Abstract
Depression, mild cognitive impairment (MCI) and dementia are highly prevalent conditions that are increasing exponentially with similarly expanding social, medical and economic burdens. While there is a clear clinical connection between these three disorders, the mechanism of action that links them is less well understood. The lack of well-accepted biomarkers results in high levels of diagnostic subjectivity, which then greatly impacts research results when attempting to further explore their association. There is also a variety of clinical presentations of depressive syndromes, particularly in the elderly; each one may be associated with a different risk in the progression from MCI to different types of dementia. The diagnostic challenges, the importance of biomarkers and the discussion of inflammation as a possible link between depression, MCI and dementia are examined in this article.
Keywords: Alzheimer’s disease, biomarker, cytokine, dementia, depression, inflammation, mild cognitive, impairment
Depression, mild cognitive impairment (MCI) and dementia are highly prevalent chronic conditions associated with social, medical and economic burdens [1]. The inter-relationship among these clinical entities is complex and not well understood. Several factors contribute to the discrepancies in findings when linking these disorders. Important factors that contribute to the contradictory findings are the challenges in diagnosing these heterogeneous disorders and the lack of understanding of the potential mechanism linking these illnesses.
In 2012, the prevalence of Alzheimer’s disease (AD) in the USA was estimated to be 5.4 million individuals with a cost of US$172 billion. Over the coming decades, the aging of the baby boom generation is projected to result in an additional 10 million people with AD. It has been projected that by the year 2050, approximately 16 million individuals will be suffering from AD [2]. Furthermore, the prevalence of depression in the USA has been estimated at 11% in the elderly [3], with a lifetime prevalence of major depressive episode of 19% [4]. Depression is the fourth leading cause of disability and disease worldwide. According to the WHO, projections indicate that depression will be the highest ranked cause of disease burden in developed countries by the year 2020 [201]. MCI is the intermediate stage between the cognitive changes of normal aging and dementia. The prevalence of MCI has been estimated to be 14–18% for individuals aged 70 years and older [5] and the progression rate to dementia, particularly AD, has been estimated at 10–15% per year from memory disorders clinics or AD centers. In epidemiologic studies where there is a broader spectrum of MCI severity and more heterogeneity as to the underlying condition, the annual rates of progression range from 6 to 10% [6].
There are a number of studies linking depression, MCI and dementia. Depression has been frequently associated with AD and MCI, but the role of depression as a risk factor for AD is a matter of disagreement. It is not clear whether a history of depression is a true risk factor for dementia or rather represents a prodromal clinical phase of the neurodegenerative changes that occur in AD. There are data to support both hypotheses. MCI has also been linked to depression. There are several studies reporting depression as a factor contributing to the progression from normal cognition to MCI and from MCI to dementia.
Interestingly, depression, dementia and MCI share some common findings: lower hippocampal volumes [7–9], vascular changes in the brain [10,11] and neurotransmitter deficits [12,13]. All of these changes have been individually linked to chronic inflammation [14–16]. Major depression has been associated with decreased responsiveness to glucocorticoids; this resistance could contribute to excessive inflammation [17]. Depressed patients have been found to have higher levels of proinflammatory cytokines suggesting that inflammatory responses have a crucial role in the pathophysiology of depression [18]. Moreover, several inflammatory markers have been linked with AD. Postmortem studies demonstrate the presence of acute-phase reactants (including C-reactive protein [CRP], proinflammatory cytokines and activated complement cascade) in senile plaques and neurofibrillary tangles [19].
It is clear that neuroinflammation increases neurodegeneration [14], and reduces both neuroprotection and neuronal repair [20]. Given that these changes are common pathological features of depression, AD and possibly MCI as a predementia phase, it is intriguing to hypothesize that inflammation could be the mediating neurobiological mechanism linking them.
This article illustrates the current diagnostic challenges impacting the understanding of the possible mechanism of action linking late-life depression with MCI and dementia, and discusses the possible role of inflammation concerning these three disorders.
The relationship of depression & dementia
A history of depression has been described in several studies as an independent risk factor for later development of AD [21–23]. Longitudinal studies have shown a strong association between the number of depressive episodes and the risk of developing dementia. Dotson et al. followed a total of 1239 subjects with depression for a median of 24.7 years. They found an increase in the risk of all-cause dementia and AD as a function of the number of depressive symptoms at baseline. Each episode of depression was associated with a 14% increased risk of the subject later developing dementia, supporting the hypothesis that depression is a risk factor for dementia [22]. In the same way, Kessing and Anderson, using hospital-based data, reported that the rate of dementia tended to increase by 13% with every episode leading to admission for patients with depressive disorder [24]. Additional studies have shown that depressive symptoms are significantly associated with AD, even when the onset of depressive symptoms preceded the onset of identified AD symptoms by 25 years or more [21]. However, despite several studies demonstrating a robust association between depression and dementia, there are some longitudinal epidemiological studies that have found no association between depression and AD [25].
Several factors may contribute to the inconsistency of findings linking depression and dementia, such as the imprecise methods used to diagnose both disorders. Depression, MCI and dementia are heterogeneous disorders that are usually diagnosed by subjective tools with a lack of specific diagnostic biomarkers. Most of the data reporting the association between these disorders are retrospective, with significant limitations regarding unifying diagnostic criteria. The absence of biomarkers in clinical practice and the insufficient research on disease mechanisms linking these disorders [26] greatly contribute to the disparate research results.
Depression has been linked to different types of dementia. Some reports link history of depression with AD and others connect depression more with vascular dementia (VD). A recent retrospective cohort study examined depressive symptoms assessed in mid- and late-life and the different types of dementia. They found that subjects with late-life depressive symptoms had a twofold increase in AD risk whereas subjects with mid- and late-life symptoms had more than a threefold increase in VD. The authors concluded that late-life depression may be a prodrome to AD while recurrent depression may be associated with VD [27].
Conversely, the glucocorticoid theory favors the hypothesis that long-term exposure to stress or depression contributes to a smaller hippocampus (HC), leading to later development of AD [28], supporting the notion that a long history of depression contributes more to AD than VD. The HC plays an important role in the regulation of the hypothalamic–pituitary–adrenal axis (HPA), which is involved in the stress response and depression. Subjects with depression have increased HPA central drive, elevated corticotrophin-releasing hormone levels and impaired negative feedback regulation due to decreased expression of corticosteroid receptors in the hypothalamus and pituitary [29]. The human HC contains large numbers of corticosteroid receptors and has a critical role in downregulating corticotrophin-releasing hormone release via a multisynaptic pathway terminating in GABAergic output to the paraventricular nucleus [30]. HPA disturbances causing prolonged hypercortisolemia may promote hippocampal atrophy and functional decline. Thus, glucocorticoids may promote hippocampal cell injury and death when chronically elevated. Meta-analyses of structural imaging studies of the HC suggest that patients with depression have small HC volumes compared with healthy subjects [31]. Repeated episodes of depression may further contribute to loss of HC volume [28] and therefore increase the risk of AD.
Substantial data exist demonstrating how interconnected depression, increased risk of stroke [32], hypercortisolemia [33,34] and lower hippocampal volumes are associated not only with AD but also with VD. Chronic inflammation plays a role in both types of dementia, either by increasing the risk of vascular events and/or by contributing to a smaller HC and increasing the risk of AD. Given that the originally described pure VD is a rather rare entity and the majority of patients with VD have AD-related neuropathology, the development of one type of dementia versus the other would be the final manifestation of the proportion of pathology present in the brain.
