Abstract
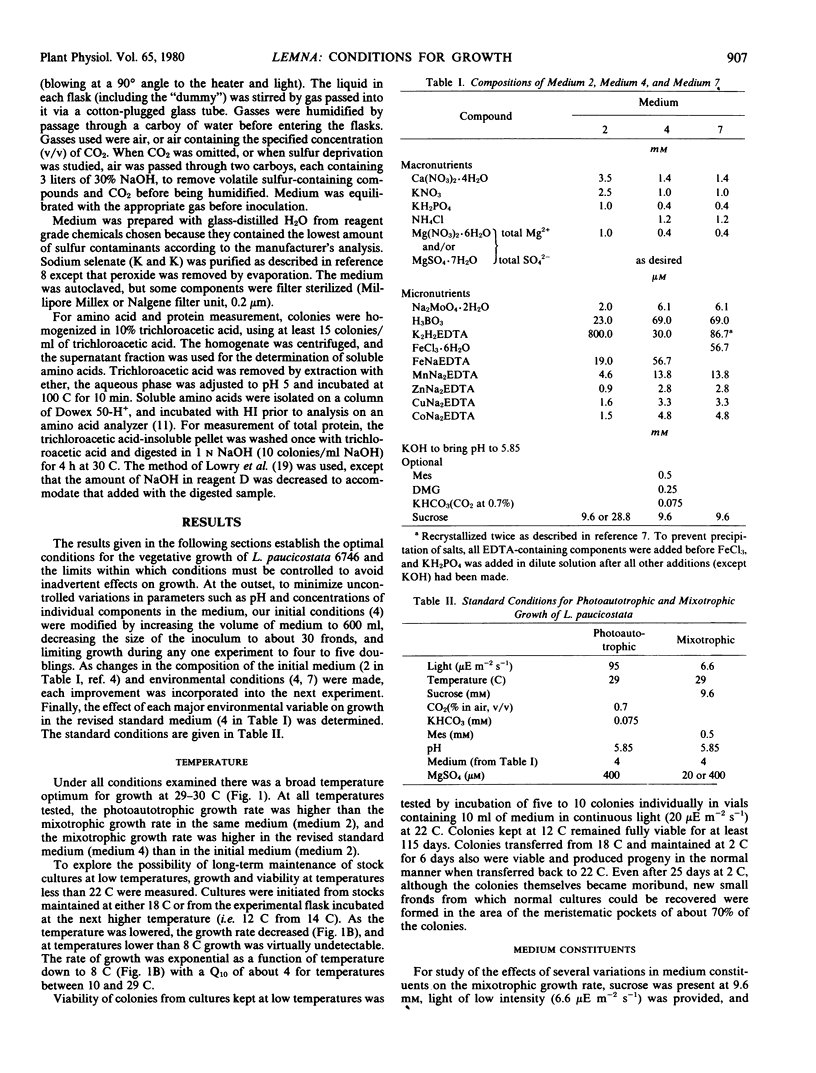
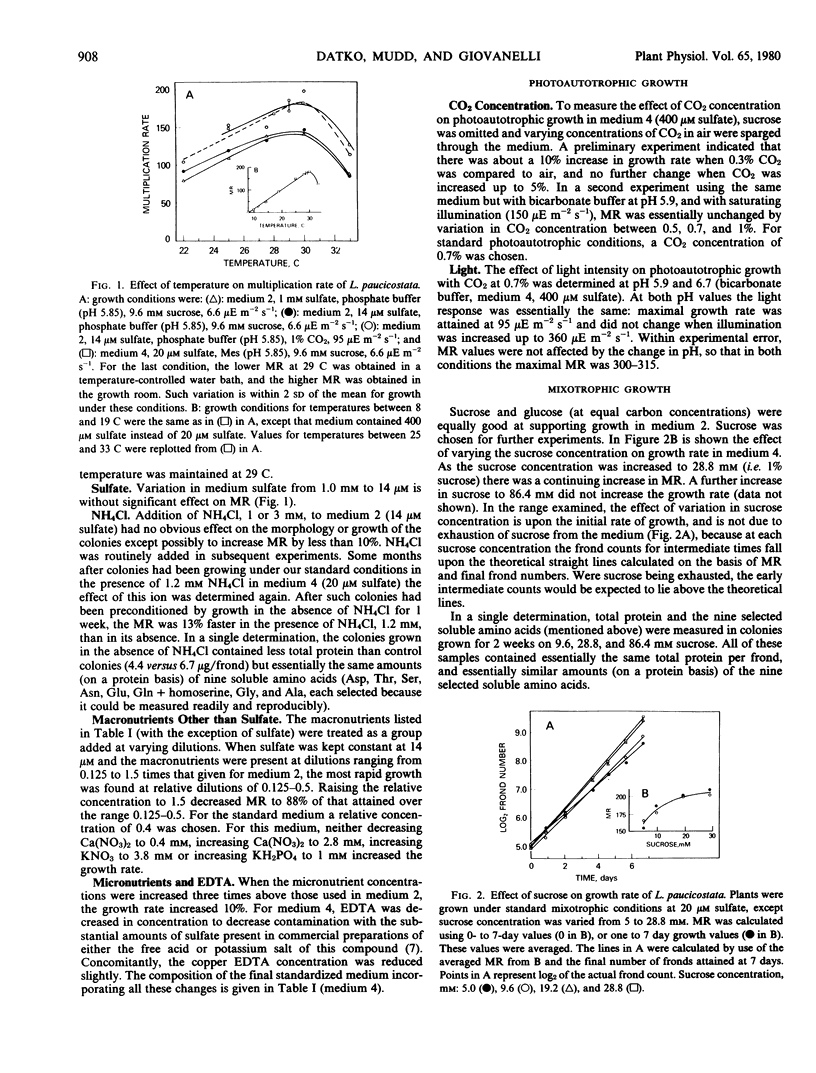
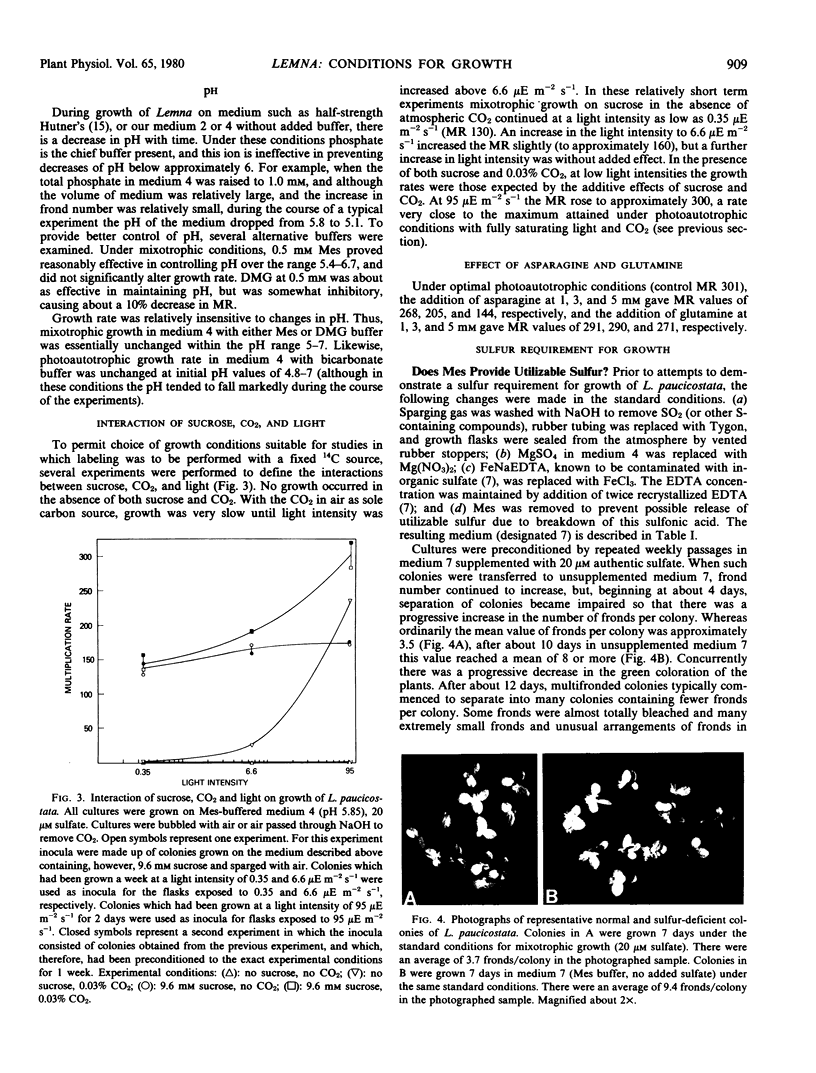
Photoautotrophic and mixotrophic growth of Lemna paucicostata Hegelm. 6746 (formerly Lemna perpusilla Torr. 6746) was investigated to establish standardized conditions for biochemical studies. Optimal temperature for growth was 29 to 30 C. The medium used previously (Datko AH, Mudd SH, Giovanelli J 1977 J Biol Chem 252: 3436-3445) was modified by inclusion of NH4Cl, decreasing macronutrient and ethylenediamine tetraacetate concentration, increasing micronutrient concentration, and inclusion of bicarbonate (for photoautotrophic growth) or 2-(N-morpholino)ethanesulfonic acid (for mixotrophic growth) buffers. Varying the sulfate concentration between 14 and 1 millimolar had no effect on growth. For photoautotrophic growth in the new medium (medium 4), the effects of CO2 concentration, light intensity, and pH were measured. Under the optimal conditions, a multiplication rate (MR) of 300 to 315, equivalent to a doubling time of 23 to 24 hours was obtained. Addition of glutamine or asparagine did