Abstract
Purpose
To determine the effect of early versus delayed initiation of a palliative care intervention for family caregivers (CGs) of patients with advanced cancer.
Patients and Methods
Between October 2010 and March 2013, CGs of patients with advanced cancer were randomly assigned to receive three structured weekly telephone coaching sessions, monthly follow-up, and a bereavement call either early after enrollment or 3 months later. CGs of patients with advanced cancer were recruited from a National Cancer Institute cancer center, a Veterans Administration Medical Center, and two community outreach clinics. Outcomes were quality of life (QOL), depression, and burden (objective, stress, and demand).
Results
A total of 122 CGs (early, n = 61; delayed, n = 61) of 207 patients participated; average age was 60 years, and most were female (78.7%) and white (92.6%). Between-group differences in depression scores from enrollment to 3 months (before delayed group started intervention) favored the early group (mean difference, −3.4; SE, 1.5; d = −.32; P = .02). There were no differences in QOL (mean difference, −2; SE, 2.3; d = −.13; P = .39) or burden (objective: mean difference, 0.3; SE, .7; d = .09; P = .64; stress: mean difference, −.5; SE, .5; d = −.2; P = .29; demand: mean difference, 0; SE, .7; d = −.01; P = .97). In decedents' CGs, a terminal decline analysis indicated between-group differences favoring the early group for depression (mean difference, −3.8; SE, 1.5; d = −.39; P = .02) and stress burden (mean difference, −1.1; SE, .4; d = −.44; P = .01) but not for QOL (mean difference, −4.9; SE, 2.6; d = −.3; P = .07), objective burden (mean difference, −.6; SE, .6; d = −.18; P = .27), or demand burden (mean difference, −.7; SE, .6; d = −.23; P = .22).
Conclusion
Early-group CGs had lower depression scores at 3 months and lower depression and stress burden in the terminal decline analysis. Palliative care for CGs should be initiated as early as possible to maximize benefits.
INTRODUCTION
Of the 13 million patients in the United States who have cancer,1 many have advanced disease requiring the assistance of family caregivers (CGs). Family CGs of patients with advanced cancer provide an average of 8 hours of daily assistance2 with symptom management, emotional and spiritual support, personal care and activities of daily living, transportation, and communication and care coordination with clinicians.3 These CGs can experience psychological distress equal to and sometimes greater than the patient with cancer.4,5 Enduring such high levels of strain has been associated with poor CG physical health3,6,7 and high mortality risk.8,9 Caregiving challenges can be further heightened by residence in a rural setting where there is a lack of convenient access to resource-rich urban centers.10,11 Hence, alleviating CGs' taxing role and improving CG support have been recognized as public health priorities.12–15
Palliative care services are aimed at reducing CGs' distress and burden by educating and activating skills in problem solving, self-care, decision making, and symptom management.16 Although the benefits of early concurrent oncology palliative care have been noted in patients with advanced cancer,17 the impact of this earlier care on CGs has not been studied. Waiting to provide services until patients are in their last weeks or days of life may not adequately address patient or CG distress.18,19 Because CG distress levels have been noted to fluctuate over the trajectory of illness, peaking at diagnosis and at death, it has been suggested that early palliative care may equally mitigate ongoing and later CG distress.4,20–22
We demonstrated improved patient quality of life (QOL) and depressed mood and lower symptom intensity23 in our previous randomized controlled trial (RCT) comparing the ENABLE (Educate, Nurture, Advise Before Life Ends) model of early palliative with usual cancer care; however, CGs were not provided with a specific intervention, and these benefits were not demonstrated among CGs.24 Our conclusion was that future palliative care studies would need to provide a specific intervention to address CGs' needs appropriate for a rural population. On the basis of those findings and exploratory work,25 we designed and offered a specific parallel CG intervention in the current trial. In this intervention, we addressed CGs' own unique self-care needs while also coaching them so they could also be supportive partners in problem solving, communication, decision making, and advance care planning. We hypothesized that CGs receiving this intervention early after patients' diagnosis would have better outcomes compared with CGs who received the intervention 3 months later (ie, delayed group). Patient outcomes are reported separately.26
PATIENTS AND METHODS
Study Design
This RCT employed a fast-track (or wait-control) design,27,28 in which patients newly diagnosed with advanced-stage recurrent or progressive metastatic cancer were randomly assigned (with their CGs) to either an early (fast track) or delayed (3 months after diagnosis) intervention group. The study protocol and data and safety monitoring plan were approved by the institutional review boards of the Norris Cotton Cancer Center/Dartmouth College (Lebanon, NH) and Veterans' Administration (VA) Medical Center (White River Junction, VT).
Participants and Setting
From October 11, 2010, to March 5, 2013, patient participants were recruited from the Norris Cotton Cancer Center, affiliated outreach clinics, and the VA Medical Center situated in New Hampshire and VT, the populations of which are 40% and 61% rural, respectively.29 Patients' eligibility criteria were as follows: age > 18 years; new diagnosis, recurrence, or progression of an advanced-stage cancer within approximately 30 to 60 days of the date the patient was informed of the diagnosis by his or her oncology clinician and oncologist-determined prognosis of 6 to 24 months; English speaking; and able to complete baseline questionnaires. Patients were excluded if they scored < 4 on the Callahan30 cognitive screen, had an untreated axis I psychiatric condition (eg, schizophrenia, bipolar disorder) or an active substance use disorder, or had uncorrectable hearing disorder or unreliable telephone service (Data Supplement provides details on disease-specific eligibility criteria, Callahan screen, and study protocol). Subsequent to enrollment, patient participants were asked to identify a CG, defined as “a person who knows you well and is involved in your medical care” to also participate in the study. Patients were not excluded if they did not have a participating CG. There were no formal CG exclusion criteria. Patients and CGs each provided signed informed consent and completed baseline data collection, and dyads were subsequently randomly assigned to either the early- or delayed-intervention group stratified by disease and recruitment site. CGs assigned to delayed intervention were able to access any of the usual support services available.
