Abstract
Objectives:
To assess the association between interleukin (IL)-10 -1082, -819, -592 polymorphisms and tuberculosis (TB) risk.
Methods:
This study was conducted between July and October 2014 in West China Hospital, Chengdu, Sichuan, China. We searched and collected data from PUBMED, EMBASE, Web of Science, China National Knowledge Infrastructure, VIP, and WANGFANG up to October 2014.
Results:
A total of 37 studies were enrolled, including 8625 TB cases, and 9928 healthy controls. The IL-10-1082G/A polymorphism was found to be associated with TB susceptibility in Caucasian (GG versus GA+AA, odds ratio [OR] - 1.83, 95% confidence interval [CI] - 1.03-3.24). The IL-10-819C/T polymorphism was related to TB susceptibility among Asians (C versus T, OR - 0.88, 95% CI - 0.81-0.97; CC versus TT: OR - 0.79, 95% CI - 0.64-0.97; CC+CT versus TT: OR - 0.87, 95% CI - 0.77-0.98; CC versus CT+TT: OR - 0.82, 95% CI - 0.68-0.98). The IL-10-592C/A polymorphism was in association with TB susceptibility in Asians (C versus A: OR - 0.74, 95% CI - 0.65-0.85; CC versus AA: OR - 0.55, 95% CI - 0.41-0.75; CA versus AA: OR - 0.73, 95% CI - 0.60-0.89; CC+CA versus AA: OR - 0.69, 95% CI 0.58-0.83; CA versus AA: OR - 0.66, 95% CI 0.51-0.86), Caucasian (C versus A: OR - 1.25, 95% CI - 1.08-1.45; CC versus CA+AA: OR-1.48, 95% CI - 1.16-1.89), and Europeans (C versus A: OR - 1.31, 95% CI - 1.02-1.67; CC versus AA: OR - 1.88, 95% CI - 1.05-3.37).
Conclusion:
This meta-analysis suggests that IL-10-1082G/A, IL-819C/T, and IL-592C/A polymorphisms might be associated with TB susceptibility in certain ethnicities.
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MT) has long plagued human beings, ranking as the second major cause of death from a single infectious agent following human immunodeficiency virus (HIV).1 According to the World Health Organization in 2012,1 there were approximately 8.6 million new TB cases, and 1.3 million deaths with the greatest burden of disease falling in developing countries. Nearly one-third of the world’s population is infected with MT, but only a small fraction (5-15%) of the population develop clinical disease during their lifetime.2 This clinical diversity on disease’ susceptibility suggests both genetic predisposition, and environmental factors may contribute to the risk of infection by this pathogen following exposure and progression to TB disease.3 Cytokines involved in the shifting of the T-helper (Th)1/Th2 balance are known to play crucial roles in the progression of MT infection.4 Interleukin (IL)-10, as an immunoregulatory cytokine functions by down-regulating the Th1 driven pro-inflammatory response.5 It can be utilized by certain intracellular pathogens, including MT, to debilitate the host immune response, which then leads to the failure of subsequent clearance from the host.6 Studies7 carried out in vivo reported that IL-10-deficient mice infected with MT exhibits enhanced anti-mycobacterial immunity, while over-expression of IL-10 reactivates chronic pulmonary TB, and reduces MT-induced apoptosis of murine macrophages.8-11 The relationship between IL-10 and TB was also proven by several research conducted in humans.12,13 For patients, especially those with anergic TB and have increased IL-10 production, it is reported that high level IL-10 plasma level was associated with shorter survival of TB patients.12,13 Up until now, several studies have identified the association of IL-10 gene polymorphisms with altered IL-10 production, especially in 3 single nucleotide polymorphisms (SNPs) at positions -1082, -819, and -592 from the transcription start site.14,15 Investigating the association of the IL-10 gene polymorphisms with TB susceptibility may promote our understanding of the pathogenesis of TB, and explain individual differences in TB risk. What more, it is worth mentioning that Tobin et al16 recently reported their success in optimizing the inflammatory response to mycobacterial infections through the host genotype-specific therapies, which may also add our attention to studies regarding the TB gene polymorphisms. Unfortunately, although in recent years, a number of molecular epidemiological studies have been conducted to evaluate the risk of TB associated with the polymorphisms of IL-10 gene, their findings are conflicting. Inconsistency of those results can be attributed to a small sample size, insufficient power, and ethnic diversity of the population. Hence, we undertook this meta-analysis to evaluate the association of IL-10 gene polymorphisms and TB risk. This report follows the PRISMA (Preferred Reporting Items For Systematic Reviews and Meta-Analyses) statement.17
Methods
Search methods
This study was conducted between July and October 2014 in West China Hospital, Chengdu, China. The following databases were searched without language restriction: PUBMED, EMBASE, Web of Science, VIP, China National Knowledge Infrastructure, and WANGFANG. The search strategies were as follows: ‘TB’ or ‘tuberculosis’ in combination with ‘polymorphism’ or ‘variant’ or ‘genotype’ or ‘allele’ or ‘mutation’, and in combination with”interleukin-10,” or “IL-10”. Furthermore, the reference lists of reports identified by this search strategy were also searched to select relevant articles. The last search was conducted in October 2014. The inclusion criteria were as follows: 1) case-control study; 2) genotype distribution in both cases and controls were available; and 3) evaluation of IL-10- 1082, -819, -592 polymorphisms and TB risk. Exclusion criteria were: 1) duplicate of previous publications; and 2) study design based on family, or sibling pairs.
Data extraction
Two investigators independently screened records initially identified by the title or abstract, and then independently reviewed these records for eligibility. Discrepancies were resolved by discussion and simultaneous reference to the relevant literature. The following variables were collected from studies: title, first author’s name, year and source of publication, country of origin, ethnic descent of the study population, numbers of eligible cases and controls, and genotype distributions in cases and controls, definition of cases, HIV status, and genotyping method. If data concerning the genotype distributions were not displayed in the article, the investigators would contact the primary author in an attempt to obtain the missing data.
Statistical analysis
Hardy-Weinberg Equilibrium (HWE) was tested in the controls via the Chi-square test, and it was considered statistically significant when p<0.05. Heterogeneity was assessed by the I2 based Q statistics, and I2 test. Fixed effect model was adopted if the result of the heterogeneity test was p>0.10. Otherwise the random effect model was used.18,19 Odds ratio (OR) and 95% confidence interval (CI) were used to assess the strength of association between IL-10 polymorphisms, and the risk of TB under allelic model, homozygote comparison, heterozygote comparison, dominant, and recessive genetic model. Stratified analyses were performed by ethnicity. Sensitivity analyses were also performed to assess the stability of the results by excluding studies deviating from HWE. Begg’s and Egger’s tests were used to test the possible publication bias each time, when more than 5 studies were pooled.20 All statistical analyses were carried out using STATA version 12.0 (Stata Corp, College Station, Texas, USA) using 2-sided p values.