The relationship of depression & MCI
Since the 1980s, researchers have struggled to find the appropriate category for patients who were not cognitively intact but were not sufficiently impaired to be considered as demented. This gray zone between ‘normal’ cognitive aging and dementia has been described as: benign forgetfulness, age-associated memory impairment, mild neurocognitive decline and questionable dementia [35]. The term ‘mild cognitive impairment’ was first used by Reisberg et al. [36] but was later established as a diagnosis with specific diagnostic criteria by Petersen et al. in 1999 [37]. The original Petersen criteria for MCI was limited to individuals with cognitive impairment in a single domain (memory). The revised published criteria include other domains besides memory and the complaint of decline can be obtained from an informant [38,39].
The two main known subtypes of MCI are amnestic MCI (aMCI) and nonamnestic MCI (naMCI). Memory deficits are the central feature of aMCI whereas naMCI patients present with deficits in other domains. Both are subdivided into single domain and multiple domains. MCI has also been classified according to probable etiology: MCI due to AD, vascular MCI, MCI – Lewy body dementia and behavioral MCI. There is an increased interest in the relationship between depression and MCI. Different mechanisms in which these two disorders have been linked range from depression being a psychological reaction to the cognitive deficits, to depression being an early manifestation of the neuropathological changes in the brain. Symptoms of depression varied according to the type of MCI and according to the classification used. Several studies have reported on the prevalence of psychiatric symptoms in MCI. Rozzini et al. reported that the predominant neuropsychiatric symptom of aMCI was depression followed by anxiety and reported a significantly higher prevalence of hallucinations and sleep disorders in the naMCI patients [40]. Other studies have reported a higher incidence of apathy in aMCI [41] and higher vascular comorbidities in naMCI [42].
The manifestation of the different depressive syndromes would differ according to the neuropathology. If the predominant neuropathology is vascular, then the depressive syndromes would fit the criteria described by the vascular depression theory. Conversely, if the predominant neuropathological substrate is amyloid, then the predominant depressive syndrome may be more related to apathy. The vascular depression hypothesis states that cerebrovascular disease can predispose, precipitate or perpetuate a depressive syndrome in older adults [43]. It has been described as a subtype of depression. The clinical presentation of vascular depression has been characterized as a medial frontal lobe syndrome with prominent psychomotor retardation, anhedonia and pronounced disability [44]. Several studies have reported that the presence of apathy as part of the depressive episode increases the risk of conversion to dementia [45].
Numerous recent studies have reported the potential role of depression in the conversion from normal cognition to MCI and from MCI to dementia. The majority of the studies found depression as an important factor in the conversion [46–54]. Table 1 provides a summary indicating whether results for depression were ‘positive’ (i.e., showed a significantly increased risk of progression to MCI or AD for those with depression vs no depression) or ‘negative’ [45,55–61].
Table 1.
Longitudinal studies of the role of depression in the development of either mild cognitive impairment or dementia among subjects either normal or mild cognitive impairment at baseline.
| Study (year) | Study type | Patients (n) | Follow-up (years) | Outcome | Results | Ref. |
|---|---|---|---|---|---|---|
| Normal to MCI and/or dementia | ||||||
| Steenland et al. (2012) | Clinic based | 8107 | 6 | MCI or dementia | + | [62] |
| Goveas et al. (2011) | Clinical trial | 6376 | 5.4 | MCI or dementia | + | [50] |
| Unverzagt et al. (2011) | Population based | 1688 | 3.3 | MCI | + | [51] |
| Köhler et al. (2010) | Population based | 479 | 6 | MCI | + | [53] |
| Dotson et al. (2010) | Population based | 1239 | 24.7 | MCI | − | [22] |
| Ravaglia et al. (2008) | Population based | 595 | 4 | MCI | + | [52] |
| Panza et al. (2008) | Population based | 2963 | 3.5 | MCI | − | [48] |
| Wilson et al. (2007) | Population based | 1256 | 12 | MCI | − | [49] |
| Barnes et al. (2006) | Population based | 2220 | 6 | MCI | + | [47] |
| Geda et al. (2006) | Clinic based | 840 | 3.5 | MCI | + | [46] |
| MCI to dementia | ||||||
| Lu et al. (2009) | Clinical trial based | 756 | 3 | Dementia | + | [57] |
| Vicini Chilovi et al. (2009) | Clinic based | 124 | 2 | Dementia | − | [59] |
| Edwards et al. (2009) | Clinic based | 521 | 1.1 | Dementia | + | [55] |
| Artero et al. (2008) | Population based | 2895 | 4 | Dementia | + | [63] |
| Panza et al. (2008) | Population based | 2963 | 3.5 | Dementia | − | [48] |
| Houde et al. (2008) | Clinic based | 60 | 4.3 | Dementia | − | [56] |
| Gabryelewicz et al. (2007) | Clinic based | 105 | 3 | Dementia | + | [60] |
| Palmer et al. (2007) | Population based | 628 | 3 | Dementia | − | [61] |
| Teng et al. (2007) | Clinic based | 51 | 2 | Dementia | + | [58] |
| Robert et al. (2006) | Clinic based | 251 | 1 | Dementia | − | [45] |
| Modrego and Ferrández (2004) | Clinic based | 114 | 3 | Dementia | + | [54] |
+: Depression was significantly associated with transition to a worse diagnosis; −: Depression was found not to be significant with the transition; MCI: Mild cognitive impairment.
Among the seven largest studies (each with over 1000 subjects [22,47–51,62]) investigating the role of depression in conversion from control to MCI, four were positive and three were negative. There were only three large studies analyzing progression from MCI to dementia (each with over 2500 subjects) with conflicting results, and the one positive study found discrepant results for men and women [63].
In a recent prospective study published by our group of 8107 subjects from 30 AD research centers in the USA between 2005 and 2011, late-life depression was shown to be a significant risk factor for subjects progressing from normal to MCI [62]. Comparisons among studies are difficult and conclusions are often limited by methodological inconsistencies (e.g., varying definitions of MCI, dementia and depression). Some of the studies were primarily designed to evaluate other outcomes or conditions, such as cardiovascular disease, with limited measures of cognition. Adjustment for baseline cognitive status, comorbidities, the timing of depression (early vs late life) and the concomitant antidepressant medication (all of which could confound or potentially modify the effect of depression) was relatively uncommon. Baseline cognitive status may act as a confounder in the association between depression and outcomes, as depressed subjects have worse cognitive function [64], which is associated with a greater risk of development of MCI and/ or dementia. Furthermore, cognitive deficits may even be present premorbidly in healthy individuals who are genetically at risk for developing depression [65]. Therefore, persistent cognitive deficits in the context of depression may reflect underlying structural changes and a common pathophysiology, increasing an individual’s risk of progression to dementia.
The underlying inflammatory process may be different in MCI subtypes as they may reflect different etiologies [66]. Dlugaj et al. detected that elevated levels of high-sensitivity CRP (hs-CRP), 5 years before diagnosis, are associated with an at least twofold increased probability of MCI [66]. hs-CRP is a sensitive marker of systemic low-grade inflammation and tissue damage. CRP has been found in and around amyloid plaques and around small-vessel damages. Stephan et al. reported in their comprehensive review of the neuropathological profile of MCI that the upregulation of complement proteins, the expression of cyclooxygenase-2, IL-6 and microglia activation do not appear to be significantly changed in the cognitive changes seen in early cognitive disorders [67]; however, most of the studies on MCI would exclude patients with comorbid conditions, in particular those related to psychiatric and/or vascular disorders [68].