Intervention
All CGs received the intervention, although the early group began right after random assignment, and the delayed group began 3 months after random assignment. The ENABLE intervention has been described in detail elsewhere31; however, this was the first early intervention trial to our knowledge that provided a specific intervention for CGs. Before this trial, we conducted two separate studies of CGs. One involved exploratory interviews with 135 CGs of patients with cancer.25 The other was a follow-up interview study of patients and CGs who had participated in our prior ENABLE II RCT.32 On the basis of these studies, we identified topics that informed the CG-specific intervention (such as cultivating communication skills with patient and health care clinicians). We refined, adapted, and embedded the newly identified components into the format of structured one-on-one telephone sessions between an advanced-practice palliative care nurse coach and a CG. A telehealth approach was used, given the largely rural populations of VT and New Hampshire, which eliminated the need for participants to travel long distances for sessions. Sessions were guided by the Charting Your Course: Caregiver (CYC-C) guidebook (available from authors). Session one addressed taking on the CG role, defined palliative and supportive care, and introduced problem-solving using the framework of the COPE (Creativity, Optimism, Planning, Expert Information) attitude33; session two covered CG self-care (eg, healthy eating, exercise, relaxation) and effective partnering in patient symptom assessment and management; session three addressed the building of a support team, decision making, decision support, and advance care planning.
Patients and CGs each were assigned a different nurse coach to promote open sharing of feelings, which oftentimes concerned each other. Following a detailed script, the nurse coaches conducted three once-per-week CG educational sessions by telephone covering the three chapters of the CYC-C guidebook. Nurse coaches also followed up at least monthly by telephone to address any ongoing or new issues until the patient participant died or the study ended. Sessions lasted 23 minutes on average. With patients' permission, CGs also were encouraged to be present for the in-person palliative care consultation and any subsequent in-person palliative care visits. If the patient participant died during the study, nurse coaches completed a bereavement call to offer condolences and address grief issues.
Seven nurse coaches underwent approximately 30 hours of training, which included didactic presentations, self-study, and role play of the three CG sessions. After these practice sessions, the principal investigator provided constructive feedback. Subsequent to training, the principal investigator, who was blinded to group assignment, listened to all sessions and met weekly with the nurse coaches to review and discuss any protocol deviations, challenges, or issues.
Data Collection and Instruments
After signing informed consent, CGs completed baseline demographic collection and a questionnaire with a research coordinator who was blinded to group assignment. Questionnaires were administered by telephone once every 6 weeks until week 24 and then every 3 months thereafter until the patient participant's death or study completion. QOL was measured using the CG QOL Scale–Cancer (CQOL-C), a 35-item self-report measure34; items measure impact of caregiving on a person's physical, emotional, and spiritual well-being and on his or her relationship with the care recipient and family. Scores range from 0 to 140; higher scores indicate worse QOL. The CQOL-C has an internal consistency of 0.91 and a test-retest reliability of 0.95. Depression was measured using the Center for Epidemiologic Study–Depression (CESD) Scale (range, 0 to 60; higher score indicates greater depressed mood; score > 16 indicates clinically significant depression).35,36 This 20-item depressive symptoms measure has been widely used in epidemiologic studies of depression and has strong validity and reliability.37 CG burden was measured using the 14-item Montgomery–Borgatta CG Burden (MBCB) Scale, which includes objective, demand, and stress burden subscales (subscales: α = .88, 0.74, and 0.84, respectively).38,39 High objective burden score (range, 6 to 30; > 23 indicates clinical significance) suggests interference with the CG's private, social, and recreational time and normal daily routine; high demand burden score (range, 4 to 20; > 15 indicates clinical significance) indicates that the CG feels overstrained by his or her caregiving demands; high stress burden score (range, 4 to 20; > 13.5 indicates clinical significance) signals strained emotional demands related to caregiving.
Statistical Analysis
Study outcomes were CG QOL (using CQOL-C), depressed mood (using CESD), and objective, stress, and demand burdens (using MBCB subscales). Two longitudinal, intention-to-treat analyses were conducted. In the first analysis, we examined the intervention effects from enrollment to 3 months. The between-group difference in change from baseline to 3 months allowed us to compare the intervention versus usual care, because delayed-group CGs had not yet started the intervention. In the second analysis, we examined only the data from CGs whose care recipient had died and fit a terminal-decline model to all the data from measurement occasions collected in the last 36 weeks of the patient's life.40
We examined patterns of missing data resulting from loss to follow-up or patient death. We conducted longitudinal analyses using models with indicators for group, time, and time by group interaction and fitted using linear mixed methods. We adjusted intervention effects in both groups for patient death by including an indicator variable for patient death as a time-varying predictor. Variance components were used to estimate pooled standard deviations, which were used to compute effect sizes in the form of standardized mean differences (Cohen's d).
RESULTS
A total of 207 patient participants were enrolled onto the trial a median of 28 days (interquartile range, 13 to 49 days) after new diagnosis with advanced cancer; 124 identified a CG; all but two CGs were enrolled and provided data (Fig 1), for a total sample of 122 CGs. Table 1 lists CGs' demographic characteristics by group. No significant differences were found between early and delayed groups except for employment status, where the early group had a higher proportion employed, fewer retired, and fewer unemployed (P = .05). On average, CGs were age 60 years, female (78.7%; n = 96), and white (92.6%; n = 113); 56% (n = 69) had completed high school, and 42% (n = 51) had completed college. Almost 50% (n = 60) were employed, and 29% (n = 35) were retired. Approximately 62% (n = 76) reported a Christian denomination (Catholic or Protestant), and 19% (n = 23) reported no religious affiliation. Most (75%; n = 92) were the patient's spouse or partner. Most cared for a patient with lung cancer (43%; n = 53), and 25% (n = 31) cared for a patient with GI cancer. As summarized in Table 2, there were no significant differences in outcome measures between groups at baseline. Approximately 32% (n = 39) of CGs did not complete all follow-up assessments; however, time-to-event analyses revealed no significant associations between attrition and measured CG characteristics or outcome (Appendix Table A1, online only).
Fig 1.
