Abstract
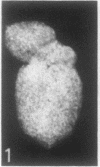
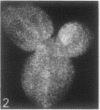
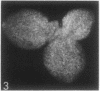
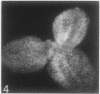
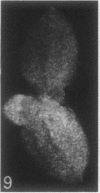
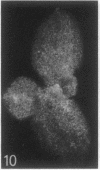
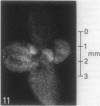
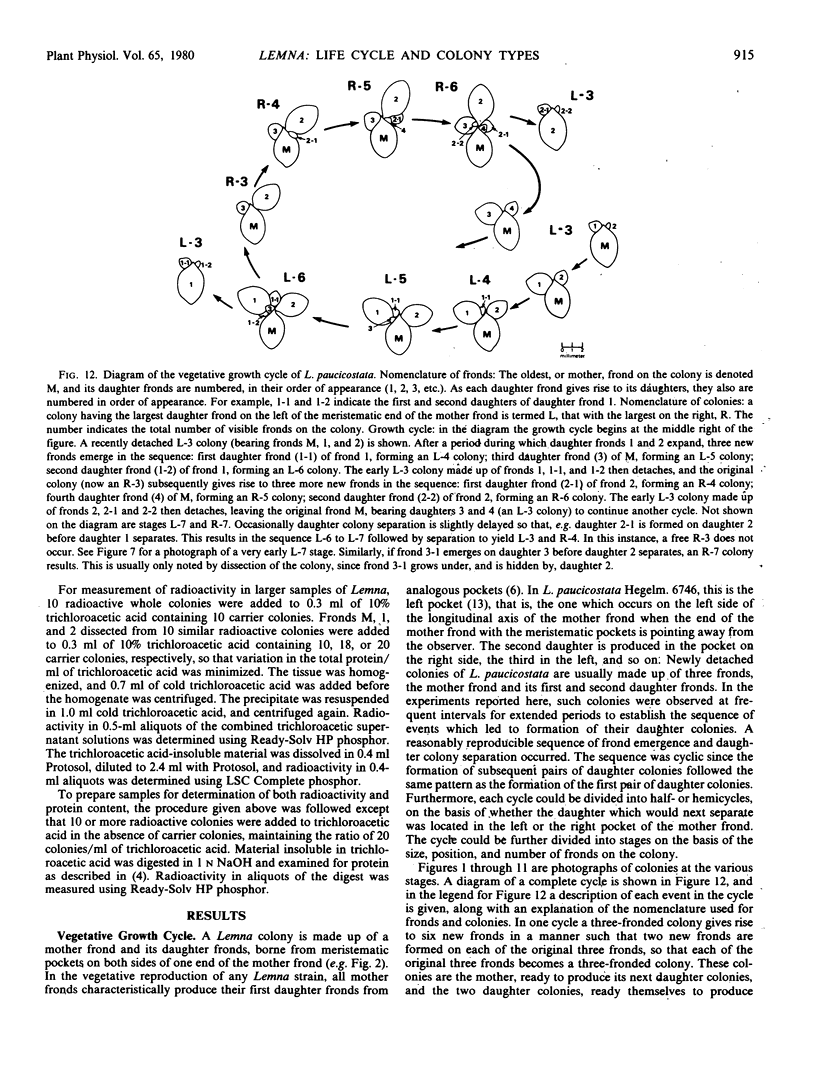
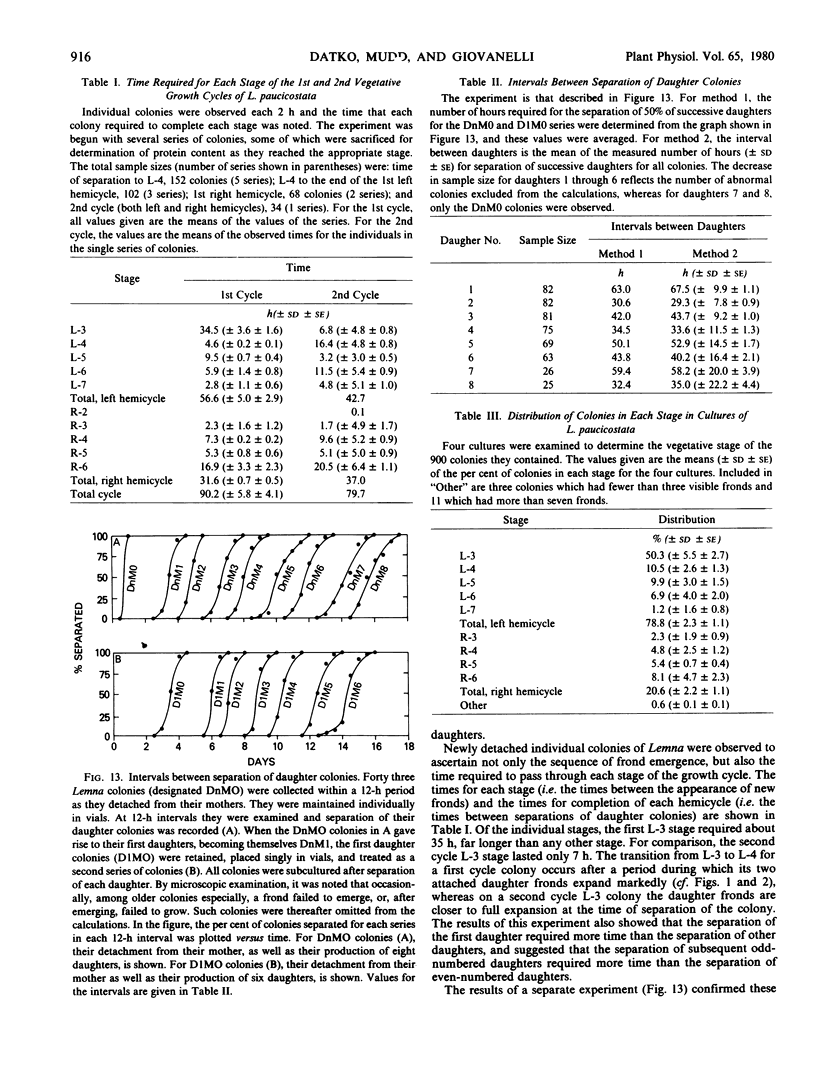
The sequence of frond emergence and the intervals required for daughter colony separation have been determined for Lemna paucicostata Hegelm. 6746 growing under standardized conditions. After separation of a new mother colony, the first daughter colony is produced from the left meristematic pocket and separates after approximately 60 hours, the second daughter, produced from the right pocket, separates after a further 30 hours, and the third daughter, again from the left, after a further 40 hours. The pattern of alternating longer and shorter intervals for separation of daughters continues throughout the life of the mother colony.
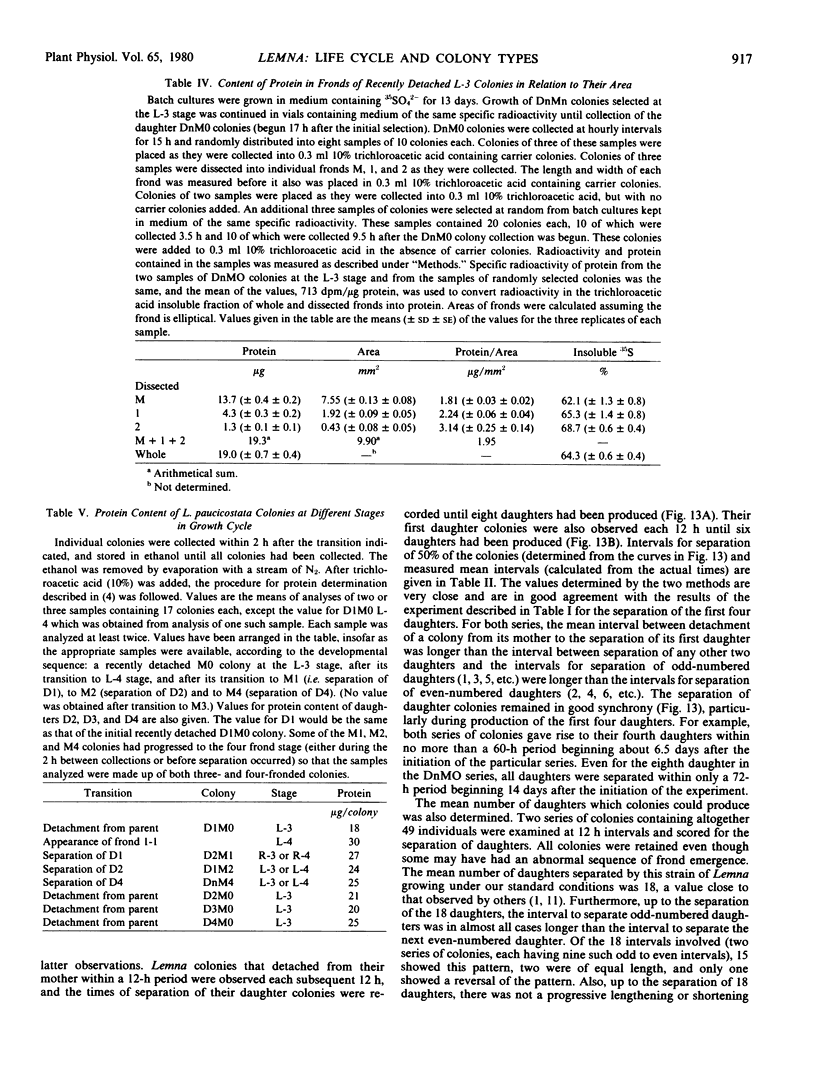
Protein contents of fronds and whole colonies were determined either by chemical methods or by labeling to isotopic equilibrium with 35SO42−. The smallest fronds measured were 5 to 6% of the