Results
Study characteristics
A total of 744 articles were initially identified. After excluding those that overlapped between the databases, 395 abstracts were retrieved for further evaluation. Then, 49 articles with full texts that met the inclusion criteria were assessed. Twelve articles were excluded for being reviews. Finally, a total of 37 studies, 31 in English, 5 in Chinese, and 1 in Turkish were included in this meta-analysis.21-57 Figure 1 provides a PRISMA flow diagram of the search. The characteristics of these 37 included studies are summarized in Table 1. They included 8625 cases and 9928 controls. Among them, there were 33 studies for the -1082G/A, 21 studies for the -819C/T, and 18 studies for the -592C/A. All the studies selected healthy people as controls. The study participants were diverse in ethnicity including African, Asian, European and Caucasian. The genotype distributions among the controls of all studies were consistent with HWE except for 11 studies for the -1082G/A, 4 studies for the -819C/T, and 4 studies for the -592C/A.
Figure 1.
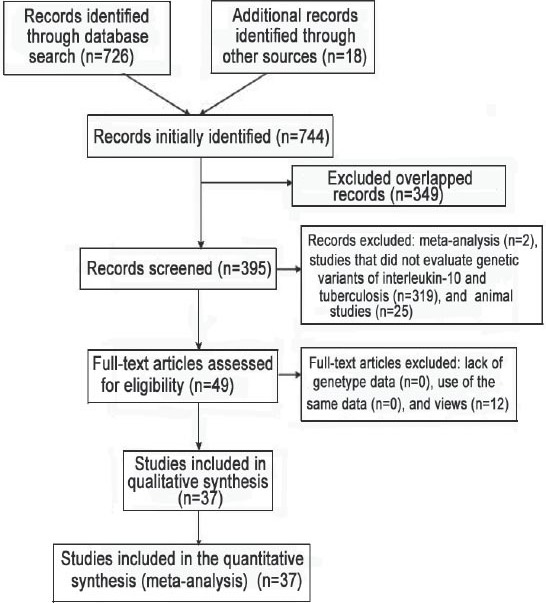
Flow diagram of studies included in a meta-analysis from China.
Table 1.
Characteristics of literature included in the meta-analysis on interleukin polymorphisms and tuberculosis risk study from China.
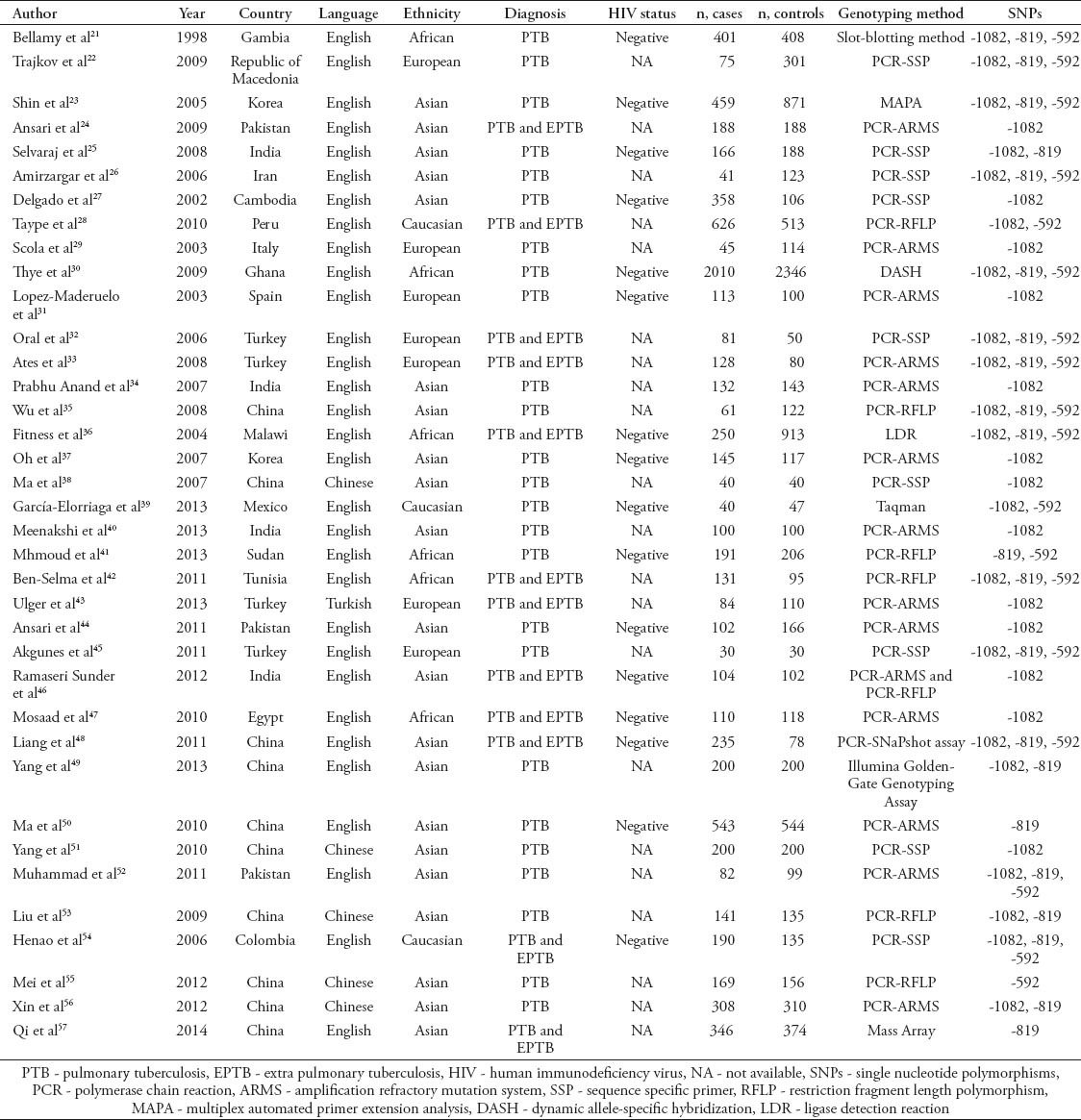
Quantitative synthesis of IL-10-1082G/A
The evaluation of association between IL-10-1082G/A polymorphism and TB risk are listed in Table 2. Overall, the results of pooling all studies showed no significant associations between the IL-10-1082 G/A polymorphism and TB risk under any of 5 genetic models (G versus A: OR - 1.04, 95% CI - 0.93-1.17; GG versus AA: OR - 1.27, 95% CI - 0.96-1.68; GA versus AA: OR - 1.02, 95% CI - 0.86-1.21; GG+GA versus AA: OR - 1.05, 95% CI - 0.88-1.25; GG versus GA+AA: OR - 1.22, 95% CI - 0.96-1.56). Excluding studies deviating from HWE did not change the results (G versus A: OR - 0.97, 95% CI - 0.84-1.13; GG versus AA: OR - 1.08, 95% CI - 0.75-1.55; GA versus AA: OR - 0.94, 95% CI - 0.78-1.14; GG+GA versus AA: OR - 0.97, 95% CI - 0.80-1.17; GG versus GA+AA: OR - 1.05, 95% CI - 0.77-1.44). We further performed subgroup analyses in IL-10-1082 G/A polymorphism by ethnicity, and no association of IL-10-1082G/A polymorphism and TB risk was observed in Asian and African descendants. While statistically significant associations were found in European descendants under 3 genetic models (G versus A: OR - 1.38, 95% CI - 1.04-1.82; GG versus AA: OR - 2.08, 95% CI 1.02-4.25; GG versus GA+AA: OR - 1.80, 95% CI - 1.28-2.54), and in Caucasian descendants under one genetic models (GG versus GA+AA: OR - 1.83, 95% CI 