Abstract
Gestational diabetes mellitus (GDM) is the most common medical complication of pregnancy. It is associated with maternal and neonatal adverse outcomes. Maintaining adequate blood glucose levels in GDM reduces morbidity for both mother and baby. There is a lack of uniform strategies for screening and diagnosing GDM globally. This review covers the latest update in the diagnosis and management of GDM. The initial treatment of GDM consists of diet and exercise. If these measures fail to achieve glycemic goals, insulin should be initiated. Insulin analogs are more physiological than human insulin, and are associated with less risk of hypoglycemia, and may provide better glycemic control. Insulin lispro, aspart, and detemir are approved to be used in pregnancy. Insulin glargine is not approved in pregnancy, but the existing studies did not show any contraindications. The use of oral hypoglycemic agents; glyburide and metformin seems to be safe and effective in pregnancy.
Pregnancy is associated with insulin resistance (IR) and hyperinsulinemia that may predispose some women to develop diabetes. Gestational diabetes has been defined as any degree of glucose intolerance with an onset, or first recognition during pregnancy.1 This definition does not exclude the possibility that unrecognized glucose intolerance may have antedated the pregnancy, and so, the term hyperglycemia in pregnancy emerges to be more appropriate as suggested lately by the Endocrine Society.2 The International Association of Diabetes and Pregnancy Study Groups (IADPSG) classify hyperglycemia first detected during pregnancy as either ‘overt diabetes’ or ‘gestational diabetes mellitus (GDM)’.3 In 2013, the World Health Organization (WHO) recommended that hyperglycemia first detected during pregnancy be classified as either ‘diabetes mellitus (DM) in pregnancy’ or ‘GDM’.4
The prevalence of GDM varies from 1-20%, and is rising worldwide, parallel to the increment in the prevalence of obesity and type 2 diabetes mellitus (T2DM).5 The amount of GDM varies in direct proportion to the prevalence of T2DM in a given population, or ethnic group. The prevalence rates for GDM are higher for African, Hispanic, Indian, and Asian women than for Caucasian women.5,6 Recently, the prevalence of GDM has increased by 2-3 folds, ranging from 8.9-53.4%.7-16 This is mainly due to the adoption of the new criteria proposed by the IADPSG on screening, and diagnosis of GDM.3 The IADPSG recommends universal screening for GDM, and requires one single glucose value above the cut-off value (instead of 2) during the oral glucose tolerance test (OGTT) for diagnosis.3 This dramatic rise in the GDM prevalence will have a major impact on health care systems. Also, the consequences of labeling a large number of women with GDM are not known. The GDM is associated with adverse maternal and neonatal sequelae.17,18 In a Hyperglycemia and Adverse Pregnancy Outcomes Study (HAPO),17 a large-scale (25,000 pregnant women) multinational epidemiologic study found significant associations between adverse pregnancy outcomes, and higher levels of maternal glucose with no defined levels, after which the risk increases. Furthermore, the Australian Carbohydrate Intolerance Study (ACHOIS) in pregnant women,19 and the US multicenter randomized trial of treatment for mild gestational diabetes studies20 indicated that maternal hyperglycemia that does not meet diagnostic criteria for overt diabetes still has a correlation with perinatal disorders and problems. This association suggests a need to re-evaluate the standard criteria for diagnosing, and treating hyperglycemia in pregnancy, and welcome the new criteria. The goal of this review is to discuss the latest update in the diagnosis and management of GDM.
Pathophysiology
During normal pregnancy, a progressive IR develops beginning around mid-pregnancy, and progresses during the third trimester.21 Hormones and adipokines secreted from the placenta, including tumor necrosis factor (TNF)-α, human placental lactogen, and human placental growth hormone are possible causes of IR in pregnancy. In addition, increased estrogen, progesterone, and cortisol during pregnancy contribute to a disruption of the glucose insulin balance.22 To compensate for the peripheral IR during pregnancy, insulin secretion increases from a woman’s pancreas. The development of GDM occurs when a woman’s pancreas does not secrete enough insulin to keep up with the metabolic stress of the IR. In addition, increased maternal adipose deposition, decreased exercise, and increased caloric intake contribute to this state of relative glucose intolerance.
Course of insulin sensitivity during pregnancy
In early pregnancy, insulin secretion increases, while insulin sensitivity is unchanged, decreased, or may even increase. At mid pregnancy, insulin sensitivity starts to decline progressively, and became worse during the rest of the pregnancy, being worst in the late third trimester. It rebounds with the delivery of the placenta. Therefore, GDM usually develops in the late second trimester and disappears, instantly, post delivery.21
Risk factors for GDM
Several risk factors are associated with the development of GDM. The most common risk factors are; obesity, older maternal age, past history of GDM, strong family history of diabetes, member of an ethnic group with a high prevalence of T2DM, polycystic ovary syndrome, and persistent glucosuria. A history of delivering big baby (birth weight ≥4000 g), history of recurrent abortions, and history of unexplained stillbirths, and history of essential hypertension, or pregnancy-related hypertension are other risk factors for GDM.23
Risks of GDM
Women with GDM have an increased incidence of hypertensive disorders during pregnancy, including gestational hypertension, pre-eclampsia, and eclampsia.17 There is an increase risk of polyhydramnios that may increase the risk of preterm labor. Excessive fetal growth remains an important perinatal concern in GDM. Consequences of excessive fetal growth include birth trauma, maternal morbidity from cesarean deliveries, shoulder dystocia, and neonatal hypoglycemia.17 Other neonatal morbidities that potentially occur more frequently in infants of women with GDM include hyperbilirubinemia, hypocalcemia, erythema, and respiratory distress syndrome.17 Long-term complications of GDM include diabetes and cardiovascular disease in the mothers,24 and obesity and diabetes in the offspring.25 Congenital anomalies do not occur at an increased rate in patients with gestational diabetes, as GDM usually occurs at the late second trimester when embryogenesis is completed.
