma concentration in the presence and absence of pitavastatin (N = 14).
PK Parameters
Statistical analysis of PK parameters of pitavastatin, EFV, and DRV is shown in Tables 1 and 2. Mean Cmax of pitavastatin at steady state was similar between pitavastatin alone and that with EFV or DRV/r coadministration. Mean AUC0-τ was lower, whereas mean Cmax was slightly higher when pitavastatin was coadministered with EFV than when pitavastatin was administered alone. Coadministration of pitavastatin with EFV decreased the AUC0-τ of pitavastatin by approximately 11% and increased mean Cmax by approximately 20% (Table 1). Median Tmax of pitavastatin was 2.0 hours (range, 0.9–3.0 hours) when it was administered alone and 1.5 hours (range, 1.0–2.3 hours) when it was coadministered with EFV. Mean AUC0-τ and Cmax for EFV decreased by approximately 10% when EFV was coadministered with pitavastatin than when it was administered alone (Table 1). In arm B, coadministration of DRV/r decreased mean AUC0-τ and mean Cmax of pitavastatin by 9% and 7%, respectively (Table 2). Median Tmax of pitavastatin was approximately 0.5 hours when it was administered alone and remained unchanged when it was coadministered with DRV/r. Mean AUC0-τ and mean Cmax of DRV increased nonsignificantly by 8% and 3% when coadministered with pitavastatin compared with DRV/r administered alone.
TABLE 1.
PK Parameter Analysis of Pitavastatin and EFV
| Geometric Mean | Geometric Mean Ratio (90% CI) | |||
|---|---|---|---|---|
| PK Parameter | N | Pitavastatin With EFV | Pitavastatin Only | Pitavastatin With EFV/Pitvastatin Only |
| AUC0-τ, ng·h·mL−1 | 14 | 76 | 85.3 | 0.89 (0.73 to 1.09) |
| Cmax, ng/mL | 14 | 18.8 | 15.6 | 1.20 (0.79 to 1.83) |
| Tmax, h* | 14 | 1.5 (1.0 to 2.3) | 2.0 (0.9 to 3.0) | |
| EFV With Pitavastatin | EFV Only | EFV With Pitavastatin/EFV Only | ||
| AUC0-τ, µg·h·mL−1 | 14 | 44.4 | 49.5 | 0.90 (0.75 to 1.06) |
| Cmax, µg·mL−1 | 14 | 3.72 | 4.15 | 0.90 (0.66 to 1.22) |
| Tmax, h* | 14 | 1.5 (1.0 to 2.3) | 2.0 (0.9 to 3.0) | |
Tmax reported as median (IQR).
TABLE 2.
PK Parameter Analysis of Pitavastatin and DRV/r
| Geometric Mean | Geometric Mean Ratio (90% CI) | |||
|---|---|---|---|---|
| PK Parameter | N | Pitavastatin With DRV/r | Pitavastatin Only | Pitavastatin With DRV/r/Pitavastatin Only |
| AUC0-τ, ng·h·mL−1 | 10 | 56.9 | 62.8 | 0.91 (0.78 to 1.06) |
| Cmax, ng/mL | 10 | 23.2 | 24 | 0.93 (0.72 to 1.19) |
| Tmax, h* | 10 | 0.5 (0.5 to 1.0) | 0.5 (0.5 to 0.5) | |
| DRV/r With Pitavastatin | DRV/r Only | DRVr With Pitavastatin/DRV/r Only | ||
| AUC0-τ, µg·h·mL−1 | 14 | 76.7 | 70.9 | 1.08 (0.96 to 1.22) |
| Cmax, µg/mL | 14 | 6.78 | 6.61 | 1.03 (0.91 to 1.15) |
| Tmax, h* | 14 | 0.5 (0.5 to 1.0) | 0.5 (0.5 to 0.5) | |
Tmax reported as median (IQR).
Pitavastatin lactone PK parameters for arms A and B are available in the Supplemental Digital Content (see Table, http://links.lww.com/QAI/A564). Mean pitavastatin lactone AUC0-τ was decreased by approximately 14%, whereas mean pitavastatin lactone Cmax was unchanged when pitavastatin was coadministered with EFV compared with pitavastatin alone. When pitavastatin was coadministered with DRV/r, there was a moderate reduction in pitavastatin lactone AUC0-τ by approximately 37%, whereas mean lactone Cmax was reduced by about 12% compared with pitavastatin alone. Median lactone Tmax decreased from 2.0 hours with pitavastatin alone to 1.0 hours with DRV/r coadministration.