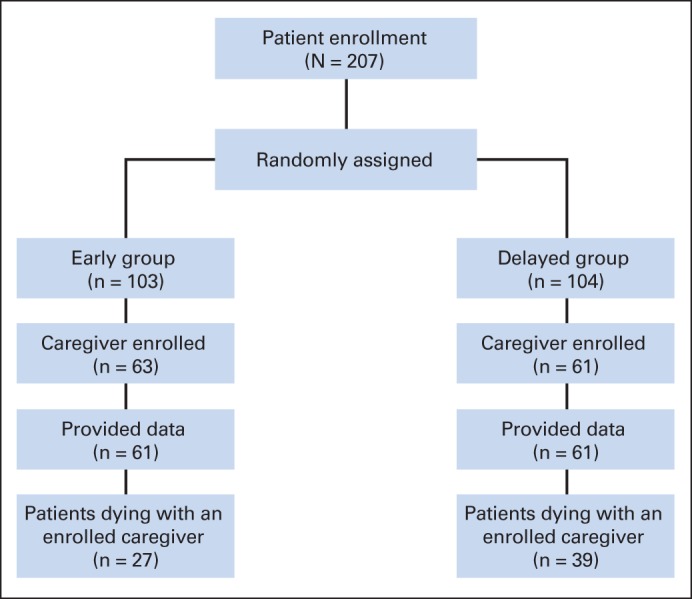
CONSORT diagram of family caregiver enrollment.
Table 1.
Demographic Characteristics of CG Participants
| Characteristic | Early Group (n = 61) |
Delayed Group (n = 61) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age, years | .15 | ||||
| Mean | 61 | 57.9 | |||
| SD | 11.6 | 11.9 | |||
| Sex | .83 | ||||
| Female | 47 | 77 | 49 | 80.3 | |
| Male | 14 | 23 | 12 | 19.7 | |
| Race | .39 | ||||
| White | 55 | 90.2 | 58 | 95.1 | |
| Other | 4 | 6.6 | 1 | 1.6 | |
| Missing/no response | 2 | 3.3 | 2 | 3.3 | |
| Marital status | .65 | ||||
| Married or living with partner | 54 | 88.5 | 58 | 95.1 | |
| Never married | 3 | 4.9 | 1 | 1.6 | |
| Divorced or separated | 2 | 3.3 | 1 | 1.6 | |
| Widowed | 1 | 1.6 | 1 | 1.6 | |
| Missing/no response | 1 | 1.6 | 0 | 0 | |
| Education | .42 | ||||
| High school or GED; some college or technical school | 37 | 60.7 | 32 | 52.5 | |
| ≥ College graduate | 23 | 37.7 | 28 | 45.9 | |
| < High school graduate | 0 | 0 | 1 | 1.6 | |
| Missing/no response | 1 | 1.6 | 0 | 0 | |
| Employment status | .05 | ||||
| Full or part time | 23 | 37.7 | 37 | 60.7 | |
| Retired | 22 | 36.1 | 13 | 21.3 | |
| Not employed | 14 | 23 | 11 | 18 | |
| Missing/no response | 2 | 3.3 | 0 | 0 | |
| Religious affiliation | .91 | ||||
| Protestant | 22 | 36.1 | 19 | 31.1 | |
| Catholic | 18 | 29.5 | 17 | 27.9 | |
| Jewish | 1 | 1.6 | 1 | 1.6 | |
| None | 12 | 19.7 | 11 | 18 | |
| Other | 6 | 9.8 | 9 | 14.8 | |
| Missing/no response | 2 | 3.3 | 4 | 6.6 | |
| Relationship to patient | .41 | ||||
| Spouse/partner | 48 | 78.7 | 44 | 72.1 | |
| Sibling | 3 | 4.9 | 4 | 6.6 | |
| Son or daughter | 4 | 6.6 | 10 | 16.4 | |
| Parent | 4 | 6.6 | 3 | 4.9 | |
| Other | 1 | 1.6 | 0 | 0 | |
| Missing/no response | 1 | 1.6 | 0 | 0 | |
| Primary disease site of patient | .98 | ||||
| Lung | 28 | 45.9 | 25 | 41.0 | |
| GI | 14 | 23.0 | 17 | 27.9 | |
| Genitourinary | 5 | 8.2 | 5 | 8.2 | |
| Breast | 5 | 8.2 | 5 | 8.2 | |
| Hematologic | 3 | 4.9 | 4 | 6.6 | |
| Other solid tumor | 6 | 9.8 | 5 | 8.2 | |
Abbreviations: CG, caregiver; GED, general educational development; SD, standard deviation.
Fisher's exact or Pearson χ2 test for categorical variables and t test for continuous variables.
Table 2.
Comparison of Baseline Measures
| Measure | Early Group (n = 61) |
Delayed Group (n = 61) |
P* | ||||
|---|---|---|---|---|---|---|---|
| No. of CGs | Mean Score | SD | No. of CGs | Mean Score | SD | ||
| CQOL-C | 61 | 58.5 | 15.6 | 61 | 62.4 | 15.1 | .16 |
| CESD | 60 | 13.4 | 10.0 | 58 | 15.9 | 11.4 | .20 |
| MBCB-OB | 61 | 22.4 | 3.5 | 59 | 22.5 | 3.5 | .87 |
| MBCB-SB | 60 | 14.3 | 2.8 | 59 | 15.0 | 2.3 | .12 |
| MBCB-DB | 46 | 11.0 | 3.6 | 40 | 12.1 | 2.8 | .11 |
Abbreviations: CESD, Center for Epidemiologic Studies–Depression Scale; CG, caregiver; CQOL-C, Caregiver Quality of Life Scale–Cancer; DB, demand burden; MBCB, Montgomery–Borgatta Caregiver Burden Scale; OB, objective burden; SB, stress burden; SD, standard deviation.
Independent-sample t test.
CG QOL, Depression, and Burden
For all outcomes, lower scores indicated better outcomes. Three months after enrollment (Table 3), between-group differences in change from baseline in depression score (CESD) were significantly better in the early compared with delayed group (mean difference, −3.4; SE, 1.5; d = −.32; P = .02). There were no statistically significant differences in QOL (mean difference, −2; SE, 2.3; d = −.13; P = .39) or burden subscale (objective burden: mean difference, 0.3; SE, .7; d = .09; P = .64; stress burden: mean difference, −.5; SE, .5; d = −.2; P = .29; demand burden: mean difference, 0; SE, .7; d = −.01; P = .97).