not increase this MR. For mixotrophic growth in low light, the effects of sucrose concentration and pH were determined. Under optimal conditions, MR was 210. A concentration of sucrose less than maximal for growth was chosen for the medium for experiments which will include 14C-labeling of intermediates. MR under these conditions was 184. Growth was equally good in medium 4 and in half-strength Hutner's medium when sulfate was high (0.4 to 1 millimolar), but better in medium 4 when sulfate was low (20 micromolar). Growth rates could be restored to normal in half-strength Hutner's with low sulfate by decreasing the molybdate concentration.
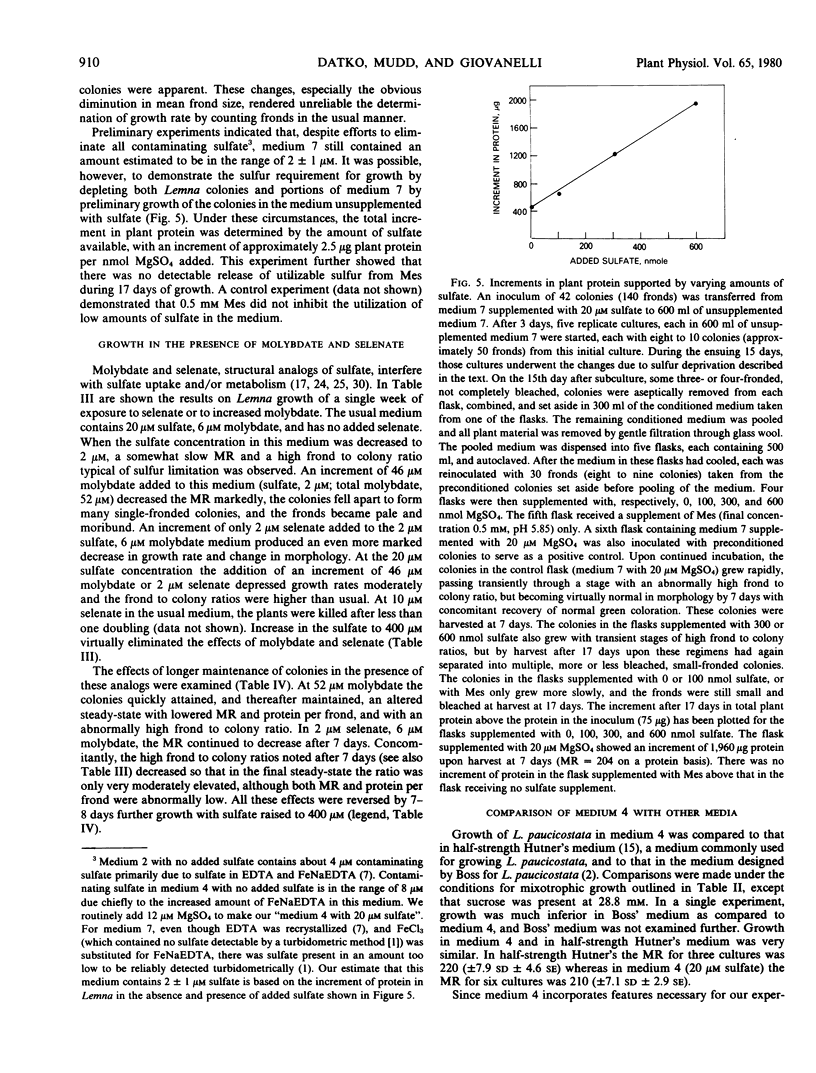
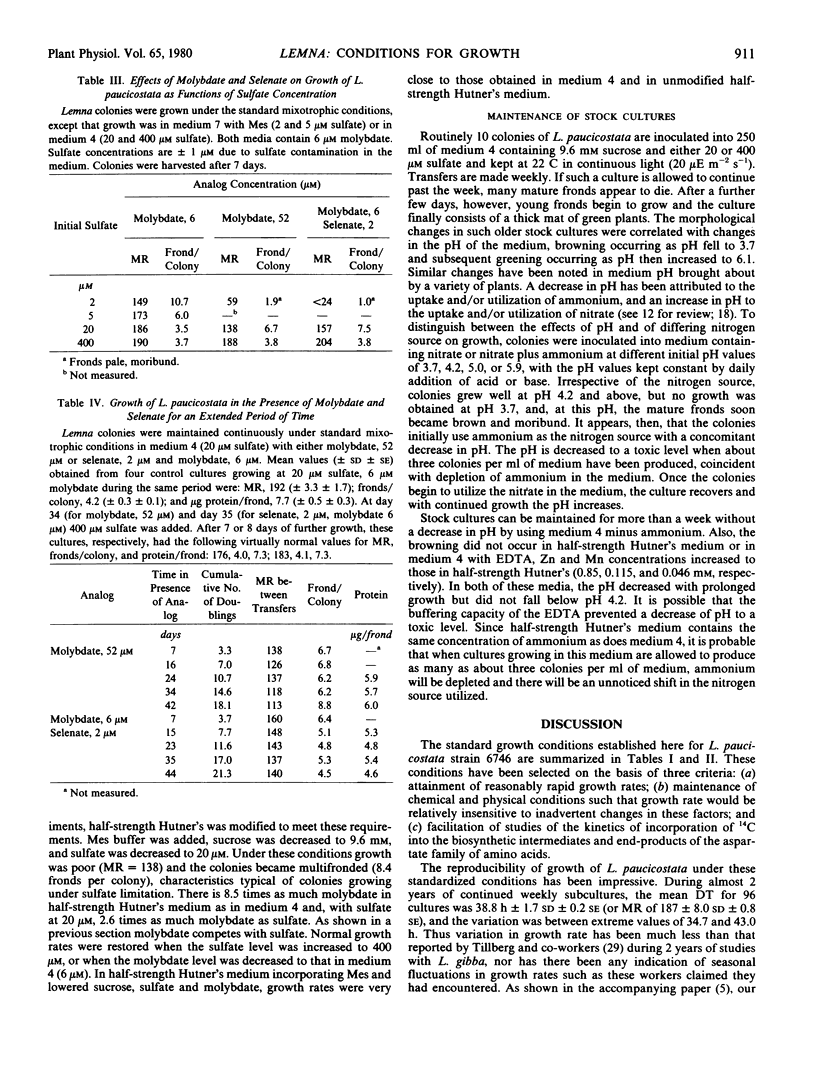
By modifying medium 4 to contain very low amounts of sulfate, and by preconditioning medium and plants, it was shown that there was an increment in plant protein of approximately 2.5 micrograms per nanomole of added MgSO4. Colonies undergoing sulfur limitation exhibited a slow growth rate and a high frond to colony ratio. Molybdate and selenate produced growth inhibition reversible by sulfate. Conditions