Screening and diagnosis of GDM
There is a lack of uniform strategies for screening and diagnosing GDM globally (Table 1). The first diagnostic criteria for gestational diabetes were provided by O´Sullivan and Mahan in the 1960’s based on a 3-hour 100 g OGTT.26 These criteria have been derived to identify women at high risk of developing diabetes after pregnancy. Many medical organizations around the world followed the original work of O’Sullivan and Mahan,26 and modified by either Carpenter and Coustan,27 or the National Diabetes Data Group (NDDG),1 although frequently selecting different thresholds for GDM diagnosis.
Table 1.
Commonly used guidelines by different study groups for gestational diabetes mellitus (GDM).
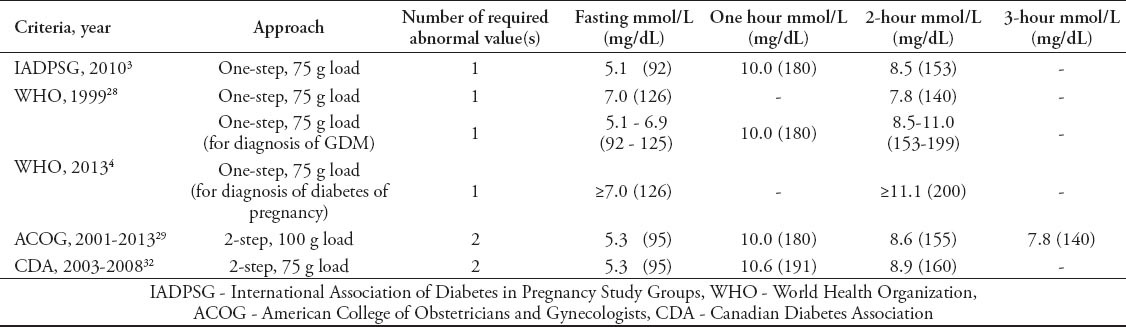
In March 2010, a consensus panel from the IADPSG; an international consensus group with representatives from multiple obstetrical and diabetes organizations including the American Diabetes Association (ADA), published new recommendations for the screening and diagnosis of GDM.3 The IADPSG recommend universal screening for gestational diabetes. At the first antenatal visit, IADPSG recommends screening pregnant women for GDM using standard criteria to diagnose diabetes in non pregnant state to identify women with overt diabetes “pre-existing diabetes”. A diagnosis of overt diabetes can be established in women who meet any of the following criteria: fasting plasma glucose level (FPG) ≥7.0 mmol/l (126 mg/dl), a casual plasma glucose of 11.1 mmol/l (≥200 mg/dl), or HbA1c ≥6.5. In the absence of unequivocal hyperglycemia, the diagnosis must be confirmed on a subsequent day. Confirmation of the diagnosis precludes the need for OGTT. If FPG is >5.0 mmol/l (90 mg/dl) but <7.0 mmol/l (126 mg/dl) at any gestational age, a diagnosis of GDM can be made. If early screening is negative, the IADPSG recommends universal screening to be performed at 24-28 weeks of gestation with a 2-hour (h), 75-g OGTT “one-step approach”. Gestational diabetes is diagnosed, if one or more values equal, or exceeds thresholds; FPG (5.1 mmol/l [92 mg/dl]), one h plasma glucose (10 mmol/l [180 mg/dl]), and 2-h plasma glucose (8.5 mmol/l [153 mg/dl]). These cut-off values were chosen arbitrary by the IADPSG based on the HAPO study17 to express an odds ratio for adverse outcomes of at least 1.75 compared with women with mean glucose levels in the HAPO study.17 The OGTT should be performed after fasting overnight for 8-14 hours, and not reducing the usual carbohydrate intake for the preceding several days.3
In January 2011, the Standards of Care of ADA endorsed the IADPSG recommendations.6 In addition, the Endocrine Society recently endorsed the IADPSG recommendations.2 The WHO updated their recommendations in 2013, and recommended glucose cut-off values for GDM corresponding to those proposed by IADPSG.4 The difference from the IADPSG guidelines is that the new WHO guidelines set a range of plasma glucose levels to distinguish diabetes in pregnancy and GDM (Table 1).4 The past diagnostic criteria recommended by the WHO in 199928 for hyperglycemia in pregnancy were those used in non-pregnant individuals (Table 1). An issue that has been problematic with these criteria relates to the FPG criterion, as the diagnostic level of ≥7.0 mmol/l is universally considered to be too high. Other organizations around the world are re-addressing their criteria for screening and diagnosis of GDM since the emergence of IADPSG recommendations. On the other side, the American Association of Obstetricians and Gynecologists (ACOG),29 and the National Institute of Health (NIH)30 have not endorsed the IADPSG recommendations, and still recommend the traditional “2-step approach”, in which an initial screening between 24-28 weeks by 50 g oral glucose challenge test (GCT), and measuring the plasma glucose concentration after one hour. Afterward, a diagnostic 3-hour 100-g OGTT is recommended for those women who exceeded the glucose threshold of ≥7.2, or ≥7.8 mmol/L (130 or 140 mg/dL) in GCT.29,30 In the 2014 Standards of Care,31 the ADA readdressed the NIH recommendation along with the IADPSG guidelines as there is insufficient data to strongly demonstrate the superiority of one strategy over the other.