final area they would attain when fully expanded and contained 10% of the protein they would finally contain. Thus, most protein accumulation occurred during the phase of rapid frond expansion rather than in an earlier primordial stage. The specific rates of protein accumulation were 0.027 to 0.030 micrograms per microgram protein per hour during rapid frond expansion and relatively constant thereafter at 0.014 to 0.019 for whole colonies until at least four daughter colonies had separated.
A substantial amount of 35S is transferred directly from the mother frond to its attached daughter fronds. The results are consistent with the transfer of soluble compounds arising through turnover of protein in the mother frond.
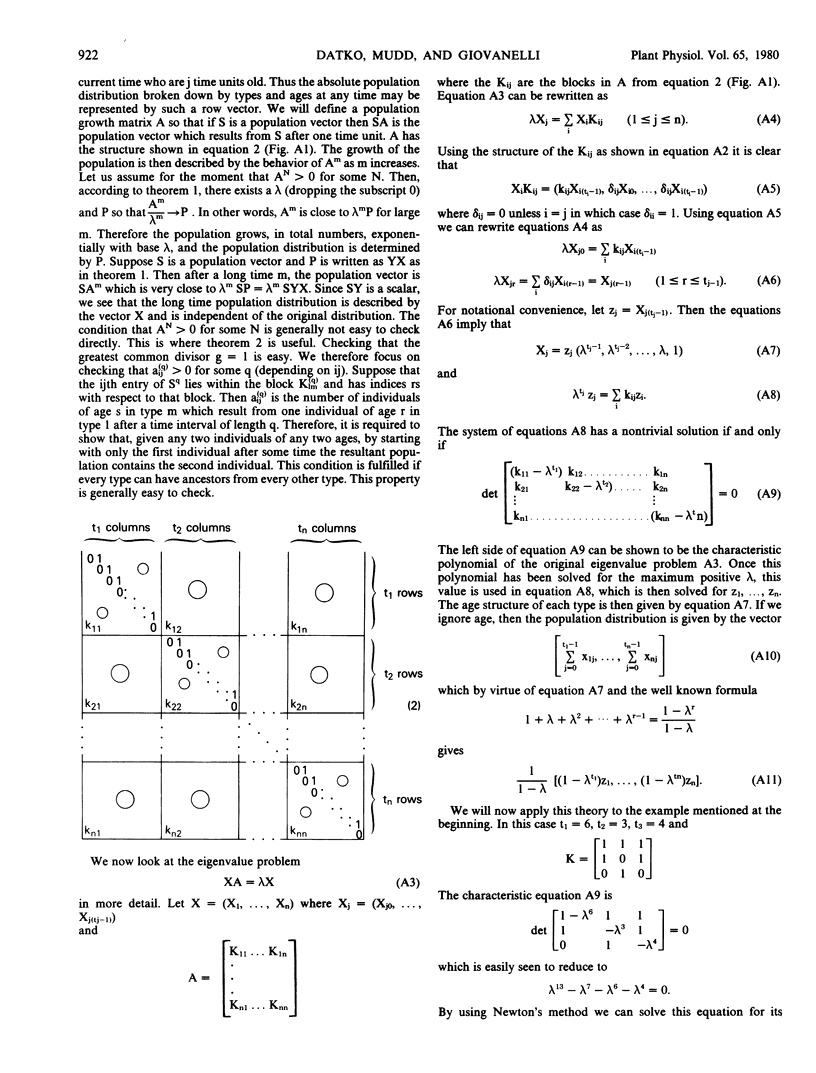
Tree diagrams and a mathematical treatment were utilized to relate the distribution of colony types and the over-all doubling time in a large population of Lemna colonies to the doubling times and modes of division of the individual colonies within that population. It was shown that a population with the growth properties described increases exponentially and that within a relatively few generations reaches an equilibrium population distribution which is independent of the initial