Abstract
Previous studies have identified the inhibitory role that the programmed death 1 (PD-1) pathway plays during chronic infection. Blockade of this pathway results in rescue of viral-specific CD8 T cells, as well as reduction of viral loads in mice chronically infected with lymphocytic choriomeningitis virus (LCMV). We tested the effect of combining PD ligand 1 (PD-L1) blockade with an agonistic regimen that induces 4-1BB costimulation during chronic LCMV infection. There is a boosting effect in the rescue of LCMV-specific CD8 T cell responses after dual treatment with PD-L1 blockade and 4-1BB agonistic Abs when the amount and timing of 4-1BB costimulation are carefully controlled. When PD-L1–blocking Abs are given together with a single low dose of anti–4-1BB agonistic Abs, there is an enhanced and stable expansion of viral-specific CD8 T cells. Conversely, when blocking Abs to PD-L1 are given with a repetitive high dose of anti–4-1BB, there is an initial synergistic expansion of viral-specific CD8 T cells by day 7, followed by dramatic apoptosis by day 14. Viral control paralleled CD8 T cell kinetics after dual treatment. By day 7 posttreatment, viral titers were lower in both of the combined regimens (compared with PD-L1 blockade alone). However, whereas the high dose of anti–4-1BB plus PD-L1 blockade resulted in rebound of viral titers to original levels, the low dose of anti–4-1BB plus PD-L1 blockade resulted in a stable reduction of viral loads. These findings demonstrate the importance of carefully manipulating the balance between activating and inhibitory signals to enhance T cell responses during chronic infection.
Upon Ag challenge, naive T cells undergo a rapid phase of proliferation that results in expansion of effector cells (1). If the Ag is cleared, T cells become bona fide memory cells that are able to respond to a secondary challenge and express IFN-γ, TNF-α, and IL-2 (1–3). However, if the Ag persists, there is a gradual loss of T cell function, resulting in progressive T cell exhaustion and inability of T cells to respond to cognate Ag (1, 3). This is the case with chronic infections such as HIV, hepatitis B virus, and hepatitis C virus.
We, and many others, have previously shown that the programmed death 1 (PD-1) pathway plays an important role in directing T cell exhaustion caused by chronic viral infection (4–8). Decreased CD8 T cell proliferative potential and high viral loads are major obstacles that limit the effectiveness of therapeutic vaccination (9). Blockade of PD ligand 1 (PD-L1) results in an increase of Ag-specific CD8 T cells, with enhanced functional capacity, and this treatment improves viral control (4, 6). Additionally, blocking PD-1 inhibitory signals results in enhancement of therapeutic vaccination during chronic infection (10). Thus, the PD-1 pathway tightly regulates T cell responses during chronic infection (7, 11, 12).
It is unclear, however, which other immune pathways may synergize during PD-L1 blockade. Dual blockade of PD-1 with other inhibitory molecules (e.g., LAG-3 and TIM-3) results in additive effects on T cell restoration and viral reduction during chronic infection (13, 14). We wanted to determine whether agonistic costimulatory signals would synergize with PD-L1 blockade and result in a more robust rescue of exhausted virus-specific CD8 T cells. 4-1BB (also known as CD137), a TNFR family member (15), is expressed by activated T cells, NK cells, NKT cells, mast cells, and neutrophils, whereas its ligand (4-1BBL) is restricted mostly to APCs (16, 17). 4-1BB interactions have been shown to be important for T cell responses to bacterial and viral infections (18–20). Interestingly, the timing and dosing amount of CD137 stimulation can result in different outcomes during viral infections. During an acute lymphocytic choriomeningitis virus (LCMV) Armstrong infection, if agonistic anti-CD137 Abs are given before viral priming, suppression of immunity occurs (21).
Conversely, if agonistic Abs to 4-1BB are administered a few days postinfection, antiviral T cell responses are enhanced (21). Such an increase in T cell responses could be beneficial during persistent infections, where severe decreases in function and absolute numbers of Ag-specific T cells are observed (1). Robertson et al. (22) demonstrated that when mice chronically infected with Friend virus are treated with an agonistic anti–4-1BB Ab, along with transfer of transgenic virus-specific CD8 T cells, there is a 99% reduction of viral loads, as well as increased numbers of transferred T cells. 4-1BB costimulation has also been shown to be important for the proliferation of human CMV-specific CD8 T cells (23) and can also regulate immune responses to allo- and autoantigens, as well as improve T cell-mediated antitumor efficacy (24–28). Even though several reports demonstrate a positive role for 4-1BB in regulating T cell responses, some evidence also shows a negative role. 4-1BB−/− mice have reduced numbers of memory CD8 T cells during latent murine CMV infection, but paradoxically they show exaggerated primary CD8 T cell responses to murine CMV, demonstrating a dual role of this co-stimulatory pathway (29).