Table 3.
Outcomes From Baseline to 3 Months
| Month After Baseline | Early Group |
Delayed Group |
Between-Group Difference in Change From Baseline* |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean† | SE | P‡ | Effect Size (d)§ | Adjusted for Patient Death |
||||||||||
| No. of CGs | Mean Score | SE | No. of CGs | Mean Score | SE | Mean† | SE | P‡ | Effect Size (d)§ | |||||
| CQOL-C | −2.1 | 2.3 | .37 | −0.13 | −2 | 2.3 | .39 | −0.13 | ||||||
| 0 | 61 | 58.5 | 2.0 | 61 | 62.4 | 2.0 | ||||||||
| 1.5 | 39 | 52.2 | 2.3 | 44 | 58 | 2.2 | ||||||||
| 3 | 35 | 50.2 | 2.4 | 34 | 55.7 | 2.4 | ||||||||
| CESD | −3.4 | 1.5 | .02 | −0.32 | −3.4 | 1.5 | .02 | −0.32 | ||||||
| 0 | 60 | 13.4 | 1.3 | 58 | 15.9 | 1.4 | ||||||||
| 1.5 | 39 | 8.8 | 1.5 | 44 | 15.9 | 1.4 | ||||||||
| 3 | 35 | 10.2 | 1.5 | 34 | 14.1 | 1.6 | ||||||||
| MBCB-OB | 0.3 | 0.7 | .62 | 0.09 | 0.3 | 0.7 | .64 | 0.09 | ||||||
| 0 | 61 | 22.4 | 0.5 | 59 | 22.5 | 0.5 | ||||||||
| 1.5 | 39 | 22.1 | 0.6 | 44 | 21.7 | 0.5 | ||||||||
| 3 | 35 | 22.2 | 0.6 | 34 | 22.2 | 0.6 | ||||||||
| MBCB-SB | −0.6 | 0.5 | .27 | −0.21 | −0.5 | 0.5 | .29 | −0.2 | ||||||
| 0 | 60 | 14.3 | 0.3 | 59 | 15.0 | 0.3 | ||||||||
| 1.5 | 38 | 13.2 | 0.4 | 44 | 14.3 | 0.4 | ||||||||
| 3 | 35 | 13.3 | 0.4 | 34 | 14.8 | 0.4 | ||||||||
| MBCB-DB | 0 | 0.7 | .99 | 0 | 0 | 0.7 | .97 | −0.01 | ||||||
| 0 | 46 | 11.0 | 0.5 | 40 | 12.1 | 0.5 | ||||||||
| 1.5 | 30 | 10.6 | 0.6 | 34 | 11.6 | 0.5 | ||||||||
| 3 | 26 | 10.3 | 0.6 | 29 | 11.7 | 0.6 | ||||||||
NOTE. Early versus delayed group (before delayed group started intervention).
Abbreviations: CESD, Center for Epidemiologic Studies–Depression Scale; CG, caregiver; CQOL-C, Caregiver Quality of Life Scale–Cancer; DB, demand burden; MBCB, Montgomery–Borgatta Caregiver Burden Scale; OB, objective burden; SB, stress burden.
Early minus delayed group.
Change represents average follow-up minus baseline.
From time by group interaction term in longitudinal models.
d represents mean difference in change from baseline divided by model-estimated pooled standard deviations.
In CGs of decedents, terminal decline models (Table 4) indicated significant time-averaged between-group differences favoring the early group for depression (CESD; mean difference, −3.8; SE, 1.5; d = −.39; P = .02) and stress burden (MBCB; mean difference, −1.1; SE, .4; d = −.44; P = .01) but not for QOL (CQOL-C; mean difference, −4.9; SE, 2.6; d = −.3; P = .07), objective burden (MBCB; mean difference, −.6; SE, .6; d = −.18; P = .27), or demand burden (MBCB; mean difference, −.7; SE, .6; d = −.23; P = .22).
Table 4.
Terminal Decline Analysis of CG Outcomes Looking Backward From Time of Patient Death
| Weeks Before Patient Death | Early Group |
Delayed Group |
Time-Averaged Difference* |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of CGs | Mean Score | SE | No. of CGs | Mean Score | SE | Mean | SE | P† | Effect Size (d)‡ | |
| CQOL-C | −4.9 | 2.6 | .07 | −0.3 | ||||||
| ≤ 6 | 8 | 58.3 | 4.3 | 11 | 60.9 | 3.7 | ||||
| 6 to 12 | 11 | 56.2 | 3.6 | 17 | 60.3 | 3 | ||||
| 12 to 24 | 23 | 50.8 | 2.5 | 23 | 59.2 | 2.4 | ||||
| 24 to 36 | 13 | 57.8 | 3.1 | 18 | 57.5 | 2.7 | ||||
| CESD | −3.8 | 1.5 | .02 | −0.39 | ||||||
| ≤ 6 | 8 | 13.7 | 2.4 | 11 | 16.5 | 2.2 | ||||
| 6 to 12 | 11 | 12.8 | 2 | 17 | 18.6 | 1.7 | ||||
| 12 to 24 | 23 | 11.1 | 1.4 | 22 | 15 | 1.3 | ||||
| 24 to 36 | 13 | 11.6 | 1.7 | 17 | 13.7 | 1.6 | ||||
| MBCB-OB | −0.6 | 0.6 | .27 | −0.18 | ||||||
| ≤ 6 | 8 | 23.8 | 1 | 11 | 24.9 | 0.9 | ||||
| 6 to 12 | 11 | 22.4 | 0.8 | 17 | 23.7 | 0.7 | ||||
| 12 to 24 | 23 | 22.5 | 0.6 | 23 | 22.6 | 0.5 | ||||
| 24 to 36 | 13 | 22.4 | 0.7 | 18 | 22.8 | 0.6 | ||||
| MBCB-SB | −1.1 | 0.4 | .01 | −0.44 | ||||||
| ≤ 6 | 8 | 13.3 | 0.7 | 11 | 14 | 0.6 | ||||
| 6 to 12 | 11 | 13.3 | 0.6 | 17 | 15.3 | 0.5 | ||||
| 12 to 24 | 23 | 13.6 | 0.4 | 22 | 14.8 | 0.4 | ||||
| 24 to 36 | 13 | 14.4 | 0.5 | 18 | 14.6 | 0.4 | ||||
| MBCB-DB | −0.7 | 0.6 | .22 | −0.23 | ||||||
| ≤ 6 | 7 | 11.9 | 1.1 | 7 | 13 | 1.2 | ||||
| 6 to 12 | 10 | 11.7 | 0.9 | 14 | 11.4 | 0.9 | ||||
| 12 to 24 | 19 | 10.7 | 0.6 | 16 | 11.7 | 0.6 | ||||
| 24 to 36 | 9 | 11.5 | 0.7 | 12 | 12.3 | 0.7 | ||||
NOTE. Minimum follow-up time was 24 weeks or until patient death if it occurred during that period. Not all caregivers were followed beyond the initial 24 week period.