Inflammation contributes to the pathogenesis of some but not all depressed patients [20] as well as some but not all MCI patients. Different studies have demonstrated the link between depression and MCI. Possibly, the depressed patients with high levels of inflammation are the ones progressing to MCI and dementia, whereas the depressed patients with no inflammation may be those who do not necessarily progress to have neuropathological changes. Inflammation may play an important role in the presence and development of MCI. Independent segments of the inflammatory cascade seem to be activated at different stages of disease. Studies exploring the relationship of inflammation between depression and MCI are needed to further understand the specific segment of the inflammatory cascade compromised when these two disorders are comorbid.
Inflammation as the mediator
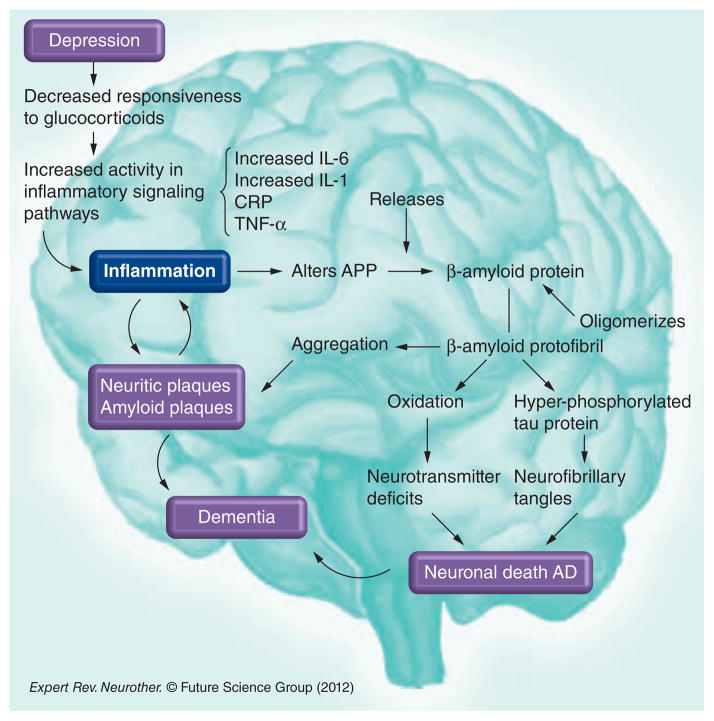
It has been hypothesized that the progression of depression to dementia could result from the chronic inflammatory changes that are linked to the activation of macrophages in the blood and microglia in the brain [20]. Several studies have reported higher serum levels of the inflammatory cytokines IL-6, IL-1 and TNF-α in depression and AD [69]. IL-1 is an immunoregulatory cytokine that is overexpressed early in plaque formation. It promotes the synthesis and processing of amyloid precursor protein and therefore promotes further amyloid production, deposition and plaque evolution. Amyloid precursor protein in turn activates microglia, inducing excessive expression of IL-1 [70]. IL-6 is a cytokine that mediates immune responses and inflammatory reactions, affecting CNS cell growth and differentiation. IL-6 overexpression is generally detrimental and destructive to cell growth. It is a proinflammatory cytokine that induces acute-phase proteins. Numerous studies have shown a relationship between IL-6 and the development of AD [71]. In addition, proinflammatory cytokines correlate with the alteration of the expression and processing of the amyloid-β (Aβ) precursor protein, triggering the amyloid cascade that leads to the neuropathology of AD. In a reciprocal fashion, once the amyloid plaques have been formed, fibrillar Aβ promotes the production of proinflamatory cytokines by microglia and monocytic cell lines (Figure 1) [69].
Figure 1. The relation between depression, inflammation and the amyloid cascade.
AD: Alzheimer’s disease; APP: Amyloid precursor protein; CRP: C-reactive protein.
Hypersecretion of the corticotropin-releasing factor (CRF) and hyperactivity of the HPA axis have been consistently demonstrated in chronic stress and depression [72]. This results in activation of inflammatory pathways, which are manifested by the increased proinflammatory cytokines and increased acute-phase proteins. Data indicate that the most reproducible finding of immune activation in patients with depression is increased plasma levels of IL-6 and its downstream product from the liver, CRP [17]. However, CRP, as well as other inflammatory markers, has been reported to be increased, decreased or normal in depression and AD. There have been conflicting reports that could be partly explained due to the heterogeneity across studies, lack of methodological standardization and differences in collection techniques. In addition, the circadian rhythm could have a significant impact on the values of the different inflammatory markers, so temporal variation must be considered when analyzing inflammatory indicators. Table 2 presents a summary of different inflammatory markers found across studies in AD and depression [16,73–84].
Table 2.
Summary of inflammatory markers found in depression, mild cognitive impairment and dementia.
| Study (year) | Patients (n) | Type of sample | Marker | Expression of inflammatory markers† | Ref. |
|---|---|---|---|---|---|
| MCI & dementia | |||||
| Olsson et al. (2012) | 65 controls, 170 MCI, 96 AD | CSF | Microglia markers, YKL-40 (+), CD 14 | YKL-40 differentiates between AD and controls | [81] |
| Doecke et al. (2012) | 754 controls, 207 AD | Blood | Cortisol (+), pancreatic polypeptide (+), IGF-binding protein 2 (+), microglobulin β2 (+), VCAM-1 (+), carcinoembryonic antigen (+), matrix metalloprotein 2 (+), CD40 (+), macrophage inflammatory protein 1α(+), superoxide dismutase (+), homocysteine (+), apoE (−), EGF receptor (−), hemoglobin (−), calcium (−), zinc (−), IL-17 (−), albumin (−) | Cortisol (+), macrophage inflammatory protein 1α(+), IL-17 (−) | [74] |
| Soares et al. (2012) | 58 controls, 396 MCI, 112 AD | Plasma | Eotaxin 3 (+), pancreatic polypeptide (+), N-terminal protein B-type brain natriuretic peptide (+), low CRP (−), ApoE (−), cortisol (+), IL-13 (+), apoB (+), IFN-γ levels (+) | CRP (−), IL-13 (+) | [82] |
| Hu et al. (2012) | 600 | Plasma | B-type natriuretic peptide, CRP, pancreatic polypeptide | CRP (−) | [78] |
| Schuitemaker et al. (2009) | 67 MCI, 145 AD | Serum and CSF | CRP (+), IL-6 (+), ACT | IL6 (+), CRP (+) in MCI | [16] |
| Zaciragic et al. (2007) | 15 AD, 15 control | Serum | CRP (+) | CRP (+) in AD | [83] |
| Kravitz et al. (2009) | 350 | Serum | CRP (+) | CRP (+) in dementia in women | [79] |
| Depression | |||||
| Dowlati et al. (2010) | Meta-analysis; 24 studies | Blood | TNF-α (+), IL-1β (=), IL-6 (+), IL-4 (=), IL-2 (=), IL-8 (=), IL-10 (=), IFN-γ (=) | IL-6 (+), TNF-α (+). Increased with depression. No differences in other cytokines studied | [75] |
| Howren et al. (2009) | Meta-analysis; 51 studies for CRP, 62 for IL-6, 14 for IL-1, nine for IL-1ra | Serum | IL-1 (+), IL-6 (+), CRP (+), IL-1ra (+) | IL-6 (+), IL-1 (+), CRP (+), IL-1ra. Increased with depression | [77] |
| Lindqvist et al. (2009) | 63 suicide attempters, 47 controls | CSF and plasma obtained at the same time | IL-6 (+) | IL-6 (+) in CSF was significantly higher in suicide attempters than in healthy control subjects | [80] |
| Zorrilla et al. (2001) | Meta-analysis | CD4/CD8 ratio (+), circulating haptoglobin (+), PGE(2) (+), IL-6 (+), NK-cell cytotoxicity (−), lymphocyte proliferative response to mitogen (−) | IL-6 (+) | [84] | |
| Carpenter et al. (2004) | 18 depressed, 26 controls | CSF | IL-6 (=) | CSF IL-6 levels did not differ between depressed and controls | [73] |
| Hestad et al. (2003) | 23 patients (15 undergoing ECT), 15 controls | Plasma | TNF-α (+) | TNF-α (+) in depressed patients. Patients treated with ECT showed decrease of TNF-α as depression improved | [76] |
YKL-40 is a marker of inflammation and endothelial dysfunction.