Management of GDM
The cornerstone of GDM management is glycemic control. The initial treatment for GDM is lifestyle interventions, which include medical nutrition therapy and daily exercise. Patients are required to check their glucose level frequently at home to assure that the glycemic targets are achieved. If the glycemic goals are not accomplished with these measurements, medical therapy should be initiated.
Blood glucose monitoring
Women are instructed to carry out self monitoring of blood glucose (SMBG) 4 times a day, fasting glucose (upon awakening), and one or 2 hour post-meals (after the first bite of a meal). In GDM, monitoring of blood glucose after meals is preferred over pre-meal testing as the risk of macrosomia increases with increased maternal glucose levels post-meals.33 This was illustrated in a randomized clinical trial,34 which compared preprandial glucose monitoring to one hour post-prandial (PP) testing, and found macrosomia, cesarean deliveries, and neonatal hypoglycemia were significantly less frequent in women who monitor their glucose post-meals. However, it is not known whether a one hour, or 2-hour PP testing is the ideal goal for the prevention of fetal risks. Therefore, patients can monitor their glucose levels at one or 2 hours post-meal, whatever is convenient, or at the estimated peak blood glucose is most likely to occur post prandial, for example, choosing the time at which glucose was elevated during OGTT.2
The glycosylated hemoglobin (HbA1C) values tend to be less in pregnant women than in non-pregnant, and this is because the average blood glucose concentration tends to be lower in pregnant women. In addition, the rise in red cell mass and the rise in red blood cell turnover during pregnancy contributes to a lower HbA1C. For this reason, frequent monitoring of HbA1C to assess glycemic control during pregnancy in women with GDM may not be useful in those with low HbA1C levels at initial visit. However, its measurement may be helpful in those with overt diabetes with an HbA1C >6.5%.35
Glycemic targets
The target glycemic goals for women with GDM is to keep the fasting glucose ≤5-5.3 mmol/l (90-95 mg/dl), and either one-hour post-meal ≤ 7.8 mmol/l (140 mg/dl), or 2-h post-meal ≤ 6.7 mmol/l (120 mg/dl).2,36 These values are more stringent for pregnant women than they are for non-pregnant patients with diabetes.
Medical nutritional therapy
Medical nutritional therapy is the keystone of treating GDM as it maintains desired glycemic goals in 80-90% of GDM women.37 The optimal dietary prescription would be a diet that provides adequate nutrition to support fetal and maternal well-being, while maintaining normoglycemia with absence of ketones, and achieving appropriate weight gain in pregnancy.38 Caloric allowance could be calculated based on ideal body weight: 30 kcal/kg for women with a BMI of 22-25; 24 kcal/kg for women with a BMI of 26-29; and 12-15 kcal/kg for women with a BMI above 30.37 The carbohydrate intake should be reduced to 35-45% of the total calories, and distributed over 3 meals, and 2-4 snacks including bedtime snack, this assists in reducing PP glucose peak, but ensures adequate nutrition to the mother and the fetus.2 Excessive weight gain should be discouraged as it increases further the risk of delivering a large-for-gestational-age infant, adverse pregnancy outcome, and childhood obesity.39 The recommended weight gain during singleton pregnancy is dependent on pre-pregnancy BMI: 12.5-18 kg of weight gain for underweight women (BMI <18.5 kg/m2); 11.5-16 kg for normal weight (BMI 18.5-24.9 kg/m2); 7-11.5 kg for overweight (BMI 25-29.9 kg/m2), and 5-9 kg for obese (BMI ≥30.0 kg/m2).40
Exercise
Exercise has been shown to improve glycemic control in GDM. Daily moderate exercise for 30 minutes or more is recommended for a woman with GDM, if she has no medical or obstetrics contraindications. Advising GDM patients to walk briskly, or do arm exercises while seated in a chair for at least 10 minutes after each meal facilitates in reducing glucose rise post-meal, and help in achieving glycemic goal.2,36
Pharmacological interventions. Insulin therapy
If the medical nutrition therapy and exercise fail to achieve glycemic goals for a woman with GDM, insulin therapy should be initiated. The type and timing of insulin should be chosen based on the specific blood glucose elevation. If the fasting glucose is greater than 90-95 mg/dl (whole blood capillary) then basal insulin, long-acting insulin analog, or neutral protamine Hagedorn (NPH); 4 units for example, should be started before bedtime. If fasting glucose level is too high, then basal insulin dose can be calculated according to the patient’s weight, 0.2 units/kg/day. In cases where glucose level is elevated following a meal, rapid-acting insulin, or regular insulin should be prescribed before that specific meal, beginning with 2-4 units, or a dose of one unit per 10-15 g of carbohydrates. If both fasting and PP glucose levels are elevated, a 4-injections-per-day regimen “basal and meal time insulin regimen” should be prescribed.37 Basal and meal time insulin regimen is preferred over twice dose regimen because it is more likely achieves, maintains target blood glucose, and allows more flexibility.2 One could start by 2-4 units of rapid-acting insulin, or regular insulin before each meal, and 2-4 units of basal insulin before bed time. Another approach to determine the insulin doses is based on a woman’s body weight and gestational week. In the first trimester, the total daily insulin requirement is 0.7 units/kg/day, in the second trimester it is 0.8 units/kg/day, and in the third trimester it is 0.9-1.0 units/kg/day.37 In a morbidly obese woman, the initial doses of insulin may need to be increased to 1.5-2.0 units/kg to overcome the combined IR of pregnancy and obesity.37 Subsequently, the calculated total daily dose of insulin should be divided into 2 halves; one half given as basal insulin at bed time, and the other half divided between 3 meals, and given as rapid-acting, or regular insulin before meals (Figure 1). As insulin requirement may increase with the progression of pregnancy, it is crucial to follow patients’ SMBG regularly, and optimize their insulin doses.