Abbreviations: CESD, Center for Epidemiologic Studies–Depression Scale; CG, caregiver; CQOL-C, Caregiver Quality of Life Scale–Cancer; DB, demand burden; MBCB, Montgomery–Borgatta Caregiver Burden Scale; OB, objective burden; SB, stress burden.
Adjusted for baseline; early minus delayed group.
From repeated measures models.
d represents mean difference in change from baseline divided by model-estimated pooled standard deviations.
DISCUSSION
To our knowledge, this is the first RCT using a fast-track design to test the timing of early versus delayed palliative care support for family CGs of patients with advanced cancer in a largely rural setting. First, this trial demonstrated that initiating a telehealth palliative care intervention early after a patient's advanced-cancer diagnosis led to lower depression but resulted in no differences in QOL or burden compared with initiation 3 months later. Second, decedents' CGs in the early compared with delayed group experienced lower depression and lower stress burden and trended toward higher QOL.
To date, RCTs of interventions designed to support CGs of patients with life-limiting illnesses have demonstrated only marginal benefits, and none have been specifically tailored to a rural population.41–45 In a meta-analysis41 of 11 RCTs including 1,836 CGs of patients in the terminal phase of illness, there were significant although small improvements in psychological distress (eg, depression, anxiety, hopelessness; standardized mean difference, −.15) and small nonsignificant improvements in QOL (standardized mean difference, −.11). Recognizing that palliative support for patients and families is often initiated late in the illness trajectory,18,19 we sought to determine whether providing this extra layer of support further upstream would result in a greater benefit. In our study, the terminal decline analysis (Table 4) showed that depression (d = −.39) and stress burden (d = −.44) had moderate effect-size differences favoring the early-intervention group. Moreover, average delayed-group scores surpassed the cut points for clinically significant depression (CESD score > 16) and high stress burden (MBCG stress subscale score > 13.5) for the last 12 weeks before death. Together, these findings suggest that providing CG support at the time of advanced-cancer diagnosis may be the essential ingredient to achieving positive outcomes.
Early palliative care for CGs may have resulted in lower stress burden and depression and higher QOL by a number of mechanisms. A meta-analysis of 29 RCTs by Northouse et al43 reported that intervention components that reduced CG psychological distress included provision of emotional, informational, and problem solving support. These components are consistent with those in our intervention. In each of their CYC-C sessions and monthly calls, nurse coaches prompted CGs to describe their day-to-day experiences and express their true thoughts and feelings about any challenges they faced. The coaches were trained not to give advice but rather to facilitate active coping and impel CGs to take control of their own problems and situations with the care recipient. This may have discouraged avoidant coping behaviors, which have been linked to anxiety and depression.46,47 Nurse coaches validated CGs' experiences and provided participants with education and tactics on how to cope with caregiving challenges by positively reframing situations, how to build and use social support networks, and how to communicate clearly and effectively with the care recipient, his or her family, and clinicians. Providing such support early after the diagnosis increased the length of support and thereby may have strengthened the nurse coach–CG relationship, facilitating enhanced emotional and problem-solving support. Earlier timing may also have increased the opportunity for integration and assimilation of the educational content and skills into CGs' own particular circumstances, thus helping CGs to be better prepared for caregiving tasks and emotional stresses.
The intervention seemed to have no significant impact on demand or objective burden. CGs in both groups reported no changes in the perceived care that was demanded of them by care recipients and no changes in interference with their daily routine and leisure time. This could be explained by the progression of the care recipients' advancing illness and functional decline, necessitating constant and potentially increasing demands on CGs, despite any strategies employed by CGs to help alleviate some of their caregiving tasks. It may also be possible that an intervention that is not solely focused on CGs' own personal needs and health may not be robust enough to help CGs relieve themselves of caregiving tasks and gain more time to themselves.43
There are several limitations to this study. As others have noted,48 the challenge in palliative care studies is to maintain an adequate sample in the face of patients becoming more ill. Similarly, for CGs, increasing patient illness will increase care demands, thus affecting their ability to participate. Our results were based on a relatively small CG sample because of both recruitment and attrition. Power estimates were based on the patients only, and patient participants were not required to nominate a CG to be eligible to participate. Because we were not able to meet patient accrual targets, these factors create a potential selection bias. The potential implication of not choosing a single primary outcome is that an inflated type I error rate may result. However, because our results are conceptually logical, it is unlikely that our findings were the result of chance. We observed 32% attrition; however, data analyses revealed no significant associations between attrition and measured CG characteristics or outcome (Appendix Table A1, online only). Even so, we advise caution in interpreting these results. CGs were white, had at least a high school education, were female spouses or partners, and lived in the same geographic area, thus limiting generalizability of the findings. Future CG interventions should be tested in populations with minorities and lower education and in different locations.7,43
Guidelines currently recommend early palliative care for patients17; however, CGs of these patients have received less attention. This study may prompt reconsideration. In our prior RCT, we learned that CGs did not benefit from early palliative care delivered solely to patients.24 In this trial, we included a parallel CG intervention and demonstrated that early initiation of palliative care provided directly to CGs of patients with advanced cancer significantly improved their outcomes. Our intervention was primarily telephone based to accommodate a rural setting and was provided shortly after patients' advanced-cancer diagnosis. These may have been key elements in making this type of support convenient and in teaching and fostering skills early on that could be successfully applied and integrated over time. To date, few interventions provided to CGs of patients with life-limiting illnesses have been able to demonstrate marked benefits, and none have been developed for the rural setting41–45; however, it is critical that researchers find ways to support burdened rural CGs. Future work should continue to devise ways to alleviate the number of tasks and hours individuals spend caregiving and focus on optimizing the physical health of CGs. Longitudinal work should also monitor the bereavement and grief outcomes of CGs who receive early palliative care. Such work should facilitate evidence-based programs and guidelines that positively enhance the lives of those caring for patients with cancer.