1.03-3.24). However, after excluding studies deviating from HWE, we found the association disappeared among European descendants (G versus A: OR - 1.61, 95% CI- 0.94-2.75; GG versus AA: OR - 2.39, 95% CI - 0.73-7.79; GG versus GA+AA: OR - 1.80, 95% CI - 0.80-4.08). Significant heterogeneity was found in the overall analyses even after removing studies deviating from HWE. However, some of the heterogeneity can be eliminated through ethnicity-specific analyses, which is observed in the studies conducted in Caucasian descendants. Among other subgroup analyses, only studies conducted in African descendants achieved improved heterogeneity after excluding studies deviating from HWE (Table 2). Results from Begg’s and Egger’s test suggested that publication bias may present in the studies investigating the association between the IL-10-1082G/A polymorphism and TB risk among Asian population (Table 2).
Table 2.
Meta-analysis of interleukin (IL)-10-1082G/A polymorphism and tuberculosis risk according to a study from China.
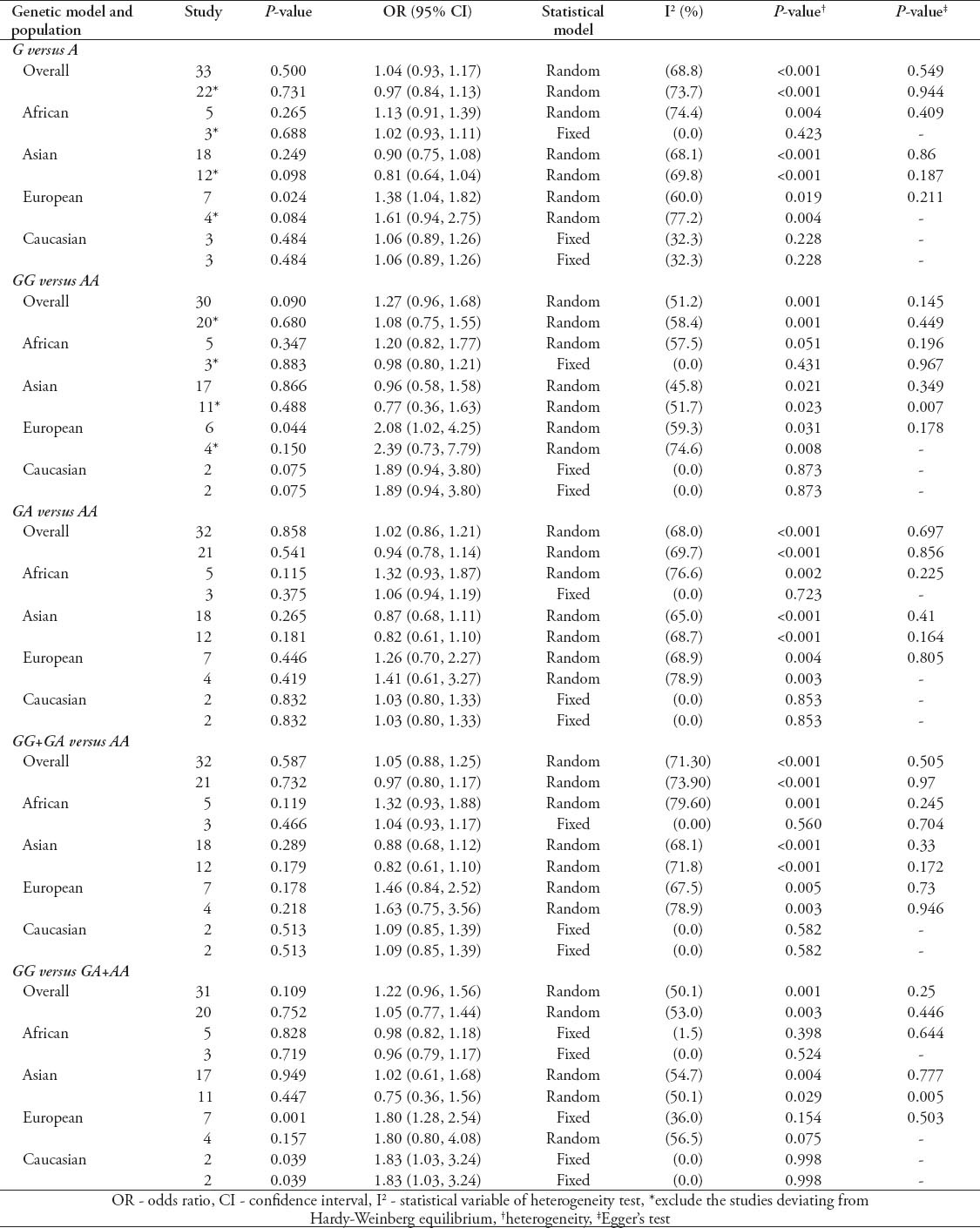
Quantitative synthesis of IL-10-819C/T
As shown in Table 3, the pooling analysis of total included studies indicated no association between IL-10-819C/T polymorphism and TB risk (C versus T: OR - 0.96, 95% CI - 0.91-1.01; CC versus TT: OR- 0.95, 95% CI - 0.85-1.07; CT versus TT: OR- 1.03, 95% CI - 0.86-1.23; CC+CT versus TT: OR - 0.98, 95% CI - 0.84-1.14; CC versus CT+TT: OR - 0.90, 95% CI - 0.78-1.05). Excluding studies deviating from HWE did not affect the results (C versus T: OR - 0.97, 95% CI - 0.92-1.03; CC versus TT: OR - 0.98, 95% CI - 0.86-1.11; CT versus TT: OR - 0.98, 95% CI - 0.89-1.08; CC+CT versus TT: OR - 0.96, 95% CI - 0.88-1.06; CC versus CT+TT: OR- 0.96, 95% CI - 0.87-1.05). In the subgroup analyses by ethnicity, IL-10-819C/T polymorphism was associated with decreased TB risk among Asian descendants under 3 genetic models (C versus T: OR - 0.89, 95% CI - 0.82-0.96; CC versus TT: OR - 0.81, 95% CI - 0.67-0.97). The association was still significant after excluding studies deviating from HWE (C versus T: OR - 0.88, 95% CI - 0.81-0.97; CC versus TT: OR - 0.79, 95% CI - 0.64-0.97; CC+CT versus TT: OR - 0.87, 95% CI - 0.77-0.98; CC versus CT+TT: OR - 0.82, 95% CI - 0.68-0.98). While no significant association was found in other ethnicity in all tested models (Table 3). The heterogeneity was significant among some genetic models in the overall and subgroup analyses. However, most of heterogeneity was eliminated after excluding studies deviating from HWE (Table 3). Begg’s and Egger’s test suggested that publication bias was not apparent in all analyses concerning IL-10-819C/T polymorphism (Table 3).