Figure 1.
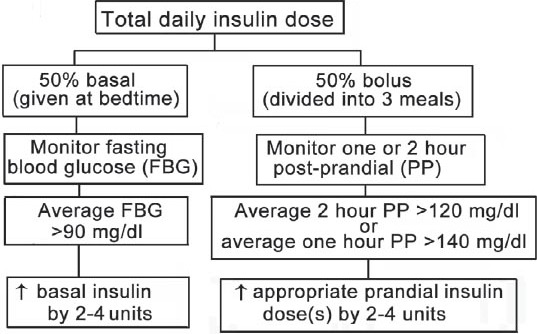
Initiation and optimization of insulin therapy in hyperglycemia during pregnancy.
In the past, regular insulin and NPH were commonly used to treat GDM. However, currently rapid-acting insulin analogs are preferred over regular insulin in pregnancy as they are associated with less risk of hypoglycemia, and may also provide better PP blood glucose control.41,42 Both lispro and aspart insulin are approved to be used in pregnancy.2 There is no data regarding the safety of glulisine in pregnancy. The long-acting insulin analogs do not have a pronounced peak effect as NPH, and therefore, cause less nocturnal hypoglycemia. Insulin detemir has not shown to have adverse maternal or neonatal effects, and has been approved by the Food and Drug Administration (FDA) to be used in pregnancy.43 The use of insulin glargine in pregnancy is not approved yet, but the existing studies did not show any contraindications, and the outcome with glargine treatment was not different from, or superior to, NPH insulin.44,45
Non-insulin antihyperglycemic agent therapy
While insulin is considered the gold standard for management of GDM, it is expensive, invasive, involves daily injections, and patient compliance is often suboptimal. On the other hand, oral hypoglycemic agents are less expensive, less invasive, and more acceptable, and could enhance patient compliance, and might achieve similar perinatal outcome as insulin.
Since GDM is characterized by IR and relatively decreased insulin secretion, a treatment with non-insulin antihyperglycemic agents could be of a potential interest. The main concerns of using non-insulin antihyperglycemic agents in pregnancy are congenital anomalies and fetal hypoglycemia. Most available data on non-insulin antihyperglycemic agents’ safety in pregnancy focuses on glyburide and metformin. In Europe and South Africa, glyburide and metformin have been used in pregnancy for years without reported adverse side effects to the fetus. Most oral antihyperglycemic agents cross the placenta and stimulate fetal hyperinsulinism, except glyburide.46
Glyburide (glibenclamide)
Clinical experience with glyburide treatment of GDM is increasing. When compared with insulin, glyburide had a similar success rate in achieving targeted glucose levels, favorable pregnancy outcomes, and significantly fewer hypoglycemic episodes than insulin.47 However, several of these patients treated with glyburide continue to require insulin, in order to maintain optimal glycemic control. The rate of glyburide failure in GDM is 20%. Failure is predicted if fasting glucose levels is greater than 115 mg/dL, higher glucose levels in the OGTT, in obese patients and if the diagnosis is made before 25 weeks’ gestation.48,49 Suggested glyburide dosing in pregnancy is to start with 2.5 mg in the morning. If glycemic control is not achieved, increase glyburide to 5 mg in the morning, then add 5 mg in the evening when advisable. If the targeted level of glycemic control is still not achieved, 5 mg should be added to the morning, and then to the evening doses for a total of 20 mg.50 Long-acting insulin can be added if glyburide dosing is not adequate.