Secondary End Point Results
For both arms A and B, mean LDL decreased from day 0–4 when subjects were taking pitavastatin only and again from day 14 to day 18 when subjects were exposed to pitavastatin and either EFV or DRV/r. Off pitavastatin, on days 5 through 14, there is an expected return of LDL-C toward baseline while on EFV or DRV/r alone (mean lipid parameter trends are shown in the Supplemental Figures: see Supplemental Digital Content, http://links.lww.com/QAI/A564). Exploratory analysis of LDL-C was a secondary objective, and the study was not powered to detect significant differences in lipids in this brief time period.
Safety
In arm A, the most common adverse events were headaches (n = 4, 22%), dizziness (n = 3, 17%), nausea (n = 2, 11%), and rhinorrhea (n = 2, 11%). One subject withdrew from the study on day 18 because of grade 2 dizziness and nausea, which resolved several hours after taking study drug.
In arm B, the most common adverse event was rash in 2 subjects (9%) who experienced a diffuse rash soon after starting DRV/r only and were withdrawn from the study. The rash resolved in both cases within a few days after discontinuing study drug. It is believed that these 2 subjects had a previously unreported sulfa allergy and had a reaction to DRV. There were no documented episodes of myopathy. There were 4 subjects who experienced asymptomatic elevations in CPK on day 18, and 1 subject (in arm B) had a CPK 4.4 times higher than that of upper limit of normal, which was considered a grade 1 toxicity per study protocol.
DISCUSSION
This study demonstrated no clinically significant PK interactions between pitavastatin 2 mg daily and EFV 600 mg daily or DRV/r 800 mg/100 mg daily in healthy volunteers. To the best of our knowledge, this represents one of the first statins to have no significant interactions with these ART regimens. This is important clinically as DRV/r and EFV are both preferred first-line antiretroviral agents and are widely used for the treatment of HIV.11 Clinically significant DDIs between ritonavir-enhanced PIs and statins are well described in the scientific literature.6,12 A majority of statins are substrates of the CYP3A pathway, whereas ritonavir-enhanced PIs are potent inhibitors of CYP3A4, and their coadministration can result in significantly increased statin exposure, associated toxicity, and rhabdomyolysis.13 For patients taking ritonavir-enhanced PIs, simvastatin and lovastatin are contraindicated, whereas atorvastatin and rosuvastatin must be started at the lowest possible dose with close monitoring and have maximum dose restrictions.4,6
Unlike other statins, pravastatin is metabolized primarily by glucuronidation and minimally by CYP3A4 and can be administered without dose adjustment with many PIs. However, the exposure of pravastatin is reduced by about 50% when combined with nelfinavir or saquinavir, decreasing its lipid-lowering efficacy.12,14 Because pravastatin is not metabolized by CYP3A4, but is inactivated by conjugation and non-CYP3A4 induced hydroxylation, the hepatic inductive effect of ritonavir on the conjugative enzymes and other CYPs may be responsible for this interaction.14 However, pravastatin exposure as indicated by AUC is increased modestly by only 33% by coadministration with lopinavir/ritonavir15 but is increased by 81% in the presence of DRV/r warranting precautionary labeling.16
Uptake transporters such as organic anion–transporting polypeptide 1B1 (OATP1B1) also play a role in the PK of most statins. Simvastatin, pitavastatin, atorvastatin, pravastatin, and rosuvastatin are substrates of OATP1B1, which is encoded by the SLCBO1B1 gene.17,18 PIs can inhibit OATP1B1 although the potency of inhibition varies between specific PIs.19 Certain single-nucleotide polymorphisms of the SLCO1B1 gene alter hepatic uptake and increase plasma concentrations of statins.20,21 Variations in genes encoding for other carriers affecting the elimination of statins from cells, such as the breast cancer resistance protein and the P-glycoprotein multidrug restiance protein, may also play a role.22 Thus, the effect of PIs on the PK of statins is mediated by multiple mechanisms including CYP- and transporter-mediated inhibition, which may be further modified in individuals by their genetic characteristics. In the case of pravastatin, subjects with low-functioning haplotype forms of the SLCO1B1 gene had significantly increased pravastatin exposure compared with those with wild-type forms, an effect that was not significantly altered by the presence of DRV/r.21