+: Increased inflammatory marker; −: decreased inflammatory marker; =: no change.
ACT: α-1-Antichymotrypsin; AD: Alzheimer’s disease; CRP: C-reactive protein; CSF: Cerebrospinal fluid; ECT: Electroconvulsive therapy; IL-1ra: IL-1 receptor antagonist; MCI: Mild cognitive impairment; NK: Natural killer; PGE2: Prostaglandin E2.
Despite inconsistencies in the data regarding the cause–effect relationship between depression, MCI and dementia, there is clear clinical relation among them. Substantial data exist supporting that neurodegenerative changes in depression and dementia are associated with an increase in proinflammatory cytokines, hypercortisolemia and other inflammatory mediators [20]. Discrepancies in the inflammatory findings reflect the lack of standardization in methodological procedures. Unifying sample collection and overcoming laboratory processing differences will help to clarify the inconsistencies with these findings.
Recent findings by Raison et al. suggest that CRP could be a biomarker in differentiating those patients with a different pathophysiological mechanism of depression (neuroinflamation). They found that by stratifying depressed patients according to the level of inflammatory markers (hs-CRP), there are differences in the antidepressant response using anticytokine therapies [85]. Identification of those patients with incipient development of neuroinflammation and increased disease risk could bring an immense opportunity for early intervention on their clinical course to potentially change the path of the events and thereby decrease the devastating epidemic of dementia.
Conclusions
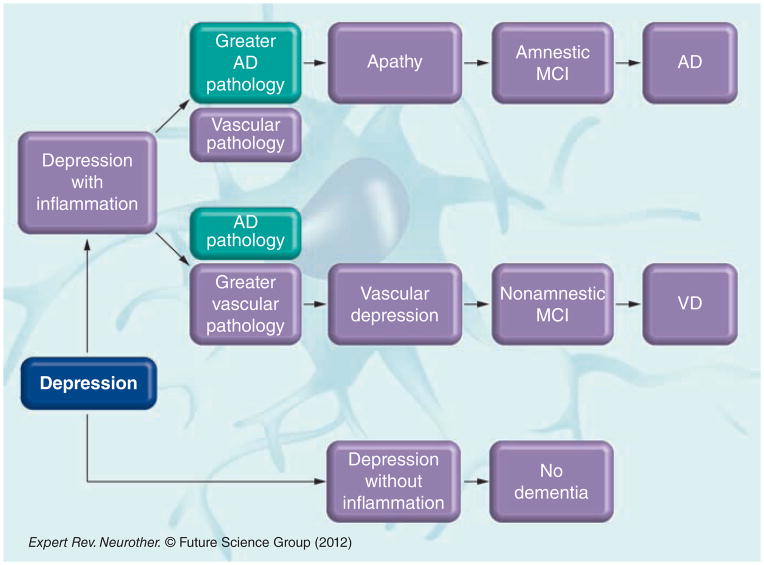
A subset of depressed individuals will develop cognitive impairment and subsequent dementia, and only some depressed patients present with high inflammation. Perhaps those with activation of the inflammatory pathway due to severity of the episode or due to genetic predisposition are the ones who are more vulnerable to either develop vascular events, trigger the amyloid cascade or both. This in turn may activate the inflammatory pathway, promoting more amyloid deposition or more vascular dysfunction according to comorbidities and/ or genetic predisposition. The extent of one type of injury (amyloid deposition) versus the other (vascular dysfunction) caused by the different pathways and the proportion of each pathology would contribute to the development of different degrees of neuropathology without being mutually exclusive. It is possible that those individuals who have a more significant vascular stigmata would develop vascular depression leading to vascular MCI and a more prominent vascular type of dementia, whereas those with more predominant AD pathology would develop a more apathetic type of depression including those nonclassifiable depressive syndromes and later progress to amnestic MCI and convert to AD (Figure 2).
Figure 2. The different pathways in which depression precedes the different types of mild cognitive impairment and dementia.
AD: Alzheimer’s disease; MCI: Mild cognitive impairment; VD: Vascular dementia.
Better understanding about the role of inflammation in the pathophysiology of these three disorders and the development of biomarkers targeting the inflammatory pathway would help advance the field tremendously, not only in the diagnosis of these heterogeneous disorders but also in the development of newer therapeutic strategies.
Expert commentary
There is inevitable discrepancy when diagnosing entities that are heterogeneous in nature in the absence of biological markers. Advances in neuroimaging techniques have advanced the accuracy of the diagnosis of dementia, such as using structural MRI (to measure hippocampal volume), PET scans and amyloid imaging as tools to differentiate and understand dementia. Neuroradiology is also slowly advancing in the field of mental illness with functional MRI and nuclear magnetic resonance spectroscopy being explored in the understanding of mental disorders. However, most of these techniques do not have the diagnostic specificity needed to be widespread clinical resources and remain research tools; they do not yet function as established biological diagnostic markers in clinical settings addressing depression, mild cognitive disorders and dementia.
In addition, depression has a wide variety of presentations in all age groups. Even the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, and International Classification of Diseases, tenth revision, nomenclature have remarkable limitations when trying to narrow a psychological diagnosis. These have been well described, particularly in the geriatric population in which depression may present with subsyndromal symptoms nonidentifiable with the strict current criteria.
While older adults with depression are less likely to complain of ‘subjective dysphoria’ than younger adults but more likely to have vegetative signs and cognitive disturbance related to depression (thus presenting with an ‘atypical signature of depression’ [86]), one could wonder if this atypical presentation may have a different etiology than depression in the younger population. This subthreshold or atypical presentation may be secondary to neuro-degenerative changes related to amyloid deposition, presenting then as the prodrome of dementia.
Several studies have reported that the presence of apathy as part of the depressive episode increases the risk of conversion to dementia [45]. The Robert et al. diagnostic criteria for apathy suggest that the lack of initiation versus responsiveness is the core symptom in the differentiation between the diagnosis of apathy versus nondysphoric depression [87,88]. Apathy could potentially be the prodrome neuropsychiatric symptom very commonly misdiagnosed as depression.
A unified diagnostic criteria for apathy and the clarification of whether this disorder differs or actually overlaps with the non-classifiable subthreshold depressive syndromes (such as depression without sadness or subsyndromal depression in the elderly) is crucial, and would shed light on the current controversy of depression as a prodrome or a risk factor of AD. Reaching a consensus on the definition and nosological position of apathy within dementia is vital to increase understanding of risk factors and enable comparisons across research and practice [89].