We wanted to determine whether synergistic effects could be achieved by combining PD-L1 blockade with an anti–4-1BB regimen during chronic LCMV infection. In this study, we show that combining PD-L1 blockade with anti–4-1BB resulted in enhanced augmentation of antiviral CD8 T cell responses during chronic LCMV infection (compared with just PD-L1 blockade alone). Improved expansion of exhausted LCMV-specific CD8 T cells was dependent on the amount and duration of 4-1BB signaling. A high, constant dose of agonistic anti–4-1BB given in combination with PD-L1 blockade resulted in a transient restoration of Ag-specific T cell responses, whereas a low, one-time dose of anti–4-1BB resulted in a sustained increase of Ag-specific CD8 T cell numbers, which correlated with faster viral control early after initiation of treatment (compared with PD-L1 blockade alone at day 7 posttreatment).
Materials and Methods
Mice and infections
Four- to 8-wk-old female C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice received 2 × 106 PFU LCMV clone 13 i.v. via lateral vein. To establish a stringent chronic infection model with life-long viremia, CD4 T cells were depleted by administration of 500 μg GK1.5 Ab (BioXCell) given i.p. 1 d before and the day of infection with LCMV clone 13. For a milder LCMV clone 13 infection model with transient viremia lasting only 2–3 mo, mice were infected with clone 13 without performing CD4 depletion prior to infection. All mice were used in accordance with National Institutes of Health and the Emory University Institutional Animal Care and Use Committee guidelines.
Viral titrations
LCMV titers were determined by plaque assays in six-well plates containing Vero cells grown in MEM media supplemented with 10% FBS. Samples for viral titration were diluted in 1% FBS DMEM and aliquoted on top of Vero cell monolayers. Plates were then incubated for 1 h, rocking them every 10 min. Wells were overlayed with a 1:1 mixture of 1% agarose in 2× 199 media (20% FBS, penicillin/streptomycin) at 45°C. Four days later, wells were overlaid with a 1:1 mixture of 1% agarose in 2× 199 media containing 1:50 neutral red. Plaques were counted the day after.
Ab treatment
For PD-L1 blockade, 200 μg rat anti-mouse PD-L1 Ab (10F.9G2) or rat IgG2b isotype control (BioXCell) was administered i.p. every 3 d (five times). For the high-dose 4-1BB stimulation, 200 μg agonist rat anti-mouse 4-1BB Ab (3H3) was administered i.p. together with PD-L1 Ab. For low-dose 4-1BB stimulation, 50 μg agonist rat anti-mouse 4-1BB Ab was delivered only once i.p. along with the first dose of anti–PD-L1 Ab. T cell responses were analyzed at day 7 or 14 posttreatment.
Cell isolation and flow cytometry
Single-cell suspensions were made from spleens and nonlymphoid organs via mechanical disruption. RBCs were lysed to isolate PBLs. MHC class I tetramers were produced and used as described previously (5). All Abs were purchased from BD Biosciences, except granzyme B (Invitrogen). Surface and intracellular staining protocols were followed as described previously (1). LCMV peptide stimulations were performed at 37°C for 5 h in a CO2 incubator in the presence of GolgiPlug and GolgiStop (BD Biosciences). LCMV peptides were purchased from the Emory Microchemical Facility (Atlanta, GA). Cells were acquired using a FACSCanto flow cytometer (BD Biosciences) and analyzed using FlowJo (Tree Star).
Statistical analysis
Statitical analysis was performed using a nonparametric Mann–Whitney U test on GraphPad Prism software.