Pitavastatin shares similar metabolism and transport characteristics with pravastatin: it is also metabolized primarily by glucuronidation and only minimally by CYP enzymes, and it is taken up into human hepatocytes by OATP1B1.18 In contrast to pravastatin, pitavastatin PK parameters do not seem to be considerably affected by coadministration of ritonavirenhanced PIs. Previous studies in healthy volunteers demonstrated only a slight effect of lopinavir/r coadministration on pitavastatin PK parameters. Pitavastatin AUC was reduced by ~20%, and Cmax was unaffected with concurrent use of pitavastatin 4 mg once daily and lopinavir/r 400/100 mg twice a day.7 When combined with ritonavir-boosted atazanavir, a 31% increase in the pitavastatin AUC was observed.23
In this study, pitavastatin peak (Cmax) and total exposures (AUC0-τ) were only marginally reduced by 7% and 9%, respectively, with concomitant use of pitavastatin 2 mg and DRV/r 800/100 mg daily. Coadministration of pitavastatin did not affect DRV total and peak exposures (increase of <10% in AUC0-τ and Cmax). A previous PK study in healthy volunteers showed similar results when pitavastatin 4 mg daily was coadministered with DRV/r 800/100 mg with a 26% reduction in pitavastatin AUC0-τ and minimal or no change in Cmax.24
NNRTIs have varied DDIs with statins. Nevirapine is a selective inducer of CYP3A4, whereas EFV is a mixed inducer/inhibitor of CYP3A4.25 The second-generation NNRTI etravirine is an inducer of CYP3A and inhibits CYP2C9 and CYP2C19. Coadministration of etravirine 200 mg twice a day with atorvastatin 40 mg daily resulted in an unchanged AUC, but a 37% reduction in Cmax of atorvastatin.26 Through induction of CYP3A4, EFV decreases the simvastatin AUC by 58% and atorvastatin AUC by 43%. Surprisingly, EFV also reduces the pravastatin AUC by 40%, although pravastatin is minimally metabolized by CYP3A4 and EFV does not induce glucuronidation.5 The exact mechanism whereby EFV coadministered with pravastatin results in a moderate reduction in pravastatin AUC is unknown. It has been suggested that EFV may induce non-CYP3A4 oxidation or organic anion transporters such as OATP1B1, MRP-2, or others, resulting in increased hepatic elimination of pravastatin.14,27,28
In this study, at steady-state pitavastatin peak concentrations (Cmax) were increased by 20%, whereas total exposure (AUC0-τ) was slightly decreased by 11% when pitavastatin 2 mg was coadministered with EFV 600 mg. Coadministration of pitavastatin had a limited effect on EFV total and peak exposures, a decrease of approximately 10% in EFV AUC0-τ and Cmax. Coadministration of EFV slightly reduced pitavastatin lactone AUC0-τ (14%), whereas DRV/r had a moderate effect on the AUC0-τ of pitavastain lactone, a 37% reduction at steady state. However, pitavastatin lactone is an inactive metabolite and is not expected to have any significant effect on the lipid-lowering efficacy or safety parameters of pitavastatin.
In summary, no significant interactions were noted between pitavastatin 2 mg daily and DRV/r or EFV; PK of pitavastatin was similar during coadministration with DRV/r and EFV. Therefore, it is unlikely that statin dosing adjustments would be required when pitavastatin 2 mg is coadministered with either EFV 600 mg or DRV/r 800/100 mg for HIV-infected individuals taking pitavastatin for the management of dyslipidemia. The exposures of DRV and EFV were also not significantly affected by concurrent dosing of pitavastatin suggesting that coadministration of pitavastatin should not interfere with dosing or virologic efficacy of these 2 widely used antiretroviral drugs. Although subjects in this study were healthy volunteers exposed only to pitavastatin and EFV or DRV-r, there may be some variation in plasma levels of these drugs when used in combination with other antiretrovirals and with additional drugs for the treatment of comorbid conditions There were no major safety concerns as a result of coadministration of pitavastatin 2 mg with EFV 600 mg or with DRV/r 800 mg/100 mg. The low drug interaction potential of pitavastatin, as demonstrated in this study, would make this agent a promising first-line treatment option in the medical management of dyslipidemia in patients with HIV infection.