Table 3.
Meta-analysis of interleukin (IL)-10-819C/T polymorphism and tuberculosis risk as found in a study from China.
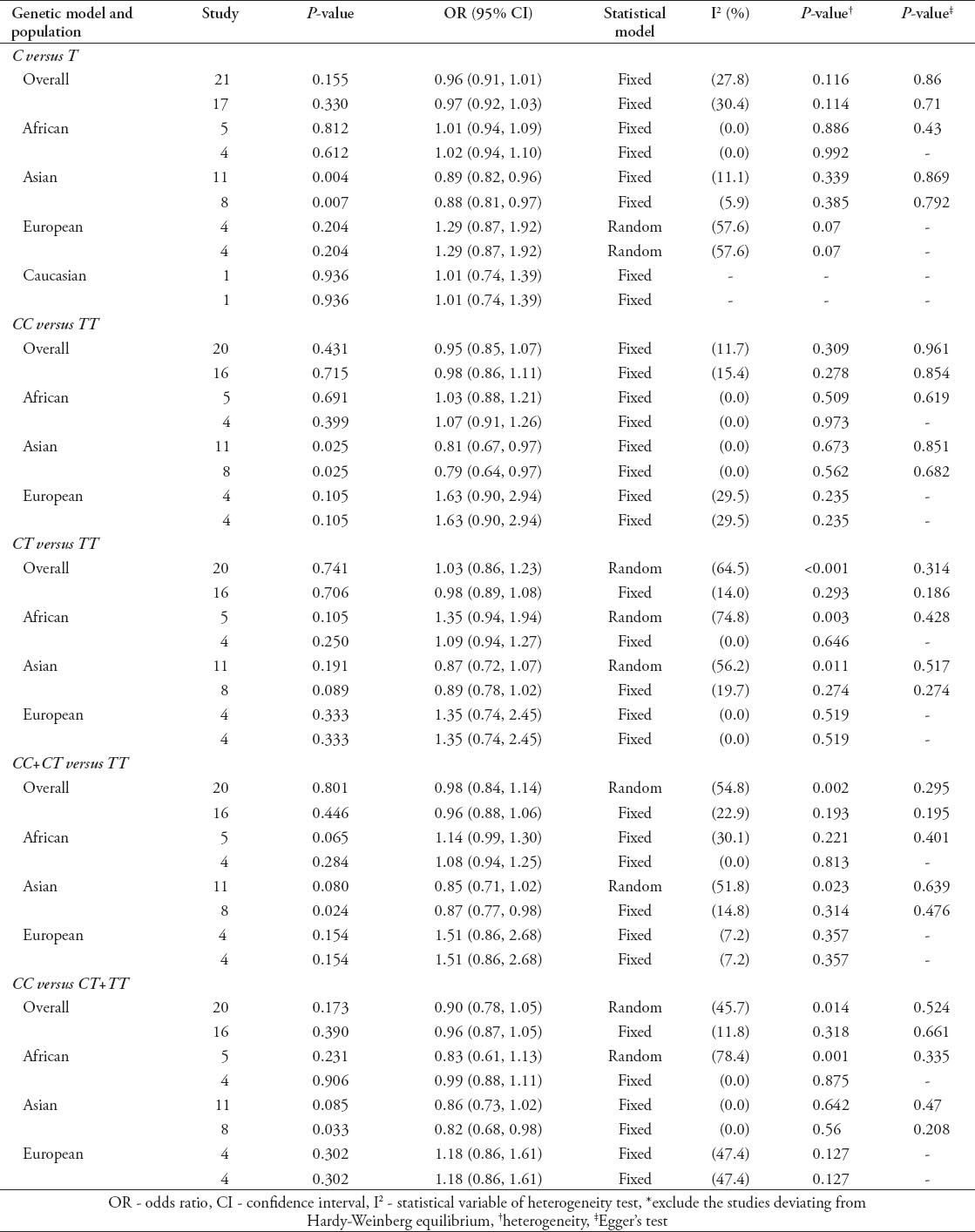
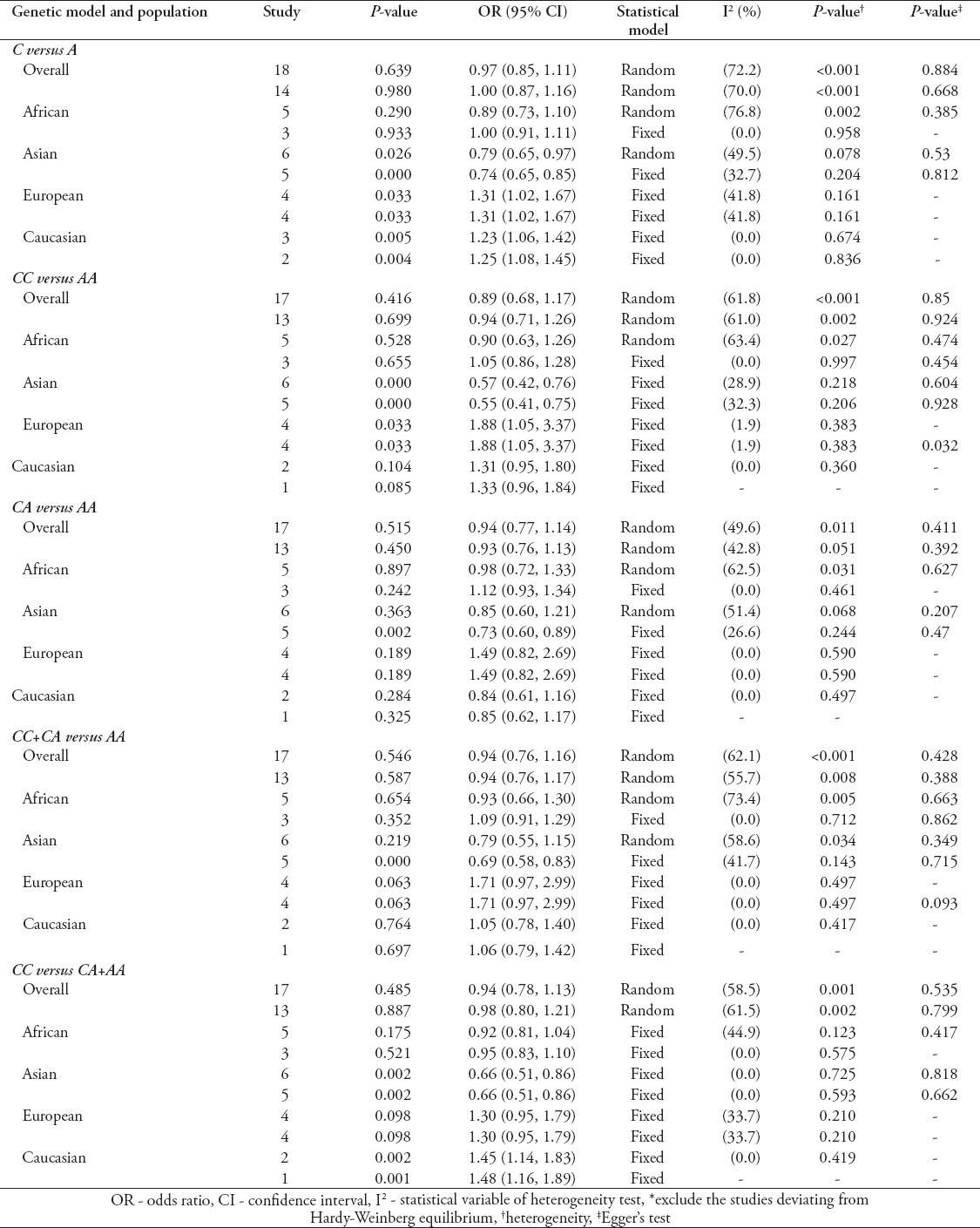
Quantitative synthesis of IL-10-592C/A
The main results of the relationship between IL-10-592C/A polymorphism and TB risk are presented in Table 4. Similar to the other 2 genetic site polymorphism, the overall analysis of IL-10-592C/A polymorphism, including all ethnic groups, showed no significant association with TB risk (C versus A: OR - 0.97, 95% CI - 0.85-1.11; CC versus AA: OR - 0.89, 95% CI - 0.68-1.17; CA versus AA: OR - 0.94, 95% CI - 0.77-1.14; CC+CA versus AA: OR - 0.94, 95% CI - 0.76-1.16; CC versus CA+AA: OR - 0.94, 95% CI - 0.78-1.13). Removing the studies deviating from HWE yielded almost similar results (C versus A: OR - 1.00, 95% CI - 0.87-1.16; CC versus AA: OR - 0.94, 95% CI - 0.71-1.26; CA versus AA: OR - 0.93, 95% CI 0.76-1.13; CC+CA versus AA: OR - 0.94, 95% CI - 0.76-1.17; CC versus CA+AA: OR - 0.98, 95% CI - 0.80-1.21). Subgroup analyses were also performed by ethnicity. Decreased risk was observed among Asians in 3 genetic models (C versus A: OR - 0.79, 95% CI - 0.65-0.97; CC versus AA: OR - 0.57, 95% CI - 0.42-0.76; CC versus CA+AA: OR - 0.66, 95% CI - 0.51-0.86). When removing the studies deviating from HWE in Asians, we found significant association in all 5 genetic models (C versus A: OR - 0.74, 95% CI - 0.65-0.85; CC versus AA: OR - 0.55, 95% CI - 0.41-0.75; CA versus AA: OR - 0.73, 95% CI - 0.60-0.89; CC+CA versus AA: OR - 0.69, 95% CI - 0.58-0.83; CC versus CA+AA: OR - 0.66, 95% CI - 0.51-0.86). While increased risk among Europeans in 2 genetic models (C versus A: OR - 1.31, 95% CI - 1.02-1.67; CC versus AA: OR - 1.88, 95% CI - 1.05-3.37) and Caucasian in 2 genetic models (C versus A: OR - 1.23, 95% CI - 1.06-1.42; CC versus CA+AA: OR - 1.45, 95% CI - 1.14-1.83) were observed in ethnicity-specific analyses. The results cannot be