Supplementary Material
Acknowledgment
We thank Tim A. Ahles, PhD, Memorial Sloan Kettering Cancer Institute, NY, for consultation on study design; members of the Dartmouth-Hitchcock Section of Palliative Medicine (Ira Byock, MD, Sharona Sachs, MD, and Sandra Knowlton-Soho, RN) and the nurse practitioners, physicians, and staff of the Section of Hematology/Oncology (James Rigas, MD, and Marc Pipas, MD); the Veterans Affairs Medical Center (VAMC) of White River Junction, VT (Stefan Balan, MD, Luann Graves, Lisa Lambert, MD, Nancy Kuemmerle, DO, PhD, and Sarah Colson, APRN); Mountainview Medical Center, Berlin, VT (John Valentine, MD, Elaine Owen, APRN); Dartmouth-Hitchcock Norris Cotton Cancer Center South staff; St Joseph Medical Center, Nashua, NH; Providence, RI, VAMC (Katherine Farisy-Anderson, MD); nurse interventionists (Peggy Bishop, MS, APRN, Peggy Plunkett, MSN, APRN, Lynn Devlin, MS, APRN, Ellen Thompson, MS, RN, Jennifer Frost, MS, RN, Nancy Redfield, MS, APRN, and Nichole L. Sorenson, MSN, APRN); and study staff (Terry Kneeland, MPH, Daphne Ellis, Ingrid Svensborn, RN, Linda Kingman, Kristen R. Allen, MPH, and Tasha Smith, PhD, MPH). Most importantly, we thank all of the patients and caregivers, who were our biggest teachers and contributed to our understanding of their needs as they live with cancer.
Appendix
Table A1.
Tests of Association Between CG Characteristics and Risk of Dropout Using Time-to-Event Models
| Characteristic | Lost to Follow-Up |
HR | 95% CI | P* | |
|---|---|---|---|---|---|
| No. | % | ||||
| Early intervention group | 22 | 36.1 | 1.33 | 0.68 to 2.59 | .40 |
| Age | 19† | 30.2 | 0.91‡ | 0.66 to 1.25 | .55 |
| Male sex | 8 | 30.8 | 0.98 | 0.43 to 2.23 | .96 |
| White race | 34 | 30.1 | 0.31 | 0.08 to 1.19 | .09 |
| Married or living with partner | 33 | 29.5 | 0.44 | 0.16 to 1.23 | .12 |
| ≥ College graduate | 15 | 29.4 | 0.85 | 0.43 to 1.69 | .64 |
| Employment status | |||||
| Full or part time | 19 | 31.7 | 0.61 | 0.27 to 1.35 | .22 |
| Retired | 8 | 22.9 | 0.41 | 0.15 to 1.06 | .07 |
| Other | 11 | 42.3 | Referent | — | |
| Religious affiliation | |||||
| Protestant/Catholic | 24 | 31.6 | 0.95 | 0.37 to 2.43 | .91 |
| None | 6 | 26.1 | 0.72 | 0.22 to 2.38 | .59 |
| Other | 6 | 35.3 | Referent | — | |
| Patient's spouse/partner | 26 | 31.7 | 1.01 | 0.49 to 2.07 | .98 |
| CQOL-C | |||||
| Baseline | 19† | 31.2 | 1.04‡ | 0.73 to 1.47 | .83 |
| Last observed | 20† | 33.3 | 1.16‡ | 0.83 to 1.63 | .39 |
| CESD | |||||
| Baseline | 19† | 29.7 | 1.1‡ | 0.77 to 1.56 | .60 |
| Last observed | 17† | 27.9 | 1.01‡ | 0.72 to 1.43 | .94 |
| MBCB-OB | |||||
| Baseline | 23† | 30.7 | 1.08‡ | 0.76 to 1.52 | .68 |
| Last observed | 17† | 27.9 | 0.86‡ | 0.61 to 1.19 | .35 |
| MBCB-SB | |||||
| Baseline | 20† | 31.8 | 0.95‡ | 0.68 to 1.34 | .77 |
| Last observed | 21† | 32.8 | 1.09‡ | 0.78 to 1.54 | .60 |
| MBCB-DB | |||||
| Baseline | 20† | 31.8 | 1.05‡ | 0.7 to 1.57 | .83 |
| Last observed | 21† | 32.8 | 1.16‡ | 0.8 to 1.7 | .43 |
| Primary disease site of patient | |||||
| Lung | 16 | 30.2 | 1.2 | 0.54 to 2.68 | .66 |
| GI | 12 | 38.7 | 1.81 | 0.76 to 4.32 | .18 |
| Other | 11 | 29 | Referent | — | |
NOTE. Event time was defined as last data collection timepoint for CGs lost to follow-up. Total participant attrition was 39 (32%).
Abbreviations: CESD, Center for Epidemiologic Studies–Depression Scale; CG, caregiver; CQOL-C, Caregiver Quality of Life Scale–Cancer; DB, demand burden; HR, hazard ratio; MBCBS, Montgomery–Borgatta Caregiver Burden Scale; OB, objective burden; SB, stress burden.
Tests conducted with discrete-time Cox proportional hazards models.
Lost to follow-up among participants above median.
HR for standard deviation increase.