Metformin
Metformin is the other oral hypoglycemic agent that is being considered as a substitute to insulin in treating patients with GDM. Metformin may be a more logical alternative to insulin for women with GDM, as it is not associated with risks of maternal hypoglycemia and weight gain. Metformin is classified as a category B drug, which implies that there is no evidence of animal, or fetal toxicity or teratogenicity. Although, metformin has been shown to pass freely across the placenta,51 there are no reported adverse side effects to the fetus when it is used to treat women with infertility caused by polycystic ovary syndrome (PCOS).52,53 In this situation, PCOS women might be exposed to metformin during part, or the entire period of embryogenesis.
The study of metformin in pregnancy revealed that the use of metformin in women with GDM was not associated with increased risk of congenital anomalies, or maternal and neonatal complications compared to insulin, except for higher rates of preterm labor.54 Metformin was associated with lower rates of neonatal hypoglycemia than insulin. Moreover, women who used metformin were more likely to say they would use metformin in a subsequent pregnancy than were women on insulin.54 The failure rate of metformin in GDM appeared to be higher than glyburide.48,54 In the aforementioned study, 46% of the women on metformin needed to be on supplemental insulin.54 Metformin is prescribed at a starting dose of 500 mg, and can be increased gradually up to 2500 mg, as tolerated, and depending on maternal glucose level. If diabetes control is not achieved, insulin should be started.54
While the use of oral hypoglycemic agents is not approved by the US FDA, many practices have successfully used glyburide,55 or metformin56 to manage GDM. Recently, the Endocrine Society Clinical Practice Guidelines in Diabetes and Pregnancy suggests that glyburide is a suitable alternative to insulin therapy for glycemic control in women with GDM, who fail to achieve sufficient glycemic control with medical nutrition therapy and exercise. It also suggests metformin therapy to be used in women with GDM, only for those who do not have satisfactory glycemic control despite medical nutrition therapy, and who refuses or cannot use insulin or glyburide, and are not in the first trimester.2 The latest practice bulletin by the ACOG on the management of GDM has indicated that insulin and oral medications are equivalent in efficacy, as pharmacologic treatment of gestational diabetes and either can be an appropriate first-line therapy.29 Before instituting glyburide or metformin to treat pregnant woman with GDM, the physician should discuss fully with her, the possible advantages and disadvantages of glyburide, or metformin compared to insulin therapy, and their lack of FDA approval for this indication.2
There is no controlled data on the use of other non-insulin antihyperglycemic agents during pregnancy, and they should not be used.
Intrapartum care
There is no universal recommendation on the ideal time for delivering mothers with GDM, and it is not known whether induction of labor, or expectant labor is more efficacious. However, there are no indications to pursue delivery before 40 weeks of gestation in patients with good glycemic control, and with no other complications. On the other hand, some studies suggested induction of labor at 38-39 weeks in the case of insulin-treated GDM patients, or when the ultrasound exam shows signs of fetal macrosomia.57
During labor and delivery, the goal is to maintain normoglycemia, that is, blood glucose level between 4-7 mmol/L (72-126 mg/dL)2 in order to prevent neonatal hypoglycemia.58 Generally, patients with diet-controlled diabetes will not require intrapartum insulin, and may simply need glucose level monitoring on admission, and then every 4-6 hours. Patients with insulin-requiring GDM need capillary glucose monitoring every one to 2 hours. There is no clear recommendation for an optimal approach for glucose/insulin management during labor. A suggested approach is to administer dextrose 5% normal saline (NS) at a rate of 125 cc/hour, and change it to NS, or Ringer Lactate when glucose level exceeds 5.6 mmol/L (100 mg/dl). Insulin infusion should be initiated when glucose level exceeds 7.8 mmol/L (140 mg/dl), and the dose should be titrated according to the capillary glucose level to keep the glucose level in the target range.59
Postpartum care
Fasting glucose level should be monitored 24-72 hours after delivery to ascertain that the mother is no longer hyperglycemic according to the criteria for non-pregnant individuals. In general, no further insulin is required postpartum for women with GDM, and they should be able to resume a normal diet, as 95% of them will return to a completely normal glucose status. If fasting glucose concentrations suggests overt diabetes (fasting ≥7 mmol/L [126 mg/dl]), or random glucose ≥11.1 mmol/L (200 mg/dL), treatment is warranted; which includes diet, exercise, reducing weight, and medication if needed. For those who have fasting glucose levels below 7 mmol/L (126 mg/dl), they should perform OGTT within 6-12 weeks after delivery to help detect the women who remain diabetic, and require further treatment. For this screening, the 75 g 2-hour OGTT is recommended, using the non-pregnant criteria.