changed after excluding studies deviating from HWE among Europeans (C versus A: OR - 1.31, 95% CI - 1.02-1.67; CC versus AA: OR - 1.88, 95% CI - 1.05-3.37) and Caucasian (C versus A: OR - 1.25, 95% CI - 1.08-1.45; CC versus CA+AA: OR - 1.48, 95% CI - 1.16-1.89). No significant association was found among other populations (Table 4). Obvious heterogeneity was found in overall analyses. However, removing studies deviating from HWE, all subgroups exhibited homogeneity in all genetic models (Table 4). Begg’s and Egger’s test showed no evidence of publication bias in all analyses concerning IL-10-592C/A polymorphism (Table 4).
Table 4.
Meta-analysis of interleukin (IL)-10-592C/A polymorphism and tuberculosis risk as found in a study from China.
Discussion
At present, 3 meta-analyses have been conducted to assess the association between IL-10 polymorphisms and TB risk. Due to a relatively smaller study sample size, it is difficult for these previous studies to arrive at solid conclusions. Pacheco et al,58 who conducted the first meta-analysis including only 8 studies did not find any significant association between IL-10-1082 G/A polymorphism and TB risk, whereas Zhang et al59 reported an increased risk of TB in Europeans with IL-10-1082 in the genetic model (GG versus AA+AG). Recently, Liang et al60 conducted a meta-analysis, and provided evidence that IL-10-1082G/A polymorphism was associated with TB risk in Europeans and Americans, and IL-10-819T/C and -592A/C polymorphisms could be risk factors for TB in Asians. Although the latter 2 meta-analyses,59,60 which included 18 European, and 31 American studies have to some extent, amplified the power of test, they did not perform sensitivity analysis by excluding studies deviating from HWE to confirm the stability of results. Therefore, we carried out this meta-analysis on the basis of 37 eligible case-control studies to comprehensively, and rigorously evaluate the association between IL-10 polymorphisms and TB risk.