Footnotes
See accompanying editorial on page 1420
Supported by Grant No. R01NR011871-01 from the National Institute for Nursing Research; by a postdoctoral fellowship supported by University of Alabama at Birmingham Cancer Prevention and Control Training Program Grant No. 5R25CA047888 (J.N.D.-O.); an NIH/NINR Small Research Grant 1R03NR014915-01 (Zhigang Li) and by Mentored Research Scholar Grant No. MRSG 12-113-01–CPPB in Applied and Clinical Research from the American Cancer Society (K.D.L.).
Presented at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01245621.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Kathleen D. Lyons, Jay G. Hull, Tor Tosteson, Mark T. Hegel, Marie A. Bakitas
Collection and assembly of data: Andres Azuero, Zhongze Li, Jennifer Frost
Data analysis and interpretation: J. Nicholas Dionne-Odom, Andres Azuero, Kathleen D. Lyons, Jay G. Hull, Tor Tosteson, Zhigang Li, Konstantin H. Dragnev, Imatullah Akyar, Mark T. Hegel, Marie A. Bakitas
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Benefits of Early Versus Delayed Palliative Care to Informal Family Caregivers of Patients With Advanced Cancer: Outcomes From the ENABLE III Randomized Controlled Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
J. Nicholas Dionne-Odom
No relationship to disclose
Andres Azuero
No relationship to disclose
Kathleen D. Lyons
No relationship to disclose
Jay G. Hull
No relationship to disclose
Tor Tosteson
No relationship to disclose
Zhigang Li
No relationship to disclose
Zhongze Li
No relationship to disclose
Jennifer Frost
No relationship to disclose
Konstantin H. Dragnev
No relationship to disclose
Imatullah Akyar
No relationship to disclose
Mark T. Hegel
Research Funding: Johnson & Johnson
Marie A. Bakitas
No relationship to disclose
REFERENCES
- 1.Howlader N, Noone A, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2010. Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 2.Yabroff KR, Kim Y. Time costs associated with informal caregiving for cancer survivors. Cancer. 2009;115(suppl):4362–4373. doi: 10.1002/cncr.24588. [DOI] [PubMed] [Google Scholar]
- 3.Bee P, Barnes P, Luker K. A systematic review of informal caregivers' need in providing home-based end-of-life care to people with cancer. J Clin Nurs. 2008;18:1379–1393. doi: 10.1111/j.1365-2702.2008.02405.x. [DOI] [PubMed] [Google Scholar]
- 4.Hodges LJ, Humphris GM, Macfarlane G. A meta-analytic investigation of the relationship between the psychological distress of cancer patients and their carers. Soc Sci Med. 2005;60:1–12. doi: 10.1016/j.socscimed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Palos GR, Mendoza TR, Liao KP, et al. Caregiver symptom burden: The risk of caring for an underserved patient with advanced cancer. Cancer. 2011;117:1070–1079. doi: 10.1002/cncr.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinquart M, Sörensen S. Correlates of physical health of informal caregivers: A meta-analysis. J Gerontol B Psychol Sci Soc Sci. 2007;62:P126–P137. doi: 10.1093/geronb/62.2.p126. [DOI] [PubMed] [Google Scholar]
- 7.Stenberg U, Ruland CM, Miaskowski C. Review of the literature on the effects of caring for a patient with cancer. Psychooncology. 2010;19:1013–1025. doi: 10.1002/pon.1670. [DOI] [PubMed] [Google Scholar]
- 8.Perkins M, Howard VJ, Wadley VG, et al. Caregiving strain and all-cause mortality: Evidence from the REGARDS study. J Gerontol B Psychol Sci Soc Sci. 2013;68:504–512. doi: 10.1093/geronb/gbs084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji J, Zöller B, Sundquist K, et al. Increased risks of coronary heart disease and stroke among spousal caregivers of cancer patients. Circulation. 2012;125:1742–1747. doi: 10.1161/CIRCULATIONAHA.111.057018. [DOI] [PubMed] [Google Scholar]
- 10.Gamm L, Hutchison L, Dabney B, et al. Rural Health People 2010: A Companion Document to Healthy People 2010 (volume 2) College Station, TX, Texas A&M University System Health Science Center, School of Rural Public Health: Southwest Rural Health Research Center; 2003. [Google Scholar]
- 11.Robinson CA, Pesut B, Bottorff JL, et al. Rural palliative care: A comprehensive review. J Palliat Med. doi: 10.1089/jpm.2008.0228. [epub ahead of print on February 13, 2009] [DOI] [PubMed] [Google Scholar]
- 12.Hudson P, Remedios C, Zordan R, et al. Guidelines for the psychosocial and bereavement support of family caregivers of palliative care patients. J Palliat Med. 2012;15:696–702. doi: 10.1089/jpm.2011.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. National Cancer Control Programmes: Policies and Managerial Guidelines (ed 2) http://www.who.int/cancer/media/en/408.pdf.