If the OGTT is negative, there should be repeat screening for diabetes every 3 years as 5-50% of women develop T2DM within 5 years after GDM. Risk factors for developing future diabetes are obesity, diagnosis of GDM in early gestational age, insulin therapy during the index pregnancy, neonatal hypoglycemia, and repeated history of GDM.60 Lifelong healthy eating and regular physical activity should be encouraged post delivery to all women with GDM. Breastfeeding should be also encouraged as it may reduce maternal and neonatal risk for the later development of T2DM.61-63 Drug therapy, like metformin or glyburide, might be considered for those with overt diabetes. The use of metformin and glyburide by breastfeeding mothers, when necessary, appears to be safe. The concentration of metformin in breast milk is generally low.64,65 The available data suggest that the levels of glyburide in milk are negligible, and hypoglycemia was not observed in nursing infants of women using glyburide.65 Recent Endocrine Society Clinical Practice Guideline on Diabetes and Pregnancy suggest women with overt diabetes who used metformin, or glyburide therapy during pregnancy can continue to use these medications, if necessary, during breastfeeding.2
In conclusion, the prevalence of GDM is raising worldwide parallel to the increment in the prevalence of obesity and T2DM. Gestational diabetes mellitus is associated with maternal and neonatal adverse outcomes. Maintaining adequate blood glucose levels in GDM reduces morbidity for both mother and baby. There is still a lack of uniform strategies for screening and diagnosing GDM worldwide. Therefore, there is a need to standardize the screening and the diagnostic criteria for GDM globally.
Treatment of GDM consists of diet and exercise. Insulin should be initiated if the initial measures fail to achieve glycemic goals. Insulin analogs are associated with less risk of hypoglycemia, and may provide better glycemic control than human insulin. The use of oral hypoglycemic agents in pregnancy might soon shift the paradigm in the treatment of GDM.
Footnotes
References
- 1.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 2.Blumer I, Hadar E, Hadden DR, Jovanovič L, Mestman JH, Murad MH, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4227–4249. doi: 10.1210/jc.2013-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. Geneva (CH): World Health Organization; 2013. Available from: http: //130.14.29.110/books/NBK169024 . [PubMed] [Google Scholar]
- 5.Bevier WC, Jovanovic-Peterson L, Peterson CM. Pancreatic disorders of pregnancy. Diagnosis, management, and outcome of gestational diabetes. Endocrinol Metab Clin North Am. 1995;24:103–138. [PubMed] [Google Scholar]
- 6.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal MM, Dhatt GS, Shah SM. Gestational Diabetes Mellitus Simplifying the International Association of Diabetes and Pregnancy diagnostic algorithm using fasting plasma glucose. Diabetes Care. 2010;33:2018–2020. doi: 10.2337/dc10-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahanayaka NJ, Agampodi SB, Ranasinghe OR, Jayaweera PM, Wickramasinghe WA, Adhikari AN, et al. Inadequacy of the risk factor based approach to detect gestational diabetes mellitus. Ceylon Med J. 2012;57:5–9. doi: 10.4038/cmj.v57i1.4193. [DOI] [PubMed] [Google Scholar]
- 9.Lacaria CS, Ghio A, Lemmi P, Battini L, Resi V, Volpe L, et al. Epidemiologic Implications of the New Diagnostic Criteria for Gestational Diabetes. International Diabetes Federation 2011. World Diabetes Congress; Dubai (UAE): 4-8 December; 2011. [Google Scholar]
- 10.Hutton E, Man G, Allan C, Soldatos G. The Changing Prevalence of GDM Post Adoption of the New Proposed International Association of the Diabetes and Pregnancy Study Groups (IADPSG) Guidelines. Available from: http: //ads-adea-2012.m.asnevents.com.au/schedule/session/453/abstract/1596 . [Google Scholar]
- 11.Jenum AK, Mørkrid K, Sletner L, Vangen S, Torper JL, Nakstad B, et al. Impact of ethnicity on gestational diabetes identified with the WHO and the modified International Association of Diabetes and Pregnancy Study Groups criteria: a population-based cohort study. Eur J Endocrinol. 2012;166:317–324. doi: 10.1530/EJE-11-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mission JF, Ohno MS, Cheng YW, Caughey AB. Gestational diabetes screening with the new IADPSG guidelines: a cost-effectiveness analysis. Am J Obstet Gynecol. 2012;207:326. doi: 10.1016/j.ajog.2012.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morikawa M, Yamada T, Akaishi R, Nishida R, Cho K, Minakami H. Change in the number of patients after the adoption of IADPSG criteria for hyperglycemia during pregnancy in Japanese women. Diabetes Res Clin Pract. 2010;90:339–342. doi: 10.1016/j.diabres.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Reyes-Muñoz E, Parra A, Castillo-Mora A, Ortega-González C. Effect of the diagnostic criteria of the International Association of Diabetes and Pregnancy Study Groups on the prevalence of gestational diabetes mellitus in urban Mexican women: a cross-sectional study. Endocr Pract. 2012;18:146–151. doi: 10.4158/EP11167.OR. [DOI] [PubMed] [Google Scholar]
