Abstract
Among 112 patients infected only by Plasmodium falciparum, WHO criteria of severity were compared with parasite load assessed by microscopy and quantitative PCR. Clinical severity was significantly correlated with higher parasite load as determined by microscopy (p < 0.001) and by PCR (p < 0.001). Hence, quantitative PCR might be useful to predict outcome.
Keywords: Diagnosis, disease severity, malaria, microscopy, Plasmodium falciparum, real-time PCR
Malaria is a severe disease associated with significant mortality, mainly attributed to Plasmodium falciparum and to delayed treatment [1]. Patients with clinical criteria of severe disease should be hospitalized and aggressively treated with intravenous anti-parasite drugs. UK recommendations suggest treating intravenously as soon as the parasitaemia is above 2% [2].
The correlation between parasitaemia and severity of disease is well established for quantification derived from microscopy [3]. Correlation between clinical severity and DNA quantification by PCR in blood has already been demonstrated for other infections, such as Streptococcus pneumoniae [4], Staphylococcus aureus [5] or Neisseria meningitidis [6], but the value of real-time PCR quantification to predict outcome is unknown for P. falciparum infection.
In our diagnostic laboratory, real-time Taqman PCR has been used to confirm species identification for every positive microscopy, as a systematic internal quality control since January 2004. This quantitative PCR targeting the 18S rRNA encoding gene [7], has also been implemented in other diagnostic microbiology laboratories [8,9]. The objective of this work was to assess (among patients with single P. falciparum infection) whether parasitaemia determined by microscopy and/or PCR correlated with the clinical severity of malaria.
From 1 January 2004 to 31 December 2011, we included in our work all patients found infected by only P. falciparum, as determined by Giemsa-stained thin smear and by a multiplex PCR performed on their first EDTA–blood sample. The included patients were adults (>16 years old) hospitalized at the University Hospital, Lausanne (Switzerland) or consulting at the Department of Ambulatory Care and Community Medicine of Lausanne's University Hospital. This project was approved by our local ethics committee.
For microscopy, parasitaemia was initially reported in %. The number of P. falciparum parasites per mL of blood was estimated assuming that 1 μL contains 5 × 106 red blood cells. Hence, 1% parasitaemia corresponded to 50 000 parasites/μL of blood and to 50 000 000 parasites/mL. Quantification was then converted in log parasites/mL.
All samples positive for P. falciparum by direct microscopy were confirmed by a multiplex Plasmodium quantitative real-time PCR [7], detecting the four most common human Plasmodium species (P. falciparum, Plasmodium ovale, Plasmodium vivax and Plasmodium malariae). Then, using the set of primers and probes specific for P. falciparum, we confirmed all positive results by a subsequent P. falciparum monoplex PCR to precisely quantify the number of parasite copies/mL of blood. Quantification was first expressed in number of DNA copies/mL and then converted in log copies/mL as described in Dormond et al. [10].
Demographic (country of exposure) and clinical characteristics were retrospectively retrieved from clinical charts. Eight characteristics, following the WHO criteria of severe malaria [1], were considered: (i) cerebral malaria (coma) or impairment of consciousness, (ii) adult respiratory distress syndrome or pulmonary oedema, (iii) haemostatic abnormalities (bleeding or disseminated intravascular coagulation), (iv) severe hypotension (<70 mmHg of systolic blood pressure) or shock, (v) severe anaemia (haemoglobin <50 g/L), (vi) acute renal failure (creatinine concentration >265 μmol/L), (vii) jaundice (total serum bilirubin ≥50 μmol/L) and severe hypoglycaemia (<2.2 mmol/L). Patients with one or more than one of these criteria were considered as severe malaria cases. The statistical analyses have been performed using the GraphPad Prism software 5.02 (GraphPad Software, La Jolla, CA, USA). Medians were compared using the Wilcoxon test.
The correlation between clinical severity and parasitaemia as well as PCR quantification was assessed for 112 patients with isolated P. falciparum infections confirmed by PCR. Most of those patients exhibited no criteria of severity (92/112; 82.1%) or one single criterion (11/112; 9.8%). Seven patients presented two criteria, one patient presented three criteria and one patient presented four criteria of severity. Neurological impairment and jaundice were the commonest observed criteria of severe malaria (Table 1). Notably, except for one patient who had returned from South America, all patients acquired P. falciparum infection in sub-Saharan Africa (60.0% in West Africa, 36.4% in Central Africa and 2.7% in East Africa).