In terms of the IL-10-1082 G/A polymorphism, the results in pooling all studies showed no significant associations between the IL-10-1082 G/A polymorphism and TB risk. In the subsequent subgroup analysis, our initial pooling analyses found that this polymorphism in European group indicated an increased TB risk in 3 genetic models (allelic model, homozygote comparison, and recessive model), which is in consistency with Zhang et al’s59 and Liang et al’s60 meta-analysis. However, after excluding studies deviating from HWE during sensitivity analysis, our research failed to replicate this positive finding. As including studies deviating from HWE may lead to false-positive finding, those positive results should be interpreted with caution. Meanwhile, it is worth emphasizing that our meta-analysis showed an association between IL-10-1082 G/A polymorphism and TB risk in Caucasian descendants without between-study heterogeneity, which is also in consistency with the research conducted by Liang et al.60 In view of the relatively small sample size of people were included in the pooling analysis of Caucasian subgroup, additional large studies are warranted to validate this finding.
Although pooled results of all the included studies demonstrated that IL-10-819C/T polymorphism has no substantial effect on the occurrence of TB, the finding that this polymorphism in 4 genetic models (allelic model, homozygote comparison, dominant model, and recessive model) was linked to protective effect on TB in Asian population was worth to be noted. It is a relatively solid result without between-study heterogeneity, and is also in accordance with the previous meta-analysis conducted by Liang et al.60 While no significant association was found in other ethnicity in all tested models, which was also consistent with previous reports.59,60 The IL-10-592C/A polymorphism was not linked to any obvious risk or protective effect on TB in all population. Interestingly, subgroup analyses showed that IL-10-592C/A polymorphism was associated with decreased susceptibility to TB in the Asian group, and increased susceptibility in European and Caucasian group, which were not reported by former meta-analyses.58-60
The reason why different association was observed in different ethnicity remained unclear. One possible explanation is the differences in the frequencies of the polymorphisms among diverse ethnicity, which may partly give rise to heterogeneity. Meanwhile, gene-gene interactions along with environmental factors could also contribute to the complexity of genetic effect. Thus, most often, variation in single genetic locus is insufficient to predict risk of disease.
The strength of our meta-analysis included a large sample size, comprehensive search strategy, and no language restrictions. As a result, our meta-analysis has more power to detect differences, and allow subgroup analysis. Additionally, we performed sensitive analysis by excluding studies deviating from HWE to confirm the stability of the results, which then may lead to more reliable conclusions.
Some limitations in this meta-analysis should also be addressed. Firstly, publication bias was apparent in the IL-10-1082 G/A genetic site under some genetic models among Asians. Secondly, some detailed information, such as HIV status, or TB severity was unavailable in most studies, which limited our further evaluation by performing stratified analysis according to those confounding factors. Thus, future studies should provide more information, such as the HIV status and TB severity, to promote further assessing of the association between IL-10 genetic polymorphism and TB risk. Thirdly, the small sample sizes in some subgroup analyses may not be the representative of the population. Therefore, the relationship between IL-10 genetic polymorphisms and TB should be confirmed in future studies.
In conclusion, this meta-analysis involving 18,553 participants evaluated the relationship between IL-10-1082/-819/-592 polymorphisms and risk of TB, which provided evidence that IL-10-819C/T and -592C/A polymorphisms are associated with TB susceptibility in Asians, and IL-10-592C/A polymorphism is related to TB susceptibility among Europeans, and IL-10-1082 G/A, -592C/A polymorphisms may be in association with TB susceptibility in Caucasian. Large-scale studies are warranted to be undertaken to confirm those relationship. Future studies should provide more information, such as the HIV status and TB severity, to promote further assessing of the association between IL-10 genetic polymorphism and TB risk.
Footnotes
References
- 1.World Health Organization. Tuberculosis (TB). Global tuberculosis report 2014. [Accessed 10 October 2014] Geneva (CH): World Health Organization; 2014. Available from URL: http: //www.who.int/tb/publications/global_report/en/index.html . [Google Scholar]
- 2.Rosman MD, Oner-Eyupoglu AF. Clinical Presentation and Treatment of Tuberculosis. In: Fishman AP, editor. Fishman's Pulmonary Diseases and Disorders. New York (NY): McGraw-Hill; 1998. pp. 2483–2502. [Google Scholar]
- 3.Bellamy R. Susceptibility to mycobacterial infection: the importance of host genetics. Genes Immun. 2003;4:4–11. doi: 10.1038/sj.gene.6363915. [DOI] [PubMed] [Google Scholar]
- 4.He XY, Xiao L, Chen HB, Hao J, Li J, Wang YJ, et al. T regulatory cells and Th1/Th2 cytokines in peripheral blood from tuberculosis patients. Eur J Clin Microbiol Infect Dis. 2010;29:643–650. doi: 10.1007/s10096-010-0908-0. [DOI] [PubMed] [Google Scholar]
- 5.Ouyang W, Rutz S, Crellin NK, Valdez PA, Hymowitz SG. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu Rev Immunol. 2011;29:71–109. doi: 10.1146/annurev-immunol-031210-101312. [DOI] [PubMed] [Google Scholar]
- 6.Redpath S, Ghazal P, Gascoigne NR. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 2001;9:86–92. doi: 10.1016/s0966-842x(00)01919-3. [DOI] [PubMed] [Google Scholar]
- 7.Murray PJ, Young RA. Increased antimycobacterial immunity in interleukin-10-deficient mice. Infect Immun. 1999;67:3087–3095. doi: 10.1128/iai.67.6.3087-3095.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gil DP, Leon LG, Correa LI, Maya JR, Paris SC, Garcia LF, et al. Differential induction of apoptosis and necrosis in monocytes from patients with tuberculosis and healthy control subjects. J Infect Dis. 2004;189:2120–2128. doi: 10.1086/386369. [DOI] [PubMed] [Google Scholar]