Results
High and repetitive doses of anti–4-1BB transiently augment LCMV-specific CD8 T cell responses in chronically infected animals during PD-L1 blockade
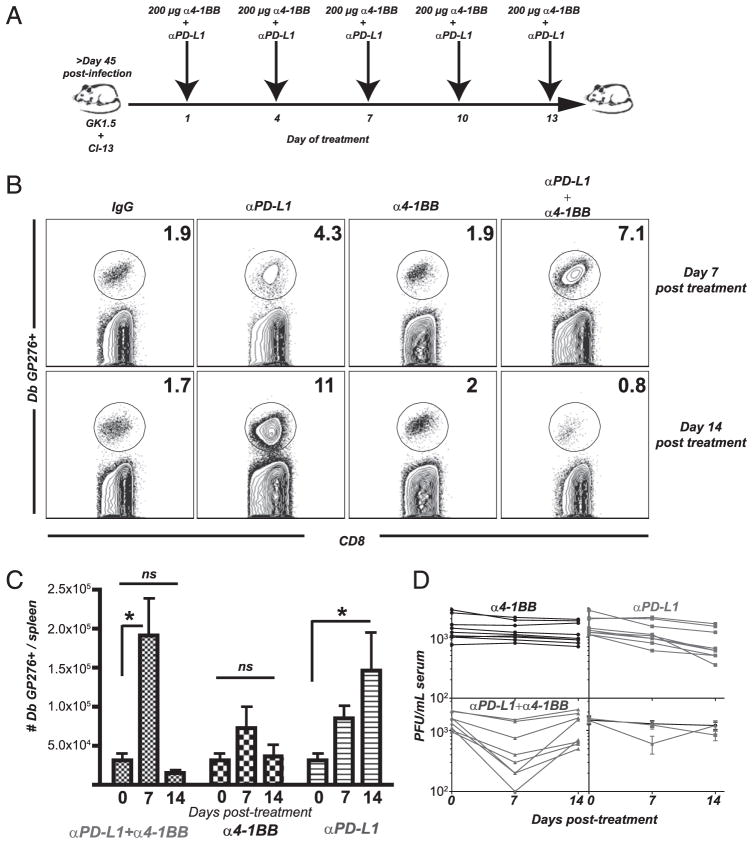
Our laboratory has previously reported that blockade of PD-1 signaling during chronic LCMV infection leads to the increase of LCMV-specific CD8 T cell numbers, increased effector function, and decreased viral loads (4, 10, 14). We sought to determine whether further enhancement in immune function was possible by combining blockade of the PD-L1 inhibitory pathway with an agonistic regimen for the 4-1BB costimulatory pathway. Mice chronically infected with LCMV were treated with a blocking Ab to PD-L1, as previously published (4). As a combination modality, these chronically infected animals also received anti–4-1BB (200 μg) every 3 d, five times (Fig. 1A). During this high-dose regimen, both anti–PD-L1 and anti–4-1BB Abs were administered on the same days. Seven days after treatment, the percentage of LCMV gp276–286-specific CD8 T cells increased >3-fold in the dual-treated mice, as compared with control (Fig. 1B). In contrast, the anti–PD-L1 alone group only increased by 2-fold by day 7 post-treatment. We continued to treat groups of mice using the same high Ab dosing protocol for another week to determine whether this constant stimulation could keep inflating the percentages of antiviral CD8 T cells. After day 14 posttreatment, however, the percentages of Ag-specific cells in the dually treated animals returned to original pretreatment values (Fig. 1B).
FIGURE 1.
High dose of 4-1BB stimulation together with PD-L1 blockade results in a transient increase of virus-specific CD8 T cells. A, Experimental set-up. Mice received CD4-depleting Ab (GK1.5) the day before and the day of infection with LCMV clone 13. After 45 d, mice received multiple doses of anti–4-1BB agonistic Abs (200 μg) together with 200 μg PD-L1–blocking Abs every 3 d (five times). B, Percentage of splenic CD8 T cells that are specific for the LCMV immunodominant Dbgp276–286 epitope at the indicated times after start of Ab treatment. C, Total numbers of splenic CD8 T cells that are specific for the LCMV immunodominant Db gp276–286 epitope at the indicated times after start of Ab treatment. D, Serum LCMV titers. *p < 0.05. Bars indicate SEM; n = 15–21; experiments were repeated five times.
The percentages of LCMV-specific CD8 T cells correlated with their absolute numbers at all the days tested. The total numbers of LCMV-specific CD8 T cells in anti–4-1BB– plus anti–PD-L1–treated mice increased ~8-fold by day 7 posttreatment, as compared with pretreatment numbers (Fig. 1C). This is a 2.5-fold increase over anti–PD-L1 alone at day 7 posttreatment. The continuous addition of anti–4-1BB and anti–PD-L1 resulted in a dramatic loss of cells, recoiling to original T cell numbers by day 14 (Fig. 1C). Because the number of antiviral T cells after 14 d was essentially the same as the starting time point, this indicated that this stimulation protocol only transiently increased numbers of antiviral T cells, whereas a stable population was generated with PD-L1 blockade alone. Spleen data are shown, and similar results were obtained from liver, lung, and blood (data not shown).
We next addressed whether such a transient increase in LCMV-specific CD8 T cell numbers affected viral titers. At day 7, there was a 5-fold drop in viral titers in animals treated with the dual Ab treatment (greater viral control than that by PD-L1 blockade alone at day 7) (Fig. 1D). However, by day 14, virus rebounded to starting levels in the dual-treated mice, mimicking the recoiled kinetics of the antiviral CD8 T cell response. Thus, the transient increase in antiviral CD8 T cell numbers by day 7 due to 4-1BB costimulation and PD-L1 blockade contributes to quick viral control, but this viral control is only transient. Viral titration was performed in several tissues with similar results (data not shown).