Table 1.
Criteria of severe malaria among 112 patients presenting single Plasmodium falciparum infection (20 patients presented at least one criteria of severe malaria)
| Criteria of severe malaria | Frequencies | |
|---|---|---|
| Cerebral malaria (coma) or impairment of consciousness | 12/112 | (11%) |
| Jaundice (total serum bilirubin ≥50 μmol/L) | 8/112 | (7%) |
| Haemostatic abnormalities (bleeding or DIC) | 4/112 | (4%) |
| Severe hypotension (<70 mmHg of systolic blood pressure) | 4/112 | (4%) |
| Acute renal failure (creatinine concentration >265 μmol/L) | 2/112 | (2%) |
| ARDS or pulmonary oedema | 2/112 | (2%) |
| Severe anaemia (haemoglobin <50 g/L) | 0/112 | (0%) |
| Severe hypoglycaemia (<2.2 mmol/L)a | 0/112 | (0%) |
ARDS, adult respiratory distress syndrome; DIC, disseminated intravascular coagulation.
Information mainly available in severe cases.
As expected, parasitaemia estimated by microscopy significantly correlated with clinical severity (Fig. 1a). Parasitaemia was significantly higher among patients with at least one criterion of severe malaria (median of 7.63 log copies/mL; interquartile range (IQR) 7.00–8.39 log copies/mL) compared with patients with 0 severity criteria (median of 7.09 log copies/mL; IQR 5.70–7.74 log copies/mL; p < 0.001). Moreover, both of the patients with at least three severity criteria exhibited a parasitaemia >100 × 106 parasites/mL (>2%; log 8 parasites/mL).
Fig. 1.
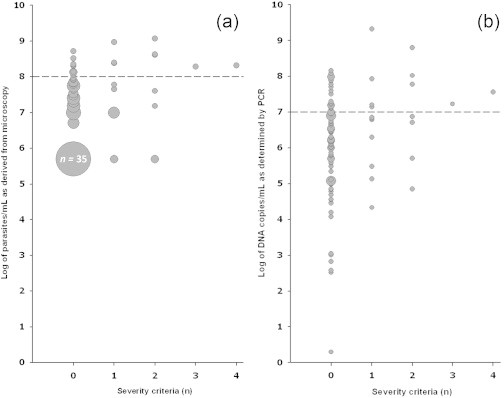
Correlation between first parasite load and severity criteria according to microscopy (a) and PCR (b). The size of each circle represents the number of subjects with a given Plasmodium level.
A similar correlation between clinical severity and parasite load determined by PCR could be demonstrated (Fig. 1b). The number of Plasmodium-specific DNA copies/mL was significantly higher among patients with one or more severity criteria (median of 6.87 log copies/mL; IQR 5.87–7.73 log copies/mL) compared with patients with 0 severity criteria (median of 6.22 log copies/mL; IQR 5.17–6.94 log copies/mL; p < 0.001). The number of plasmodial DNA copies was high, i.e. above 16 900 000 copies/mL (log 7 copies/mL), among both patients with at least three criteria of severe malaria. These two patients with at least three criteria of severe malaria both exhibited a parasitaemia ≥2%. This correlates with a previous study where patients with parasitaemia of ≥2% had an odds ratio of severe P. falciparum malaria that was 12-fold higher than in patients with <2% parasitaemia [11], and this threshold was proposed to decide on the choice of parenteral anti-malarials to administer [2]. According to our results, when PCR is used for quantification, a threshold of parasitaemia of >log 7 might be proposed for such a decision. Moreover, an added value of PCR quantification is also present for low DNA load, as all six patients with a parasitaemia of <log 4 exhibited no criteria of severity. However, interpretation of quantitative PCR results should take into account the overall clinical presentation, as low parasitaemia has been reported in patients with severe malaria, due to splenic sequestration [12], especially in non-immune patients. This <log 4 cut-off should be challenged in future larger studies, because our work relies on a small number of patients and we observed a large dynamic range of parasitaemia among patients without criteria of severity. Practically, the quantitative PCR will not replace microscopy, which provides an earlier result and is available 24/24 and 7/7. The main advantage of having such an additional quantitative approach is for clinical studies [10] and for specific cases, such as estimating the parasitaemia retrospectively in a dead patient when full blood is no longer available [13].