were developed in which the plants could be maintained indefinitely in the presence of either molybdate or selenate in altered metabolic steady-states with lowered growth rates and protein per frond.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGLUND F., SORBO B. Turbidimetric analysis of inorganic sulfate in serum, plasma and urine. Scand J Clin Lab Invest. 1960;12:147–153. doi: 10.3109/00365516009062416. [DOI] [PubMed] [Google Scholar]
- Datka A. H., Mudd S. H., Giovanelli J. Homocysteine biosynthesis in green plants: studies of the homocysteine-forming sulfhydrylase. J Biol Chem. 1977 May 25;252(10):3436–3445. [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J. Lemna paucicostata Hegelm. 6746: LIFE CYCLE AND CHARACTERIZATION OF THE COLONY TYPES IN A POPULATION. Plant Physiol. 1980 May;65(5):913–923. doi: 10.1104/pp.65.5.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J., Macnicol P. K. Sulfur-containing Compounds in Lemna perpusilla 6746 Grown at a Range of Sulfate Concentrations. Plant Physiol. 1978 Oct;62(4):629–635. doi: 10.1104/pp.62.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Macnicol P. K., Giovanelli J. Phytostat for the growth of lemna in semicontinuous culture with low sulfate. Plant Physiol. 1978 Oct;62(4):622–628. doi: 10.1104/pp.62.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth G. L., Bandurski R. S. Activation of selenate by adenosine 5'-triphosphate sulphurylase from Saccharomyces cerevisiae. Biochem J. 1977 Jun 1;163(3):521–529. doi: 10.1042/bj1630521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanelli J., Mudd S. H., Datko A. H. Homocysteine biosynthesis in green plants. Physiological importance of the transsulfuration pathway in Chlorella sorokiniana growing under steady state conditions with limiting sulfate. J Biol Chem. 1978 Aug 25;253(16):5665–5677. [PubMed] [Google Scholar]
- Hillman W. S. Calibrating duckweeds: light, clocks, metabolism, flowering. Science. 1976 Aug 6;193(4252):453–458. doi: 10.1126/science.193.4252.453. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leggett J. E., Epstein E. Kinetics of Sulfate Absorption by Barley Roots. Plant Physiol. 1956 May;31(3):222–226. doi: 10.1104/pp.31.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner H. B. Inhibitory Effect of Carbohydrate on Flowering in Lemna perpusilla: II. Reversal by Glycine and l-Aspartate. Correlation with Reduced Levels of beta-Carotene and Chlorophyll. Plant Physiol. 1970 Jun;45(6):687–690. doi: 10.1104/pp.45.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramus J. In vivo molybdate inhibition of sulfate transfer to porphyridium capsular polysaccharide. Plant Physiol. 1974 Dec;54(6):945–949. doi: 10.1104/pp.54.6.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuveny Z. Derepression of ATP sulfurylase by the sulfate analogs molybdate and selenate in cultured tobacco cells. Proc Natl Acad Sci U S A. 1977 Feb;74(2):619–622. doi: 10.1073/pnas.74.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON L. G., BANDURSKI R. S. Enzymatic reactions involving sulfate, sulfite, selenate, and molybdate. J Biol Chem. 1958 Oct;233(4):975–981. [PubMed] [Google Scholar]
- Wong K. F., Dennis D. T. Aspartokinase in Lemna minor L: Studies on the in Vivo and in Vitro Regulation of the Enzyme. Plant Physiol. 1973 Feb;51(2):327–331. doi: 10.1104/pp.51.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]