Furthermore, vascular pathology contributes to the pathogenesis of depression in late life. Indeed, cerebrovascular disease, observed in neuroimaging studies controlled for white matter lesion severity and increased arterial stiffness, assessed using carotid artery distensibility and pulse wave velocity, has been observed in patients with depressive symptoms in large epidemiologic studies [10,47,90]. Inversely, vascular events have been linked with a past history of depression, a prior history of depression, or depressive symptoms have been strongly associated with subsequent development of ischemic heart disease and myocardial infarction [91]. A significant correlation has been described between depression and vascular disease; in particular, cardiovascular mortality, coronary disease and heart failure [10]. Inflammation and immune dysregulation have been critically involved in vascular disease. Elevated CRF concentration in cerebrospinal fluid, nonsuppression of cortisol secretion after dexamethasone administration and overexpression of hypothalamic CRF neurons in depressed patients have all been well documented [92–94]. In addition, chronic inflammation has a significant direct impact on vascular endothelial cells [10] and, in the same way, vascular disease could be a consequence of the ‘glucocorticoid cascade’. Inflammation can follow, precipitate or aggravate vascular events. Therefore, inflammation has a bidirectional role in the link between depression and vascular pathology.
There is robust evidence that inflammation plays a crucial role in some types of depression. However, some depressive episodes are the result of a transient psychological stress not causing permanent neuronal injury. Nevertheless, increased depression burden (either by severity or number of episodes) could cause a neuronal insult with detrimental consequences to brain cells. Decreased hippocampal size has been described in some depressed individuals [28]. Chronic stress has been correlated with hypercortisolemic states, hypersecretion of the CRF, hyperactivity of the HPA axis, increased proinflammatory cytokines and increased acute-phase reactants impacting negatively on hippocampal size [29], one of the main neuroanatomical changes in AD. The use of specific inflammatory biomarkers to identify those depressive states at risk of developing dementia could be revolutionary in the way we diagnose and treat major depression and impact the progression to dementia.
The clinical syndrome of dementia takes place several years after the neuropathological changes have occurred, so early identification of preclinical stages and risk factors is critical in order to modify the course of illness. Identification of biomarkers as diagnostic aids to the recognition of patients in predementia stages will add precision to the incipient current diagnostic work-up of AD.
Five-year view
There are several areas that should be addressed with targeted research to further define the link between depression, MCI and dementia.
First, as discussed in this article, the fundamental challenge when trying to link heterogeneous disorders is the lack of unifying diagnostic criteria and the highly subjective tools used in research and clinical practice. New diagnostic criteria based more on underlying neurobiology rather than clinical syndromes would be the first step to progress in establishing a biological connection between these three disorders.
Second, the use of advanced technology in research and clinical practice, such as the implementation of neuroimaging and CSF markers in the diagnosis of different types of dementia, would help to understand the end point of the progression from depression, MCI and dementia. Biomarkers are necessary to enable more rigorous identification and quantitation of underlying neurobiological mechanisms such as inflammation, amyloid deposition, vascular insults and other processes that may variably contribute to cognitive and depressive symptoms. It would help to better categorize the different pathological substrates that may be present as a result of inflammation. Reduced concentrations of Aβ, increased total tau and increased phosphotau in CSF [95] along with positive amyloid result on PET imaging (11C and 18F PET imaging [96]) and reduced hippocampal size in structural imaging [97] are current useful tools that have not been incorporated into the diagnostic criteria for AD. The use of cost-effective novel markers and neuroradiology techniques to detect the early pathological manifestations of the predementia states in AD would help define the role of risk factors such as depression in the progression to dementia.
Third, given that specific inflammatory mechanisms, including the cytokine-driven acute-phase response, complement activation and microglial activation [69,98], contribute to neurodegeneration and have been shown to play a role in the pathogenesis of depression and dementia [99], more research should focus on identifying the mediating inflammatory factors in the progression from depression to MCI and dementia. The discovery of biomarkers related to the inflammatory pathway would help with the identification of specific steps in the sequence of events that contribute to such progression and the discovery of disease-modifying factors that may impact the course of illness of these three interlinked disorders.
Fourth, further research is needed to elucidate the impact of early and adequate treatment of depression. A number of reports suggest that antidepressants have neuroprotective properties by increasing the proliferation of neural progenitors in the subgranulate zone of the HC [100,101].
It would be important to determine if long-term continued and adequate treatment with antidepressants may decrease the risk of developing dementia among individuals with recurrent depression and to determine the impact of such treatment on the inflammatory pathways.
A final and most important issue for research would be the development of novel therapeutic strategies targeting the inflammatory pathway in order to reduce the neurotoxic effects of neuroinflammation. Despite much evidence linking inflammation and AD, the initial attempts to apply anti-inflammatory therapies for the treatment of AD have not been successful. Brain tissue from elderly individuals with a history of chronic NSAID use was compared with tissue from age-matched controls [102]. The two groups showed a similar degree of senile plaque and neurofibrillary tangle pathology, but the use of NSAIDS was associated with a significant reduction in the number of senile plaques associated with activated microglia. Tepoxalin, another NSAID, inhibits the synthesis of IL-1β in microglial cells and α-1-antichymotrypsin in astrocytes by preventing NF-kB activation [103], all factors that are involved in the inflammatory events of AD. The use of corticosteroids has also been the subject of epidemiological and clinical research in AD. The potential benefit from nonselective COX inhibitors (such as indomethacin, naproxen, ibuprofen and diclofenac) and/or selective COX-2 inhibitors (such as celecoxib and meloxicam) has also been supported by several studies [104]. Therapeutic mechanisms targeting key glial activation pathways have also been reported supporting the neuroinflammation hypothesis of disease progression [105]. Unfortunately, none of these therapeutic strategies targeting the inflammatory pathway have reached the level of evidence to modify clinical practice. A number of anti-inflammatory strategies have also been studied in the treatment of depression, including the direct administration of anticytokine therapies [85]. Despite numerous attempts to identify possible anti-inflammatory treatments for depression and dementia, further research is necessary in order to find effective therapies that could ameliorate, treat and even reverse the neurodegenerative consequences of inflammation. Because neuroinflammation can be friend or foe, depending on many factors including the nature of the immune response and stage of disease, better understanding of the role of inflammation in depression and AD will inform more rational development of immunomodulatory approaches.
Key issues.
The identification of specific biomarkers to differentiate the diverse types of depressive disorders as well as the types of mild cognitive impairment and dementia would help to elucidate the potential mechanism linking these illnesses.
The most reproducible findings of immune activation in patients with depression are increased plasma levels of IL-6 and its downstream product from the liver, C-reactive protein, which could serve as biomarkers to identify those depressive syndromes at risk of progression to dementia.
There are various types of depressive syndromes and each one may possess a different risk in the progression from mild cognitive impairment to different types of dementia.
Apathy may be an earlier clinical manifestation of the neuropathological changes in the brain related to Alzheimer’s disease, which are commonly misdiagnosed as depression.
High levels of cortisol have been linked to depression, and prolonged hypercortisolemia has been linked to smaller hippocampal volumes, increasing the risk of dementia.
Inflammation has been associated with both Alzheimer’s pathology and vascular changes in the brain. The inter-relation between inflammation and other factors such as genetic, environmental and comorbid conditions will ultimately determine the type of predominant neuropathology and clinical manifestation of dementia.