- 15.Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, et al. Frequency of Gestational Diabetes Mellitus at Collaborating Centers Based on IADPSG Consensus Panel-Recommended Criteria The Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012;35:526–528. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moses RG. Gestational diabetes mellitus: implications of an increased frequency with IADPSG criteria. Diabetes Care. 2012;35:461–462. doi: 10.2337/dc11-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 18.Kjos SL, Buchanan TA. Gestational diabetes mellitus. N Engl J Med. 1999;341:1749–1756. doi: 10.1056/NEJM199912023412307. [DOI] [PubMed] [Google Scholar]
- 19.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 20.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Catalano PM, Tyzbir ED, Roman NM, Amini SB, Sims EA. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol. 1991;165:1667–1672. doi: 10.1016/0002-9378(91)90012-g. [DOI] [PubMed] [Google Scholar]
- 22.Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S112–S119. doi: 10.2337/dc07-s202. [DOI] [PubMed] [Google Scholar]
- 23.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278:1078–1083. [PubMed] [Google Scholar]
- 24.Kim C, Newton KM, Knopp RH. Gestational Diabetes and the Incidence of Type 2 Diabetes A systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 25.Dabelea D, Pettitt D. Intrauterine diabetic environment confers risks for type 2 diabetes mellitus and obesity in the offspring, in addition to genetic susceptibility. J Pediatr Endocrinol Metab. 2001;14:1085–1091. doi: 10.1515/jpem-2001-0803. [DOI] [PubMed] [Google Scholar]
- 26.O’Sullivan J, Mahan C. Criteria for the oral glucose tolerance test in pregnancy. Diabetes. 1964;13:278–285. [PubMed] [Google Scholar]
- 27.Carpenter M, Coustan DRW. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144:768–773. doi: 10.1016/0002-9378(82)90349-0. [DOI] [PubMed] [Google Scholar]
- 28.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 29.Committee on Practice Bulletins--Obstetrics. Practice Bulletin No. 137: Gestational diabetes mellitus. Obstet Gynecol. 2013;12:406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 30.National Institutes of Health consensus development conference statement: diagnosing gestational diabetes mellitus, March 4-6, 2013. Obstet Gynecol. 2013;122:358–369. doi: 10.1097/AOG.0b013e31829c3e64. [DOI] [PubMed] [Google Scholar]
- 31.American Diabetes Association. Standards of Medical Care in Diabetes--2014. Diabetes Care. 2014;37(Suppl 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 32.Canadian Diabetes Association. Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Toronto (Canada): Canadian Diabetes Association; 2008. [Google Scholar]
- 33.Jovanovic-Peterson L, Peterson CM, Reed GF, Metzger BE, Mills JL, Knopp RH, et al. Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development--Diabetes in Early Pregnancy Study. Am J Obstet Gynecol. 1991;164:103–111. doi: 10.1016/0002-9378(91)90637-7. [DOI] [PubMed] [Google Scholar]
- 34.de Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333:1237–1241. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 35.Jovanovič L, Savas H, Mehta M, Trujillo A, Pettitt DJ. Frequent monitoring of A1C during pregnancy as a treatment tool to guide therapy. Diabetes Care. 2011;34:53–54. doi: 10.2337/dc10-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and Recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Supplement 2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 37.Jovanovic L. Role of diet and insulin treatment of diabetes in pregnancy. Clin Obstet Gynecol. 2000;43:46–55. doi: 10.1097/00003081-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 38.American Diabetes Association. Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31:S61–S78. doi: 10.2337/dc08-S061. [DOI] [PubMed] [Google Scholar]
- 39.Cheng YW, Chung JH, Kurbisch-Block I, Inturrisi M, Shafer S, Caughey AB. Gestational weight gain and gestational diabetes mellitus: perinatal outcomes. Obstet Gynecol. 2008;112:1015–1022. doi: 10.1097/AOG.0b013e31818b5dd9. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol. 2009;21:521–526. doi: 10.1097/GCO.0b013e328332d24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pettitt DJ, Ospina P, Kolaczynski JW, Jovanovic L. Comparison of an insulin analog, insulin aspart, and regular human insulin with no insulin in gestational diabetes mellitus. Diabetes Care. 2003;26:183–186. doi: 10.2337/diacare.26.1.183. [DOI] [PubMed] [Google Scholar]
- 42.Mecacci F, Carignani L, Cioni R, Bartoli E, Parretti E, La Torre P, et al. Maternal metabolic control and perinatal outcome in women with gestational diabetes treated with regular or lispro insulin: comparison with non-diabetic pregnant women. Eur J Obstet Gynecol Reprod Biol. 2003;111:19–24. doi: 10.1016/s0301-2115(03)00157-x. [DOI] [PubMed] [Google Scholar]
- 43.Mathiesen ER, Hod M, Ivanisevic M, Garcia SD, Brøndsted L, Jovanovič L, et al. Maternal efficacy and safety outcomes in a randomized, controlled trial comparing insulin detemir with NPH insulin in 310 pregnant women with type 1 diabetes. Diabetes Care. 2012;35:2012–2017. doi: 10.2337/dc11-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollex E, Moretti ME, Koren G, Feig DS. Safety of insulin glargine use in pregnancy: a systematic review and meta-analysis. Ann Pharmacother. 2011;45:9–16. doi: 10.1345/aph.1P327. [DOI] [PubMed] [Google Scholar]