proportions of the members of the population. The measured distribution of colony types producing odd-numbered as compared to those producing even-numbered daughters was the same as that predicted by the theoretical analyses. Once a mass culture of Lemna paucicostata has attained its equilibrium distribution of colony types, under our standard conditions the proportions of colonies in which the indicated daughter will be next to separate will be: 60%, first; 15%, second; 11%, third; 5%, fourth; 8% fifth or higher. Thus, about 91% of colonies will have produced no more than four daughter colonies. For at least this period the specific rate of protein accumulation is relatively constant and senescent changes in mother fronds are rare. In the equilibrium distribution, 39% of colonies will themselves be first daughters, 25% will be second daughters, 14% will be third daughters, 9% fourth, and 13% greater than fourth daughters. These combined results suggest that obtaining representative samples of colonies from such a population for biochemical studies should be relatively simple.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland C. F., Briggs W. R. Flowering Responses of the Long-day Plant Lemna gibba G3. Plant Physiol. 1967 Nov;42(11):1553–1561. doi: 10.1104/pp.42.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datka A. H., Mudd S. H., Giovanelli J. Homocysteine biosynthesis in green plants: studies of the homocysteine-forming sulfhydrylase. J Biol Chem. 1977 May 25;252(10):3436–3445. [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J. Lemna paucicostata Hegelm. 6746: DEVELOPMENT OF STANDARDIZED GROWTH CONDITIONS SUITABLE FOR BIOCHEMICAL EXPERIMENTATION. Plant Physiol. 1980 May;65(5):906–912. doi: 10.1104/pp.65.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Mudd S. H., Giovanelli J., Macnicol P. K. Sulfur-containing Compounds in Lemna perpusilla 6746 Grown at a Range of Sulfate Concentrations. Plant Physiol. 1978 Oct;62(4):629–635. doi: 10.1104/pp.62.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERBERT D., ELSWORTH R., TELLING R. C. The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol. 1956 Jul;14(3):601–622. doi: 10.1099/00221287-14-3-601. [DOI] [PubMed] [Google Scholar]
- Humphrey T. J., Davies D. D. A sensitive method for measuring protein turnover based on the measurement of 2-3H-labelled amino acids in protein. Biochem J. 1976 Jun 15;156(3):561–568. doi: 10.1042/bj1560561. [DOI] [PMC free article] [PubMed] [Google Scholar]