Acknowledgments
The authors acknowledge the assistance of Gisella Klekamp with the design of the figures.
Footnotes
Financial and competing interests disclosure
WM McDonald is supported by Health Resources and Services Administration grant (6UB4HP19215). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Turró-Garriga O, López-Pousa S, Vilalta-Franch J, et al. Annual economic cost of informal care in Alzheimer’s disease. Rev Neurol. 2010;51(4):201–207. [PubMed] [Google Scholar]
- 2.2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. No authors listed. [DOI] [PubMed] [Google Scholar]
- 3.Steffens DC, Fisher GG, Langa KM, Potter GG, Plassman BL. Prevalence of depression among older Americans: the Aging, Demographics and Memory Study. Int Psychogeriatr. 2009;21(5):879–888. doi: 10.1017/S1041610209990044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, Shahly V. Age differences in major depression: results from the National Comorbidity Survey Replication (NCS-R) Psychol Med. 2010;40(2):225–237. doi: 10.1017/S0033291709990213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farias ST, Mungas D, Reed BR, Harvey D, DeCarli C. Progression of mild cognitive impairment to dementia in clinic- vs community-based cohorts. Arch Neurol. 2009;66(9):1151–1157. doi: 10.1001/archneurol.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hickie I, Naismith S, Ward PB, et al. Reduced hippocampal volumes and memory loss in patients with early- and late-onset depression. Br J Psychiatry. 2005;186:197–202. doi: 10.1192/bjp.186.3.197. [DOI] [PubMed] [Google Scholar]
- 8.MacQueen GM, Campbell S, McEwen BS, et al. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci USA. 2003;100(3):1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161(4):598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 10.Camus V, Kraehenbühl H, Preisig M, Büla CJ, Waeber G. Geriatric depression and vascular diseases: what are the links? J Affect Disord. 2004;81(1):1–16. doi: 10.1016/j.jad.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Hickie I, Naismith S, Ward PB, et al. Vascular risk and low serum B12 predict white matter lesions in patients with major depression. J Affect Disord. 2005;85(3):327–332. doi: 10.1016/j.jad.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Rodríguez JJ, Noristani HN, Verkhratsky A. The serotonergic system in ageing and Alzheimer’s disease. Prog Neurobiol. 2012 doi: 10.1016/j.pneurobio.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Wixey JA, Reinebrant HE, Buller KM. Inhibition of neuroinflammation prevents injury to the serotonergic network after hypoxia–ischemia in the immature rat brain. J Neuropathol Exp Neurol. 2011;70(1):23–35. doi: 10.1097/NEN.0b013e3182020b7b. [DOI] [PubMed] [Google Scholar]
- 14.Hurley LL, Tizabi Y. Neuroinflammation, neurodegeneration, and depression. Neurotox Res. 2012 doi: 10.1007/s12640-012-9348-1. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mondelli V, Cattaneo A, Belvederi Murri M, et al. Stress and inflammation reduce brain-derived neurotrophic factor expression in first-episode psychosis: a pathway to smaller hippocampal volume. J Clin Psychiatry. 2011;72(12):1677–1684. doi: 10.4088/JCP.10m06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuitemaker A, Dik MG, Veerhuis R, et al. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging. 2009;30(11):1885–1889. doi: 10.1016/j.neurobiolaging.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21(1):9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duong T, Nikolaeva M, Acton PJ. C-reactive protein-like immunoreactivity in the neurofibrillary tangles of Alzheimer’s disease. Brain Res. 1997;749(1):152–156. doi: 10.1016/s0006-8993(96)01359-5. [DOI] [PubMed] [Google Scholar]
- 20.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32(10):1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 21.Green RC, Cupples LA, Kurz A, et al. Depression as a risk factor for Alzheimer disease: the MIRAGE Study. Arch Neurol. 2003;60(5):753–759. doi: 10.1001/archneur.60.5.753. [DOI] [PubMed] [Google Scholar]
- 22.Dotson VM, Beydoun MA, Zonderman AB. Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology. 2010;75(1):27–34. doi: 10.1212/WNL.0b013e3181e62124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, Au R. Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology. 2010;75(1):35–41. doi: 10.1212/WNL.0b013e3181e62138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessing LV, Andersen PK. Does the risk of developing dementia increase with the number of episodes in patients with depressive disorder and in patients with bipolar disorder? J Neurol Neurosurg Psychiatr. 2004;75(12):1662–1666. doi: 10.1136/jnnp.2003.031773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker JT, Chang YF, Lopez OL, et al. Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am J Geriatr Psychiatry. 2009;17(8):653–663. doi: 10.1097/jgp.0b013e3181aad1fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geda YE. Blowing hot and cold over depression and cognitive impairment. Neurology. 2010;75(1):12–14. doi: 10.1212/WNL.0b013e3181e8cc2f. [DOI] [PubMed] [Google Scholar]
- 27.Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, Whitmer RA. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69(5):493–498. doi: 10.1001/archgenpsychiatry.2011.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry. 2011;16(3):252–264. doi: 10.1038/mp.2010.80. [DOI] [PubMed] [Google Scholar]
- 29.Butters MA, Young JB, Lopez O, et al. Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues Clin Neurosci. 2008;10(3):345–357. doi: 10.31887/DCNS.2008.10.3/mabutters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 31.MacQueen GM. Magnetic resonance imaging and prediction of outcome in patients with major depressive disorder. J Psychiatry Neurosci. 2009;34(5):343–349. [PMC free article] [PubMed] [Google Scholar]
- 32.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol. 2007;6(7):611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 33.Vogelzangs N, Beekman AT, Boelhouwer IG, et al. Metabolic depression: a chronic depressive subtype? Findings from the InCHIANTI study of older persons. J Clin Psychiatry. 2011;72(5):598–604. doi: 10.4088/JCP.10m06559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogelzangs N, Suthers K, Ferrucci L, et al. Hypercortisolemic depression is associated with the metabolic syndrome in late-life. Psychoneuroendocrinology. 2007;32(2):151–159. doi: 10.1016/j.psyneuen.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geda YE. Mild cognitive impairment in older adults. Curr Psychiatry Rep. 2012;14(4):320–327. doi: 10.1007/s11920-012-0291-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reisberg B, Ferris SH, de Leon MJ, et al. The stage specific temporal course of Alzheimer’s disease: functional and behavioral concomitants based upon cross-sectional and longitudinal observation. Prog Clin Biol Res. 1989;317:23–41. [PubMed] [Google Scholar]
- 37.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 38.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rozzini L, Vicini Chilovi B, Conti M, et al. Neuropsychiatric symptoms in amnestic and nonamnestic mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;25(1):32–36. doi: 10.1159/000111133. [DOI] [PubMed] [Google Scholar]