- 45.Price N, Bartlett C, Gillmer M. Use of insulin glargine during pregnancy: a case–control pilot study. BJOG. 2007;114:453–457. doi: 10.1111/j.1471-0528.2006.01216.x. [DOI] [PubMed] [Google Scholar]
- 46.Elliott BD, Langer O, Schenker S, Johnson RF. Insignificant transfer of glyburide occurs across the human placenta. Am J Obstet Gynecol. 1991;165:807–812. doi: 10.1016/0002-9378(91)90421-m. [DOI] [PubMed] [Google Scholar]
- 47.Langer O, Conway DL, Berkus MD, Xenakis EM-J, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134–1138. doi: 10.1056/NEJM200010193431601. [DOI] [PubMed] [Google Scholar]
- 48.Kahn BF, Davies JK, Lynch AM, Reynolds RM, Barbour LA. Predictors of glyburide failure in the treatment of gestational diabetes. Obstet Gynecol. 2006;107:1303–1309. doi: 10.1097/01.AOG.0000218704.28313.36. [DOI] [PubMed] [Google Scholar]
- 49.Chmait R, Dinise T, Moore T. Prospective observational study to establish predictors of glyburide success in women with gestational diabetes mellitus. J Perinatol. 2004;24:617–622. doi: 10.1038/sj.jp.7211147. [DOI] [PubMed] [Google Scholar]
- 50.Langer O, Yogev Y, Xenakis EM, Rosenn B. Insulin and glyburide therapy: dosage, severity level of gestational diabetes, and pregnancy outcome. Am J Obstet Gynecol. 2005;192:134–139. doi: 10.1016/j.ajog.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Eyal S, Easterling TR, Carr D, Umans JG, Miodovnik M, Hankins GD, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010;38:833–840. doi: 10.1124/dmd.109.031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nawaz FH, Khalid R, Naru T, Rizvi J. Does continuous use of metformin throughout pregnancy improve pregnancy outcomes in women with polycystic ovarian syndrome? J Obstet Gynaecol Res. 2008;34:832–837. doi: 10.1111/j.1447-0756.2008.00856.x. [DOI] [PubMed] [Google Scholar]
- 53.Bolton S, Cleary B, Walsh J, Dempsey E, Turner M. Continuation of metformin in the first trimester of women with polycystic ovarian syndrome is not associated with increased perinatal morbidity. Eur J Pediatr. 2009;168:203–206. doi: 10.1007/s00431-008-0737-7. [DOI] [PubMed] [Google Scholar]
- 54.Rowan JA, Hague WM, Gao W, Battin MR, Moore MP. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008;358:2003–2015. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 55.Gabbe SG, Gregory RP, Power ML, Williams SB, Schulkin J. Management of diabetes mellitus by obstetrician-gynecologists. Obstet Gynecol. 2004;103:1229–1234. doi: 10.1097/01.AOG.0000128045.50439.89. [DOI] [PubMed] [Google Scholar]
- 56.Swaminathan K. Experience of metformin use in gestational diabetes: Contribution to the debate. Apollo Medicine. 2013;10:113–115. [Google Scholar]
- 57.Lurie S, Insler V, Hagay ZJ. Induction of labor at 38 to 39 weeks of gestation reduces the incidence of shoulder dystocia in gestational diabetic patients class A2. Am J Perinatol. 1996;13:293–296. doi: 10.1055/s-2007-994344. [DOI] [PubMed] [Google Scholar]
- 58.Flores-le Roux JA, Sagarra E, Benaiges D, Hernandez-Rivas E, Chillaron JJ, Puig de Dou J, et al. A prospective evaluation of neonatal hypoglycaemia in infants of women with gestational diabetes mellitus. Diabetes Res Clin Pract. 2012;97:217–222. doi: 10.1016/j.diabres.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 59.Jovanovic L, Peterson CM. Management of the pregnant, insulin-dependent diabetic woman. Diabetes Care. 1980;3:63–68. doi: 10.2337/diacare.3.1.63. [DOI] [PubMed] [Google Scholar]
- 60.Baptiste-Roberts K, Barone BB, Gary TL, Golden SH, Wilson LM, Bass EB, et al. Risk factors for type 2 diabetes among women with gestational diabetes: a systematic review. Am J Med. 2009;122:207–214. doi: 10.1016/j.amjmed.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gunderson EP. Breastfeeding After Gestational Diabetes Pregnancy Subsequent obesity and type 2 diabetes in women and their offspring. Diabetes Care. 2007;30(Supplement 2):S161–S168. doi: 10.2337/dc07-s210. [DOI] [PubMed] [Google Scholar]
- 62.O’Reilly MW, Avalos G, Dennedy MC, O’Sullivan EP, Dunne F. Atlantic DIP: high prevalence of abnormal glucose tolerance post partum is reduced by breast-feeding in women with prior gestational diabetes mellitus. Eur J Endocrinol. 2011;165:953–959. doi: 10.1530/EJE-11-0663. [DOI] [PubMed] [Google Scholar]
- 63.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life?A quantitative analysis of published evidence. Am J Clin Nutr. 2006;84:1043–1054. doi: 10.1093/ajcn/84.5.1043. [DOI] [PubMed] [Google Scholar]
- 64.Glueck CJ, Wang P. Metformin before and during pregnancy and lactation in polycystic ovary syndrome. Expert Opin Drug Saf. 2007;6:191–198. doi: 10.1517/14740338.6.2.191. [DOI] [PubMed] [Google Scholar]
- 65.Glatstein MM, Djokanovic N, Garcia-Bournissen F, Finkelstein Y, Koren G. Use of hypoglycemic drugs during lactation. Can Fam Physician. 2009;55:371–373. [PMC free article] [PubMed] [Google Scholar]