Supplementary Material
Acknowledgments
This study was funded by Kowa Pharmaceuticals America. This study was also supported in part by NIAID AI069532 (J.A.A.) and NIH 1UL1RR029893 from the National Center for Research Resources, the Grunebaum AIDS Scholarship Award, and the NYU CTSA grant UL1TR000038 from the National Center for the Advancement of Translational Science (NCATS), the Saperstein Medical Scholars Research Fund Award (C.D.M.), and NIMH K08MH098794 (Q.M.).
C.D.M. has received funding in the form of a grant paid to his institution from Kowa Pharmaceuticals America, Inc. Q.M. received salary support from NIH grant K08MH098794. G.D.M. received funding in the form of a grant paid to his institution from NIH. J.A.U. received honoraria for consulting work for Liposcience, Aegerion, Novartis, and AstraZeneca and also received honoraria for serving on speaker bureaus for Kowa, GSK, Amarin, AstraZeneca, Liposcience, Merck, and Genzyme. J.A.A. also received payment as a member of the scientific advisory boards for AbbVie, Janssen, and Merck.
Footnotes
Presented at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC), September 10–13, 2013, Denver, CO.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
REFERENCES
- 1.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:e1–e34. doi: 10.1093/cid/cit665. [DOI] [PubMed] [Google Scholar]
- 2.Masana L. Pitavastatin in cardiometabolic disease: therapeutic profile. Cardiovasc Diabetol. 2013;12(suppl 1):S2. doi: 10.1186/1475-2840-12-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sponseller CA, Morgan R, Campbell S, et al. After 52 Weeks, Pitavastatin Is Superior to Pravastatin for LDL-C Lowering in Patients With HIV; 14th Annual Conference on Retroviruses and Opportunistic Infections (CROI); March 3–6, 2014; Boston, MA. [Google Scholar]
- 4.Food and Drug Administration. FDA Drug Safety Communication: interactions between certain HIV or hepatitis C drugs and cholesterol-lowering statin drugs can increase the risk of muscle injury. [Accessed March 21, 2014]; Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293877.htm.
- 5.Gerber J, Rosenkranz S, Fichtenbaum C, et al. Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: results of AIDS Clinical Trials Group 5108 Study. J Acquir Immune Defic Syndr. 2005;39:307–312. doi: 10.1097/01.qai.0000167156.44980.33. [DOI] [PubMed] [Google Scholar]
- 6.Chauvin B, Drouot S, Barrail-Tran A, et al. Drug-drug interactions between HMG-CoA reductase inhibitors (statins) and antiviral protease inhibitors. Clin Pharmacokinet. 2013;52:815–831. doi: 10.1007/s40262-013-0075-4. [DOI] [PubMed] [Google Scholar]
- 7.Morgan RE, Campbell SE, Suehira K, et al. Effects of steady-state lopinavir/ritonavir on the pharmacokinetics of pitavastatin in healthy adult volunteers. J Acquir Immune Defic Syndr. 2012;60:158–164. doi: 10.1097/QAI.0b013e318251addb. [DOI] [PubMed] [Google Scholar]
- 8.Ma Q, Okusanya OO, Smith PF, et al. Pharmacokinetic drug interactions with non-nucleoside reverse transcriptase inhibitors. Expert Opin Drug Metab Toxicol. 2005;1:473–485. doi: 10.1517/17425255.1.3.473. [DOI] [PubMed] [Google Scholar]
- 9.Keil K, Hochreitter J, DiFrancesco R, et al. Integration of atazanavir into an existing liquid chromatography UV method for protease inhibitors: validation and application. Ther Drug Monit. 2007;29:103–109. doi: 10.1097/FTD.0b013e3180318ef3. [DOI] [PubMed] [Google Scholar]
- 10.Holland DT, DiFrancesco R, Connor JD, et al. Quality assurance program for pharmacokinetic assay of antiretrovirals: ACTG proficiency testing for pediatric and adult pharmacology support laboratories, 2003 to 2004: a requirement for therapeutic drug monitoring. Ther Drug Monit. 2006;28:367–374. doi: 10.1097/01.ftd.0000211817.58052.b8. [DOI] [PubMed] [Google Scholar]