- 41.Ellison JM, Harper DG, Berlow Y, Zeranski L. Beyond the “C” in MCI: noncognitive symptoms in amnestic and non-amnestic mild cognitive impairment. CNS Spectr. 2008;13(1):66–72. doi: 10.1017/s1092852900016175. [DOI] [PubMed] [Google Scholar]
- 42.Ihle-Hansen H, Thommessen B, Wyller TB, et al. Incidence and subtypes of MCI and dementia 1 year after first-ever stroke in patients without pre-existing cognitive impairment. Dement Geriatr Cogn Disord. 2011;32(6):401–407. doi: 10.1159/000335361. [DOI] [PubMed] [Google Scholar]
- 43.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60(12):1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Krishnan KR, Taylor WD, McQuoid DR, et al. Clinical characteristics of magnetic resonance imaging-defined subcortical ischemic depression. Biol Psychiatry. 2004;55(4):390–397. doi: 10.1016/j.biopsych.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 45.Robert PH, Berr C, Volteau M, et al. PréAL study. Apathy in patients with mild cognitive impairment and the risk of developing dementia of Alzheimer’s disease: a one-year follow-up study. Clin Neurol Neurosurg. 2006;108(8):733–736. doi: 10.1016/j.clineuro.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Geda YE, Knopman DS, Mrazek DA, et al. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch Neurol. 2006;63(3):435–440. doi: 10.1001/archneur.63.3.435. [DOI] [PubMed] [Google Scholar]
- 47.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63(3):273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 48.Panza F, Capurso C, D’Introno A, et al. Impact of depressive symptoms on the rate of progression to dementia in patients affected by mild cognitive impairment. The Italian Longitudinal Study on Aging. Int J Geriatr Psychiatry. 2008;23(7):726–734. doi: 10.1002/gps.1967. [DOI] [PubMed] [Google Scholar]
- 49.Wilson RS, Schneider JA, Boyle PA, Arnold SE, Tang Y, Bennett DA. Chronic distress and incidence of mild cognitive impairment. Neurology. 2007;68(24):2085–2092. doi: 10.1212/01.wnl.0000264930.97061.82. [DOI] [PubMed] [Google Scholar]
- 50.Goveas JS, Espeland MA, Woods NF, Wassertheil-Smoller S, Kotchen JM. Depressive symptoms and incidence of mild cognitive impairment and probable dementia in elderly women: the Women’s Health Initiative Memory Study. J Am Geriatr Soc. 2011;59(1):57–66. doi: 10.1111/j.1532-5415.2010.03233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Unverzagt FW, Ogunniyi A, Taler V, et al. Incidence and risk factors for cognitive impairment no dementia and mild cognitive impairment in African Americans. Alzheimer Dis Assoc Disord. 2011;25(1):4–10. doi: 10.1097/WAD.0b013e3181f1c8b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ravaglia G, Forti P, Lucicesare A, et al. Prevalent depressive symptoms as a risk factor for conversion to mild cognitive impairment in an elderly Italian cohort. Am J Geriatr Psychiatry. 2008;16(10):834–843. doi: 10.1097/JGP.0b013e318181f9b1. [DOI] [PubMed] [Google Scholar]
- 53.Köhler S, Thomas AJ, Barnett NA, O’Brien JT. The pattern and course of cognitive impairment in late-life depression. Psychol Med. 2010;40(4):591–602. doi: 10.1017/S0033291709990833. [DOI] [PubMed] [Google Scholar]
- 54.Modrego PJ, Ferrández J. Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol. 2004;61(8):1290–1293. doi: 10.1001/archneur.61.8.1290. [DOI] [PubMed] [Google Scholar]
- 55.Edwards ER, Spira AP, Barnes DE, Yaffe K. Neuropsychiatric symptoms in mild cognitive impairment: differences by subtype and progression to dementia. Int J Geriatr Psychiatry. 2009;24(7):716–722. doi: 10.1002/gps.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Houde M, Bergman H, Whitehead V, Chertkow H. A predictive depression pattern in mild cognitive impairment. Int J Geriatr Psychiatry. 2008;23(10):1028–1033. doi: 10.1002/gps.2028. [DOI] [PubMed] [Google Scholar]
- 57.Lu PH, Edland SD, Teng E, Tingus K, Petersen RC, Cummings JL Alzheimer’s Disease Cooperative Study Group. Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology. 2009;72(24):2115–2121. doi: 10.1212/WNL.0b013e3181aa52d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teng E, Lu PH, Cummings JL. Neuropsychiatric symptoms are associated with progression from mild cognitive impairment to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24(4):253–259. doi: 10.1159/000107100. [DOI] [PubMed] [Google Scholar]
- 59.Vicini Chilovi B, Conti M, Zanetti M, Mazzù I, Rozzini L, Padovani A. Differential impact of apathy and depression in the development of dementia in mild cognitive impairment patients. Dement Geriatr Cogn Disord. 2009;27(4):390–398. doi: 10.1159/000210045. [DOI] [PubMed] [Google Scholar]
- 60.Gabryelewicz T, Styczynska M, Luczywek E, et al. The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. Int J Geriatr Psychiatry. 2007;22(6):563–567. doi: 10.1002/gps.1716. [DOI] [PubMed] [Google Scholar]
- 61.Palmer K, Berger AK, Monastero R, Winblad B, Bäckman L, Fratiglioni L. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 2007;68(19):1596–1602. doi: 10.1212/01.wnl.0000260968.92345.3f. [DOI] [PubMed] [Google Scholar]
- 62.Steenland K, Karnes C, Seals R, Carnevale C, Hermida A, Levey A. Late-life depression as a risk factor for mild cognitive impairment or Alzheimer’s disease in 30 US Alzheimer’s disease centers. J Alzheimers Dis. 2012;31(2):265–275. doi: 10.3233/JAD-2012-111922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Artero S, Ancelin ML, Portet F, et al. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatr. 2008;79(9):979–984. doi: 10.1136/jnnp.2007.136903. [DOI] [PubMed] [Google Scholar]
- 64.Köhler S, van Boxtel M, Jolles J, Verhey F. Depressive symptoms and risk for dementia: a 9-year follow-up of the Maastricht Aging Study. Am J Geriatr Psychiatry. 2011;19(10):902–905. doi: 10.1097/JGP.0b013e31821f1b6a. [DOI] [PubMed] [Google Scholar]
- 65.Christensen MV, Kyvik KO, Kessing LV. Cognitive function in unaffected twins discordant for affective disorder. Psychol Med. 2006;36(8):1119–1129. doi: 10.1017/S0033291706007896. [DOI] [PubMed] [Google Scholar]
- 66.Dlugaj M, Gerwig M, Wege N, et al. Elevated levels of high-sensitivity C-reactive protein are associated with mild cognitive impairment and its subtypes: results of a population-based case-control study. J Alzheimers Dis. 2012;28(3):503–514. doi: 10.3233/JAD-2011-111352. [DOI] [PubMed] [Google Scholar]
- 67.Stephan BC, Hunter S, Harris D, et al. The neuropathological profile of mild cognitive impairment (MCI): a systematic review. Mol Psychiatry. 2012;17(11):1059–1076. doi: 10.1038/mp.2011.147. [DOI] [PubMed] [Google Scholar]
- 68.Stephan BC, Brayne C, Savva GM, Matthews FE Medical Research Council Cognitive Function and Ageing Study. Occurrence of medical co-morbidity in mild cognitive impairment: implications for generalisation of MCI research. Age Ageing. 2011;40(4):501–507. doi: 10.1093/ageing/afr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akiyama H, Barger S, Barnum S, et al. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buxbaum JD, Oishi M, Chen HI, et al. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer β/A4 amyloid protein precursor. Proc Natl Acad Sci USA. 1992;89(21):10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wood JA, Wood PL, Ryan R, et al. Cytokine indices in Alzheimer’s temporal cortex: no changes in mature IL-1 β or IL-1RA but increases in the associated acute phase proteins IL-6, α 2-macroglobu-lin and C-reactive protein. Brain Res. 1993;629(2):245–252. doi: 10.1016/0006-8993(93)91327-o. [DOI] [PubMed] [Google Scholar]