- 9.Gong JH, Zhang M, Modlin RL, Linsley PS, Iyer D, Lin Y, et al. Interleukin-10 downregulates Mycobacterium tuberculosis-induced Th1 responses and CTLA-4 expression. Infect Immun. 1996;64:913–918. doi: 10.1128/iai.64.3.913-918.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner J, Gonzalez-Juarrero M, Ellis DL, Basaraba RJ, Kipnis A, Orme IM, et al. In vivo IL-10 production reactivates chronic pulmonary tuberculosis in C57BL/6 mice. J Immunol. 2002;169:6343–6351. doi: 10.4049/jimmunol.169.11.6343. [DOI] [PubMed] [Google Scholar]
- 11.Rojas M, Olivier M, Gros P, Barrera LF, Garcia LF. TNF-alpha and IL-10 modulate the induction of apoptosis by virulent Mycobacterium tuberculosis in murine macrophages. J Immunol. 1999;162:6122–6131. [PubMed] [Google Scholar]
- 12.Lin Y, Zhang M, Hofman FM, Gong J, Barnes PF. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect Immun. 1996;64:1351–1356. doi: 10.1128/iai.64.4.1351-1356.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JY, Chang HC, Liu JL, Shu CC, Lee CH, Wang JT, et al. Expression of toll-like receptor 2 and plasma level of interleukin-10 are associated with outcome in tuberculosis. Eur J Clin Microbiol Infect Dis. 2012;31:2327–2333. doi: 10.1007/s10096-012-1572-3. [DOI] [PubMed] [Google Scholar]
- 14.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, et al. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest. 2000;105:1317–1325. doi: 10.1172/JCI9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63. doi: 10.1615/critrevimmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tobin DM, Roca FJ, Oh SF, McFarland R, Vickery TW, Ray JP, et al. Host genotype-specific therapies can optimize the inflammatory response to mycobacterial infections. Cell. 2012;148:434–446. doi: 10.1016/j.cell.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellamy R, Ruwende C, Corrah T, McAdam KP, Whittle HC, Hill AV. Assessment of the interleukin 1 gene cluster and other candidate gene polymorphisms in host susceptibility to tuberculosis. Tuber Lung Dis. 1998;79:83–89. doi: 10.1054/tuld.1998.0009. [DOI] [PubMed] [Google Scholar]
- 22.Trajkov D, Trajchevska M, Arsov T, Petlichkovski A, Strezova A, Efinska-Mladenovska O, et al. Association of 22 cytokine gene polymorphisms with tuberculosis in Macedonians. Indian J Tuberc. 2009;56:117–131. [PubMed] [Google Scholar]
- 23.Shin HD, Park BL, Kim YH, Cheong HS, Lee IH, Park SK. Common interleukin 10 polymorphism associated with decreased risk of tuberculosis. Exp Mol Med. 2005;37:128–132. doi: 10.1038/emm.2005.17. [DOI] [PubMed] [Google Scholar]
- 24.Ansari A, Talat N, Jamil B, Hasan Z, Razzaki T, Dawood G, et al. Cytokine gene polymorphisms across tuberculosis clinical spectrum in Pakistani patients. PLoS One. 2009;4:e4778. doi: 10.1371/journal.pone.0004778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvaraj P, Alagarasu K, Harishankar M, Vidyarani M, Nisha Rajeswari D, Narayanan PR. Cytokine gene polymorphisms and cytokine levels in pulmonary tuberculosis. Cytokine. 2008;43:26–33. doi: 10.1016/j.cyto.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Amirzargar AA, Rezaei N, Jabbari H, Danesh AA, Khosravi F, Hajabdolbaghi M, et al. Cytokine single nucleotide polymorphisms in Iranian patients with pulmonary tuberculosis. Eur Cytokine Netw. 2006;17:84–89. [PubMed] [Google Scholar]
- 27.Delgado JC, Baena A, Thim S, Goldfeld AE. Ethnic-specific genetic associations with pulmonary tuberculosis. J Infect Dis. 2002;186:1463–1468. doi: 10.1086/344891. [DOI] [PubMed] [Google Scholar]
- 28.Taype CA, Shamsuzzaman S, Accinelli RA, Espinoza JR, Shaw MA. Genetic susceptibility to different clinical forms of tuberculosis in the Peruvian population. Infect Genet Evol. 2010;10:495–504. doi: 10.1016/j.meegid.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Scola L, Crivello A, Marino V, Gioia V, Serauto A, Candore G, et al. IL-10 and TNF-alpha polymorphisms in a sample of Sicilian patients affected by tuberculosis: implication for ageing and life span expectancy. Mech Ageing Dev. 2003;124:569–572. doi: 10.1016/s0047-6374(03)00038-1. [DOI] [PubMed] [Google Scholar]
- 30.Thye T, Browne EN, Chinbuah MA, Gyapong J, Osei I, Owusu-Dabo E, et al. IL10 haplotype associated with tuberculin skin test response but not with pulmonary TB. PLoS One. 2009;4:e5420. doi: 10.1371/journal.pone.0005420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopez-Maderuelo D, Arnalich F, Serantes R, Gonzalez A, Codoceo R, Madero R, et al. Interferon-gamma and interleukin-10 gene polymorphisms in pulmonary tuberculosis. Am J Respir Crit Care Med. 2003;167:970–975. doi: 10.1164/rccm.200205-438BC. [DOI] [PubMed] [Google Scholar]
- 32.Oral HB, Budak F, Uzaslan EK, Basturk B, Bekar A, Akalin H, et al. Interleukin-10 (IL-10) gene polymorphism as a potential host susceptibility factor in tuberculosis. Cytokine. 2006;35:143–147. doi: 10.1016/j.cyto.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 33.Ates O, Musellim B, Ongen G, Topal-Sarikaya A. Interleukin-10 and tumor necrosis factor-alpha gene polymorphisms in tuberculosis. J Clin Immunol. 2008;28:232–236. doi: 10.1007/s10875-007-9155-2. [DOI] [PubMed] [Google Scholar]
- 34.Prabhu Anand S, Selvaraj P, Jawahar MS, Adhilakshmi AR, Narayanan PR. Interleukin-12B and interleukin-10 gene polymorphisms in pulmonary tuberculosis. Indian J Med Res. 2007;126:135–138. [PubMed] [Google Scholar]
- 35.Wu F, Qu Y, Tang Y, Cao D, Sun P, Xia Z. Lack of association between cytokine gene polymorphisms and silicosis and pulmonary tuberculosis in Chinese iron miners. J Occup Health. 2008;50:445–454. doi: 10.1539/joh.l8006. [DOI] [PubMed] [Google Scholar]
- 36.Fitness J, Floyd S, Warndorff DK, Sichali L, Malema S, Crampin AC, et al. Large-scale candidate gene study of tuberculosis susceptibility in the Karonga district of northern Malawi. Am J Trop Med Hyg. 2004;71:341–349. [PubMed] [Google Scholar]