- 11.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; 2013. Available at: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 12.Fichtenbaum C, Gerber J, Rosenkranz S, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG Study A5047. AIDS. 2002;16:569–577. doi: 10.1097/00002030-200203080-00008. [DOI] [PubMed] [Google Scholar]
- 13.Hare CB, Vu MP, Grunfeld C, et al. Simvastatin-nelfinavir interaction implicated in rhabdomyolysis and death. Clin Infect Dis. 2002;35:e111–e112. doi: 10.1086/344179. [DOI] [PubMed] [Google Scholar]
- 14.Aberg J, Rosenkranz S, Fichtenbaum C, et al. Pharmacokinetic interaction between nelfinavir and pravastatin in HIV-seronegative volunteers: ACTG Study A5108. AIDS. 2006;20:725–729. doi: 10.1097/01.aids.0000216373.53819.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.PRAVACHOL (pravastatin sodium) Tablets [package insert] Bristol Myers-Squibb; 2013. [Accessed February 16, 2014]. Available at: packageinserts.bms.com/pi/pi_pravachol.pdf. [Google Scholar]
- 16.Prezista (darunavir) [package insert] Tibotec, Inc.; 2013. [Accessed March 27, 2014]. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/.../021976s003s004lbl.pdf. [Google Scholar]
- 17.Ieiri I, Higuchi S, Sugiyama Y. Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs. Expert Opin Drug Metab Toxicol. 2009;5:703–729. doi: 10.1517/17425250902976854. [DOI] [PubMed] [Google Scholar]
- 18.Hirano M, Maeda K, Shitara Y, et al. Drug-drug interaction between pitavastatin and various drugs via OATP1B1. Drug Metab Dispos. 2006;34:1229–1236. doi: 10.1124/dmd.106.009290. [DOI] [PubMed] [Google Scholar]
- 19.Annaert P, Ye ZW, Stieger B, et al. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica. 2010;40:163–176. doi: 10.3109/00498250903509375. [DOI] [PubMed] [Google Scholar]
- 20.Romaine SP, Bailey KM, Hall AS, et al. The influence of SLCO1B1 (OATP1B1) gene polymorphisms on response to statin therapy. Pharmacogenomics J. 2010;10:1–11. doi: 10.1038/tpj.2009.54. [DOI] [PubMed] [Google Scholar]
- 21.Aquilante CL, Kiser JJ, Anderson PL, et al. Influence of SLCO1B1 polymorphisms on the drug-drug interaction between darunavir/ritonavir and pravastatin. J Clin Pharmacol. 2012;52:1725–1738. doi: 10.1177/0091270011427907. [DOI] [PubMed] [Google Scholar]
- 22.Needham M, Mastaglia FL. Statin myotoxicity: a review of genetic susceptibility factors. Neuromuscular Disord. 2014;24:4–15. doi: 10.1016/j.nmd.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Livalo (pitavastatin) Tablet; 1 mg, 2 mg, 4 mg [package insert], Vol Version 8.0. Montgomery, AL: Kowa Pharmaceuticals America, Inc.; 2013. [Accessed March 26, 2014]. Available at: www.kowapharma.com/documents/LIVALO_PI_CURRENT.pdf. [Google Scholar]
- 24.Yu CC, Campbell SE, Sponseller CA, et al. Steady-state pharmacokinetic interactions of darunavir/ritonavir with pitavastatin in healthy adult volunteers; Poster presented at XIX International AIDS Conference; July 22–27, 2012–2013; Washington, DC. [Google Scholar]
- 25.Sustiva (efavirenz) [package insert] Bristol-Myers Squibb; 2013. [Accessed June 2, 2014]. Available at: packageinserts.bms.com/pi/pi_sustiva.pdf. [Google Scholar]
- 26.Intelence (etravirine) [package insert] Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2008. [Accessed June 2, 2014]. Available at: http://www.intelence.com/hcp/full-prescribing-information. [Google Scholar]
- 27.Kivisto KT, Niemi M. Influence of drug transporter polymorphisms on pravastatin pharmacokinetics in humans. Pharm Res. 2007;24:239–247. doi: 10.1007/s11095-006-9159-2. [DOI] [PubMed] [Google Scholar]
- 28.Weiss J, Herzog M, Konig S, et al. Induction of multiple drug transporters by efavirenz. J Pharmacol Sci. 2009;109:242–250. doi: 10.1254/jphs.08209fp. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.