- 72.O’Brien D, Skelton KH, Owens MJ, Nemeroff CB. Are CRF receptor antagonists potential antidepressants? Hum Psychopharmacol. 2001;16(1):81–87. doi: 10.1002/hup.187. [DOI] [PubMed] [Google Scholar]
- 73.Carpenter LL, Heninger GR, Malison RT, Tyrka AR, Price LH. Cerebrospinal fluid interleukin (IL)-6 in unipolar major depression. J Affect Disord. 2004;79(1–3):285–289. doi: 10.1016/S0165-0327(02)00460-3. [DOI] [PubMed] [Google Scholar]
- 74.Doecke JD, Laws SM, Faux NG, et al. The Alzheimer’s Disease Neuroimaging Initiative and Australian Imaging Biomarker and Lifestyle Research Group. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol. 2012:1–8. [Google Scholar]
- 75.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 76.Hestad KA, Tønseth S, Støen CD, Ueland T, Aukrust P. Raised plasma levels of tumor necrosis factor α in patients with depression: normalization during electroconvulsive therapy. J ECT. 2003;19(4):183–188. doi: 10.1097/00124509-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 77.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 78.Hu WT, Holtzman DM, Fagan AM, et al. For the Alzheimer’s Disease Neuroimaging Initiative. Plasma multianalyte profiling in mild cognitive impairment and Alzheimer disease. Neurology. 2012;79(9):897–905. doi: 10.1212/WNL.0b013e318266fa70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kravitz BA, Corrada MM, Kawas CH. Elevated C-reactive protein levels are associated with prevalent dementia in the oldest-old. Alzheimers Dement. 2009;5(4):318–323. doi: 10.1016/j.jalz.2009.04.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lindqvist D, Janelidze S, Hagell P, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66(3):287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 81.Olsson B, Hertze J, Lautner R, et al. Microglial markers are elevated in the prodromal phase of Alzheimer’s disease and vascular dementia. J Alzheimers Dis. 2012 doi: 10.3233/JAD-2012-120787. Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 82.Soares HD, Potter WZ, Pickering E, et al. The Biomarkers Consortium Alzheimer’s Disease Plasma Proteomics Project. Plasma biomarkers associated with the apolipoprotein E genotype and Alzheimer disease. Arch Neurol. 2012;69(10):1310–1317. doi: 10.1001/archneurol.2012.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaciragic A, Lepara O, Valjevac A, et al. Elevated serum C-reactive protein concentration in Bosnian patients with probable Alzheimer’s disease. J Alzheimers Dis. 2007;12(2):151–156. doi: 10.3233/jad-2007-12204. [DOI] [PubMed] [Google Scholar]
- 84.Zorrilla EP, Luborsky L, McKay JR, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15(3):199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]
- 85.Raison CL, Rutherford RE, Woolwine BJ, et al. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. Arch Gen Psychiatry. 2012:1–11. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gallo JJ, Rabins PV, Lyketsos CG, Tien AY, Anthony JC. Depression without sadness: functional outcomes of nondysphoric depression in later life. J Am Geriatr Soc. 1997;45(5):570–578. doi: 10.1111/j.1532-5415.1997.tb03089.x. [DOI] [PubMed] [Google Scholar]
- 87.Robert P, Onyike CU, Leentjens AF, et al. Proposed diagnostic criteria for apathy in Alzheimer’s disease and other neuropsychiatric disorders. Eur Psychiatry. 2009;24(2):98–104. doi: 10.1016/j.eurpsy.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 88.Robert PH. For a unified definition of apathy. J Psychosom Res. 2011;71(3):197. doi: 10.1016/j.jpsychores.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 89.Mortby ME, Maercker A, Forstmeier S. Apathy: a separate syndrome from depression in dementia? A critical review. Aging Clin Exp Res. 2011 doi: 10.3275/8105. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 90.Hakim AM. Depression, strokes and dementia: new biological insights into an unfortunate pathway. Cardiovasc Psychiatry Neurol. 2011;2011:649629. doi: 10.1155/2011/649629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ariyo AA, Haan M, Tangen CM, et al. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102(15):1773–1779. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- 92.Evans DL, Nemeroff CB. The dexamethasone suppression test in organic affective syndrome. Am J Psychiatry. 1984;141(11):1465–1467. doi: 10.1176/ajp.141.11.1465. [DOI] [PubMed] [Google Scholar]
- 93.Nemeroff CB, Musselman DL. Are platelets the link between depression and ischemic heart disease? Am Heart J. 2000;140(Suppl 4):57–62. doi: 10.1067/mhj.2000.109978. [DOI] [PubMed] [Google Scholar]
- 94.Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60(4):436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 95.Mattsson N, Rosén E, Hansson O, et al. Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology. 2012;78(7):468–476. doi: 10.1212/WNL.0b013e3182477eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knight WD, Okello AA, Ryan NS, et al. Carbon-11-Pittsburgh compound B positron emission tomography imaging of amyloid deposition in presenilin 1 mutation carriers. Brain. 2011;134(Pt 1):293–300. doi: 10.1093/brain/awq310. [DOI] [PubMed] [Google Scholar]
- 97.Holland D, Brewer JB, Hagler DJ, Fennema-Notestine C, Fenema-Notestine C, Dale AM Alzheimer’s Disease Neuroimaging Initiative. Subregional neuroanatomical change as a biomarker for Alzheimer’s disease. Proc Natl Acad Sci USA. 2009;106(49):20954–20959. doi: 10.1073/pnas.0906053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Aisen PS. Inflammation and Alzheimer’s disease: mechanisms and therapeutic strategies. Gerontology. 1997;43(1–2):143–149. doi: 10.1159/000213842. [DOI] [PubMed] [Google Scholar]
- 99.Wuwongse S, Chang RC, Law AC. The putative neurodegenerative links between depression and Alzheimer’s disease. Prog Neurobiol. 2010;91(4):362–375. doi: 10.1016/j.pneurobio.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 100.Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol Psychiatry. 2006;59(12):1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kessing LV. Depression and the risk for dementia. Curr Opin Psychiatry. 2012 doi: 10.1097/YCO.0b013e328356c368. [DOI] [PubMed] [Google Scholar]
- 102.Mackenzie IR, Munoz DG. Nonsteroidal anti-inflammatory drug use and Alzheimer-type pathology in aging. Neurology. 1998;50(4):986–990. doi: 10.1212/wnl.50.4.986. [DOI] [PubMed] [Google Scholar]
- 103.Fiebich BL, Hofer TJ, Lieb K, et al. The non-steroidal anti-inflammatory drug tepoxalin inhibits interleukin-6 and α1-anti-chymotrypsin synthesis in astrocytes by preventing degradation of IkappaB-α. Neuropharmacology. 1999;38(9):1325–1333. doi: 10.1016/s0028-3908(99)00055-6. [DOI] [PubMed] [Google Scholar]
- 104.Jaturapatporn D, Isaac MG, McCleery J, Tabet N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst Rev. 2012;2:CD006378. doi: 10.1002/14651858.CD006378.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ralay Ranaivo H, Craft JM, Hu W, et al. Glia as a therapeutic target: selective suppression of human amyloid-β-induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration. J Neurosci. 2006;26(2):662–670. doi: 10.1523/JNEUROSCI.4652-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Website
- 201.WHO. Revised global burden of disease 2002 estimates. www.who.int/healthinfo/global_burden_disease/en/index.html.