- 37.Oh JH, Yang CS, Noh YK, Kweon YM, Jung SS, Son JW, et al. Polymorphisms of interleukin-10 and tumour necrosis factor-alpha genes are associated with newly diagnosed and recurrent pulmonary tuberculosis. Respirology. 2007;12:594–598. doi: 10.1111/j.1440-1843.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 38.Ma ZM, Xiao P, Tang LG. [A study on the correlation between the polymorphism of interleukin 10 gene and susceptibility to pulmonary tuberculosis] Guang Dong Yi Xue. 2007;28:1243–1245. Chinese. [Google Scholar]
- 39.Garcia-Elorriaga G, Vera-Ramirez L, del Rey-Pineda G, Gonzalez-Bonilla C. -592 and -1082 interleukin-10 polymorphisms in pulmonary tuberculosis with type 2 diabetes. Asian Pac J Trop Med. 2013;6:505–509. doi: 10.1016/S1995-7645(13)60086-3. [DOI] [PubMed] [Google Scholar]
- 40.Meenakshi P, Ramya S, Shruthi T, Lavanya J, Mohammed HH, Mohammed SA, et al. Association of IL-1beta +3954 C/T and IL-10-1082 G/A cytokine gene polymorphisms with susceptibility to tuberculosis. Scand J Immunol. 2013;78:92–97. doi: 10.1111/sji.12055. [DOI] [PubMed] [Google Scholar]
- 41.Mhmoud N, Fahal A, van de Sande WJ. Association of IL-10 and CCL5 single nucleotide polymorphisms with tuberculosis in the Sudanese population. Trop Med Int Health. 2013;18:1119–1127. doi: 10.1111/tmi.12141. [DOI] [PubMed] [Google Scholar]
- 42.Ben-Selma W, Harizi H, Boukadida J. Association of TNF-alpha and IL-10 polymorphisms with tuberculosis in Tunisian populations. Microbes Infect. 2011;13:837–843. doi: 10.1016/j.micinf.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 43.Ulger M, Emekdas G, Aslan G, Tas D, Ilvan A, Tezcan S, et al. Determination of the cytokine gene polymorphism and genetic susceptibility in tuberculosis patients. Mikrobiyol Bul. 2013;47:250–264. doi: 10.5578/mb.4699. Turkish. [DOI] [PubMed] [Google Scholar]
- 44.Ansari A, Hasan Z, Dawood G, Hussain R. Differential combination of cytokine and interferon- gamma +874 T/A polymorphisms determines disease severity in pulmonary tuberculosis. PLoS One. 2011;6:e27848. doi: 10.1371/journal.pone.0027848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akgunes A, Coban AY, Durupinar B. Human leucocyte antigens and cytokine gene polymorphisms and tuberculosis. Indian J Med Microbiol. 2011;29:28–32. doi: 10.4103/0255-0857.76520. [DOI] [PubMed] [Google Scholar]
- 46.Ramaseri Sunder S, Hanumanth SR, Nagaraju RT, Venkata SK, Suryadevara NC, Pydi SS, et al. IL-10 high producing genotype predisposes HIV infected individuals to TB infection. Hum Immunol. 2012;73:605–611. doi: 10.1016/j.humimm.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 47.Mosaad YM, Soliman OE, Tawhid ZE, Sherif DM. Interferon-gamma +874 T/A and interleukin-10 -1082 A/G single nucleotide polymorphism in Egyptian children with tuberculosis. Scand J Immunol. 2010;72:358–364. doi: 10.1111/j.1365-3083.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 48.Liang L, Zhao YL, Yue J, Liu JF, Han M, Wang H, et al. Interleukin-10 gene promoter polymorphisms and their protein production in pleural fluid in patients with tuberculosis. FEMS Immunol Med Microbiol. 2011;62:84–90. doi: 10.1111/j.1574-695X.2011.00791.x. [DOI] [PubMed] [Google Scholar]
- 49.Yang Y, Li X, Cui W, Guan L, Shen F, Xu J, et al. Potential association of pulmonary tuberculosis with genetic polymorphisms of toll-like receptor 9 and interferon-gamma in a Chinese population. BMC Infect Dis. 2013;13:511. doi: 10.1186/1471-2334-13-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma MJ, Xie LP, Wu SC, Tang F, Li H, Zhang ZS, et al. Toll-like receptors, tumor necrosis factor-alpha, and interleukin-10 gene polymorphisms in risk of pulmonary tuberculosis and disease severity. Hum Immunol. 2010;71:1005–1010. doi: 10.1016/j.humimm.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Yang H, Liang ZH, Liu XL, Wang F. [Association between polymorphisms of interleukin-10, interferon-gamma gene and the susceptibility to pulmonary tuberculosis] Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31:155–158. Chinese. [PubMed] [Google Scholar]
- 52.Muhammad SA, Sadia A, Amna S, Sajjad A, Zia URF, Tahir A, et al. Interleukin-10 gene promoter polymorphism as a potential host susceptibility factor in Pakistani patients with pulmonary tuberculosis. African Journal of Biotechnology. 2011;10:14706–14710. [Google Scholar]
- 53.Liu XX, Sun YH, Guo M, Feng FM. [Study on the relationship of interleukin-10 genetic polymorphisms with the susceptibility of pulmonary tuberculosis] Modern Preventive Medicine. 2009;36:1827–1830. Chinese. [Google Scholar]
- 54.Henao MI, Montes C, Paris SC, Garcia LF. Cytokine gene polymorphisms in Colombian patients with different clinical presentations of tuberculosis. Tuberculosis (Edinb) 2006;86:11–19. doi: 10.1016/j.tube.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 55.Mei LQC, Zha D, Huang LP, Duo LN, Lu X.J, Ying BW, Fan H. [Study on association between interleukin-10 gene polymorphism with pulmonary tuberculosis in Tibetans] Modern Preventive Medicine. 2012;39:3607–3610. Chinese. [Google Scholar]
- 56.Xin DS, Jiang LF, Yang XM, Guo W, Fu FX. [A case-control study on the association between IL-10 genetic polymorphisms and susceptibility to pulmonary tuberculosis] China Healthcare & Nutrition. 2012;11:68–70. Chinese. [Google Scholar]
- 57.Qi H, Sun L, Jin YQ, Shen C, Chu P, Wang SF, et al. rs2243268 and rs2243274 of Interleukin-4 (IL-4) gene are associated with reduced risk for extrapulmonary and severe tuberculosis in Chinese Han children. Infect Genet Evol. 2014;23:121–128. doi: 10.1016/j.meegid.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 58.Pacheco AG, Cardoso CC, Moraes MO. IFNG +874T/A, IL10 -1082G/A and TNF -308G/A polymorphisms in association with tuberculosis susceptibility: a meta-analysis study. Hum Genet. 2008;123:477–484. doi: 10.1007/s00439-008-0497-5. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J, Chen Y, Nie XB, Wu WH, Zhang H, Zhang M, et al. Interleukin-10 polymorphisms and tuberculosis susceptibility: a meta-analysis. Int J Tuberc Lung Dis. 2011;15:594–601. doi: 10.5588/ijtld.09.0703. [DOI] [PubMed] [Google Scholar]
- 60.Liang B, Guo Y, Li Y, Kong H. Association between IL-10 gene polymorphisms and susceptibility of tuberculosis: evidence based on a meta-analysis. PLoS One. 2014;9:e88448. doi: 10.1371/journal.pone.0088448. [DOI] [PMC free article] [PubMed] [Google Scholar]