In conclusion, we observed a significant correlation between first parasite load as determined by microscopy and the number of severity criteria. We also observed a significantly higher parasite load as determined by PCR in patients with at least one criterion of severe malaria compared with those without any criteria of severity. Hence, quantitative PCR might be useful to predict the outcome of patients with P. falciparum infection and to guide prescription of intravenous anti-malarial treatment.
Conflicts of interest
None declared.
Acknowledgements
We thank R. Brouillet, F. Dusserre, D. Dulon, and Y. Vonlanthen for technical help.
References
- 1.World Health Organization, Communicable Diseases Cluster Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl. 1):S1–S90. [PubMed] [Google Scholar]
- 2.Lalloo D.G., Shingadia D., Pasvol G., Chiodini P.L., Whitty C.J., Beeching N.J. UK malaria treatment guidelines. J Infect. 2007;54:111–121. doi: 10.1016/j.jinf.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Field J.W. Blood examination and prognosis in acute falciparum malaria. Trans R Soc Trop Med Hyg. 1949;43:33–48. doi: 10.1016/0035-9203(49)90022-x. [DOI] [PubMed] [Google Scholar]
- 4.Peters R.P., de Boer R.F., Schuurman T., Gierveld S., Kooistra-Smid M., van Agtmael M.A. Streptococcus pneumoniae DNA load in blood as a marker of infection in patients with community-acquired pneumonia. J Clin Microbiol. 2009;47:3308–3312. doi: 10.1128/JCM.01071-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho Y.C., Chang S.C., Lin S.R., Wang W.K. High levels of mecA DNA detected by a quantitative real-time PCR assay are associated with mortality in patients with methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. 2009;47:1443–1451. doi: 10.1128/JCM.01197-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darton T., Guiver M., Naylor S., Jack D.L., Kaczmarski E.B., Borrow R. Severity of meningococcal disease associated with genomic bacterial load. J Clin Microbiol. 2009;48:587–594. doi: 10.1086/596707. [DOI] [PubMed] [Google Scholar]
- 7.Rougemont M., Van Saanen M., Sahli R., Hinrikson H.P., Bille J., Jaton K. Detection of four Plasmodium species in blood from humans by 18S rRNA gene subunit-based and species-specific real-time PCR assays. J Clin Microbiol. 2004;42:5636–5643. doi: 10.1128/JCM.42.12.5636-5643.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bialasiewicz S., Whiley D.M., Nissen M.D., Sloots T.P. Impact of competitive inhibition and sequence variation upon the sensitivity of malaria PCR. J Clin Microbiol. 2007;45:1621–1623. doi: 10.1128/JCM.02145-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shokoples S.E., Ndao M., Kowalewska-Grochowska K., Yanow S.K. Multiplexed real-time PCR assay for discrimination of Plasmodium species with improved sensitivity for mixed infections. J Clin Microbiol. 2009;47:975–980. doi: 10.1128/JCM.01858-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dormond L., Jaton-Ogay K., De Vallière S., Genton B., Bille J., Greub G. Multiplex real-time PCR for the diagnosis of malaria: correlation with microscopy. Clin Microbiol Infect. 2011;17:469–475. doi: 10.1111/j.1469-0691.2010.03218.x. [DOI] [PubMed] [Google Scholar]
- 11.Phillips A., Bassett P., Zeki S., Newman S., Pasvol G. Risk factors for severe disease in adults with falciparum malaria. Clin Infect Dis. 2009;48:871–878. doi: 10.1086/597258. [DOI] [PubMed] [Google Scholar]
- 12.White N.J. Malaria. In: Farrar J., Hotez P., Junghanss T., Kang G., Lalloo D., White N., editors. Manson’s Tropical Diseases. 23rd ed. WB Saunders; Philadelphia: 2014. pp. 532–601. Section 9, Chapter 43. [Google Scholar]
- 13.Palmiere C., Jaton K., Lobrinus A., Schrag B., Greub G. Post-mortem diagnosis of malaria. New Microbe. New Infect. 2014;2:154–155. doi: 10.1002/nmi2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]