- 14.Centers for Disease Control and Prevention. Assuring Healthy Caregivers: A Public Health Approach to Translating Research Into Practice—The RE-AIM Framework. Atlanta, GA: Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 15.Institute of Medicine. Delivering High Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: National Academies Press; 2013. [PubMed] [Google Scholar]
- 16.Dahlin C (ed) Clinical Practice Guidelines for Quality Palliative Care. Pittsburgh, PA: National Consensus Project; 2013. [Google Scholar]
- 17.Smith T, Temin S, Alesi E, et al. American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol. 2012;30:880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 18.Hui D, Kim SH, Kwon JH, et al. Access to palliative care among patients treated at a comprehensive cancer center. Oncologist. 2012;17:1574–1580. doi: 10.1634/theoncologist.2012-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osta BE, Palmer JL, Paraskevopoulos T, et al. Interval between first palliative care consult and death in patients diagnosed with advanced cancer at a comprehensive cancer center. J Palliat Med. 2008;11:51–57. doi: 10.1089/jpm.2007.0103. [DOI] [PubMed] [Google Scholar]
- 20.Stamataki Z, Ellis JE, Costello J, et al. Chronicles of informal caregiving in cancer: Using “The Cancer Family Caregiving Experience” model as an explanatory framework. Support Care Cancer. 2014;22:435–444. doi: 10.1007/s00520-013-1994-1. [DOI] [PubMed] [Google Scholar]
- 21.Wadhwa D, Burman D, Swami N, et al. Quality of life and mental health in caregivers of outpatients with advanced cancer. Psychooncology. 2013;22:403–410. doi: 10.1002/pon.2104. [DOI] [PubMed] [Google Scholar]
- 22.Schulz R. Research priorities in geriatric palliative care: Informal caregiving. J Palliat Med. 2013;16:1008–1012. doi: 10.1089/jpm.2013.9483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakitas M, Lyons K, Hegel M, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Hara RE, Hull JG, Lyons KD, et al. Impact on caregiver burden of a patient-focused palliative care intervention for patients with advanced cancer. Palliat Support Care. 2010;8:395–404. doi: 10.1017/S1478951510000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams AL, Bakitas M. Cancer family caregivers: A new direction for interventions. J Palliat Med. 2012;15:775–783. doi: 10.1089/jpm.2012.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakitas M, Tosteson T, Li Z, et al. The ENABLE III randomized controlled trial of concurrent palliative oncology care. J Clin Oncol. 2014;32(suppl 15s):605s. doi: 10.1200/JCO.2014.58.6362. abstr 9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higginson IJ, Vivat B, Silber E, et al. Study protocol: Delayed intervention randomised controlled trial within the Medical Research Council (MRC) framework to assess the effectiveness of a new palliative care service. BMC Palliat Care. 2006;5:7. doi: 10.1186/1472-684X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farquhar M, Higginson IJ, Booth S. Fast-track trials in palliative care: An alternative randomized controlled trial design. J Palliat Med. 2009;12:213. doi: 10.1089/jpm.2008.0267. [DOI] [PubMed] [Google Scholar]
- 29.US Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. https://www.census.gov/geo/reference/ua/urban-rural-2010.html.
- 30.Callahan CM, Unverzagt FW, Hui SL, et al. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Bakitas M, Lyons KD, Hegel MT, et al. The project ENABLE II randomized controlled trial to improve palliative care for rural patients with advanced cancer: Baseline findings, methodological challenges, and solutions. Palliat Support Care. 2009;7:75–86. doi: 10.1017/S1478951509000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maloney C, Lyons KD, Li Z, et al. Patient perspectives on participation in the ENABLE II randomized controlled trial of a concurrent oncology palliative care intervention: Benefits and burdens. Palliat Med. 2013;27:375–383. doi: 10.1177/0269216312445188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McMillan SC, Small BJ, Weitzsner MA, et al. Impact of coping skills intervention with family caregivers of hospice patients with cancer: A randomized clinical trial. Cancer. 2006;106:214–222. doi: 10.1002/cncr.21567. [DOI] [PubMed] [Google Scholar]
- 34.Weitzner M, Jacobsen PB, Wagner H, et al. The caregiver quality of life index-cancer (CQOL-C) scale: Development and validation of an instrument to measure quality of life of the family of caregiver of patients with cancer. Qual Life Res. 1999;8:55–63. doi: 10.1023/a:1026407010614. [DOI] [PubMed] [Google Scholar]
- 35.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 36.Okun A, Stein RE, Bauman LJ, et al. Content validity of the Psychiatric Symptom Index, CES-depression Scale, and State-Trait Anxiety Inventory from the perspective of DSM-IV. Psychol Rep. 1996;79:1059–1069. doi: 10.2466/pr0.1996.79.3.1059. [DOI] [PubMed] [Google Scholar]
- 37.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients: Evaluation of the Center for Epidemiological Studies Depression Scale (CES-D) J Psychosom Res. 1999;46:437–443. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery R, Gonyea J, Hooyman N. Caregiving and the experience of subjective and objective burden. Fam Relat. 1985;34:19–26. [Google Scholar]
- 39.Montgomery R, Borgatta E, Borgatta M. Societal and family change in the burden of care. In: Liu B, Kendig H, editors. Who Should Care of the Elderly? An East-West Value Divide. Singapore: The National University of Singapore Press; 2000. pp. 27–54. [Google Scholar]
- 40.Kurland BF, Johnson LL, Egleston BL, et al. Longitudinal data with follow-up truncated by death: Match the analysis method to research aims. Stat Sci. 2009;24:211. doi: 10.1214/09-STS293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Candy B, Jones L, Drake R, et al. Interventions for supporting informal caregivers of patients in the terminal phase of a disease. Cochrane Database Syst Rev. 2011;6:CD007617. doi: 10.1002/14651858.CD007617.pub2. [DOI] [PubMed] [Google Scholar]
- 42.Lorenz KA, Lynn J, Dy SM, et al. Evidence for improving palliative care at the end of life: A systematic review. Ann Intern Med. 2008;148:147–159. doi: 10.7326/0003-4819-148-2-200801150-00010. [DOI] [PubMed] [Google Scholar]
- 43.Northouse LL, Katapodi MC, Song L, et al. Interventions with family caregivers of cancer patients: Meta-analysis of randomized trials. CA Cancer J Clin. 2010;60:317–339. doi: 10.3322/caac.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Adelman RD, Tmanova LL, Delgado D, et al. Caregiver burden: A clinical review. JAMA. 2014;311:1052–1060. doi: 10.1001/jama.2014.304. [DOI] [PubMed] [Google Scholar]
- 45.Gomes B, Calanzani N, Curiale V, et al. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2013;6:CD007760. doi: 10.1002/14651858.CD007760.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goetzinger AM, Blumenthal JA, O'Hayer CV, et al. Stress and coping in caregivers of patients awaiting solid organ transplantation. Clin Transplant. 2012;26:97–104. doi: 10.1111/j.1399-0012.2011.01431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lambert SD, Girgis A, Lecathelinais C, et al. Walking a mile in their shoes: Anxiety and depression among partners and caregivers of cancer survivors at 6 and 12 months post-diagnosis. Support Care Cancer. 2013;21:75–85. doi: 10.1007/s00520-012-1495-7. [DOI] [PubMed] [Google Scholar]
- 48.Hui D, Glitza I, Chisholm G, et al. Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer. 2013;119:1098–1105. doi: 10.1002/cncr.27854. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