Reducing the dose and frequency of anti–4-1BB treatment improves T cell longevity
We wanted to determine whether modulating the dosing of 4-1BB costimulation would enhance T cell longevity during combined treatment with anti–PD-L1. We had the same experimental PD-L1 blockade regimen as before, but we decreased the dosing of 4-1BB agonistic Ab. The new treatment consisted of only one injection of anti–4-1BB (50 μg) along with the first dose of anti–PD-L1 Ab (Fig. 2A). At day 7 after treatment, the percentages of LCMV-specific CD8 T cells were increased in the dual-treated (low-dose anti–4-1BB) group, similar to the high dose of anti–4-1BB plus PD-L1 blockade (Fig. 2B), and there was an ~8-fold increase compared with untreated mice. Interestingly, at day 14, animals receiving low-dose 4-1BB costimulatory regimen plus PD-L1 blockade were able to maintain the high percentages of LCMV-specific CD8 T cells that were seen at day 7 (Fig. 2B). Absolute numbers of Ag-specific CD8 T cells were also higher in the dual-treated group (Fig. 2C). Spleen data are shown, and similar results were obtained from liver, lung, and blood (data not shown).
FIGURE 2.
Low dose of 4-1BB stimulation together with PD-L1 blockade results in a permanent increase in virus-specific CD8 T cells. A, Experimental set-up. Mice received CD4-depleting Ab (GK1.5) the day before and the day of infection with LCMV clone 13. After 45 d, mice received a single dose of anti–4-1BB agonistic Abs (50 μg) together with 200 μg PD-L1–blocking Abs at day 1. PD-L1 blockade alone was continued every 3 d (five times). B, Percentage of splenic CD8 T cells that are specific for the LCMV immunodominant Dbgp276–286 epitope at the indicated times after start of Ab treatment. C, Total numbers of splenic CD8 T cells that are specific for the LCMV immunodominant Dbgp276–286 epitope at the indicated times after start of Ab treatment. D, Serum LCMV titers. *p < 0.05. Bars indicate SEM; n = 15–21; experiments were repeated six times.
Acceleration of viral control with a reduced dose of anti–4-1BB together with PD-L1 blockade
A significant drop in viral titer was detected in dual-treated animals at day 7 (Fig. 2D), similar to what was seen in the high-dose protocol. This control of viremia was maintained to day 14 posttreatment, correlating well with sustained numbers of antiviral CD8 T cells. By day 14, both sera and tissue viral titers were also similar to PD-L1 blockade alone (Supplemental Fig. 1). Thus, the amount of stimulation is an important factor in longevity of T cells rescued from exhaustion. Activating signals should be carefully controlled during chronic infection to ensure T cell viability.
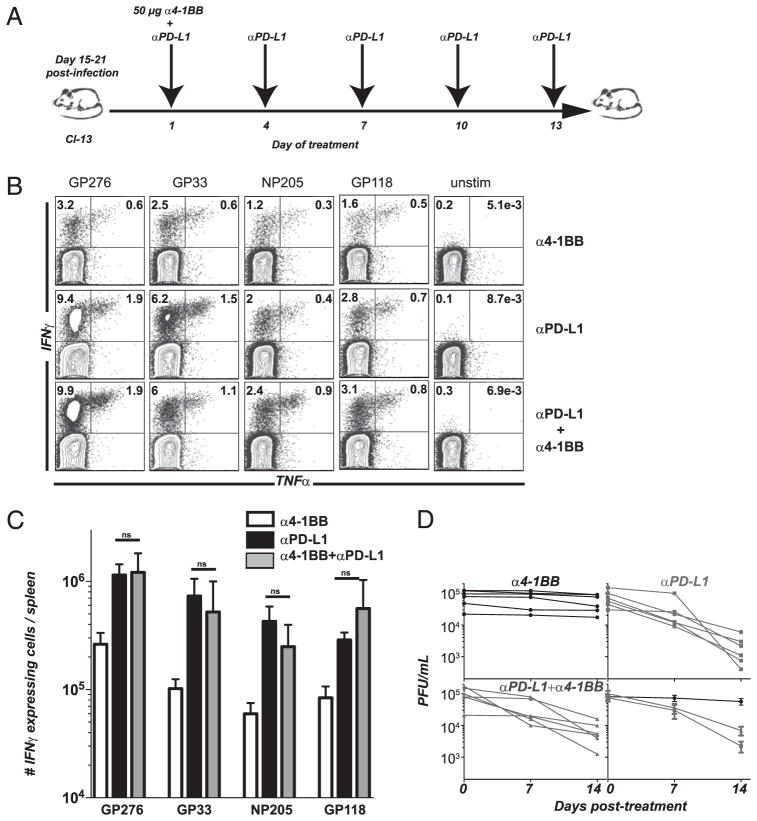
Functional and phenotypic changes in viral-specific CD8 T cells after a reduced 4-1BB stimulation paired with PD-L1 blockade
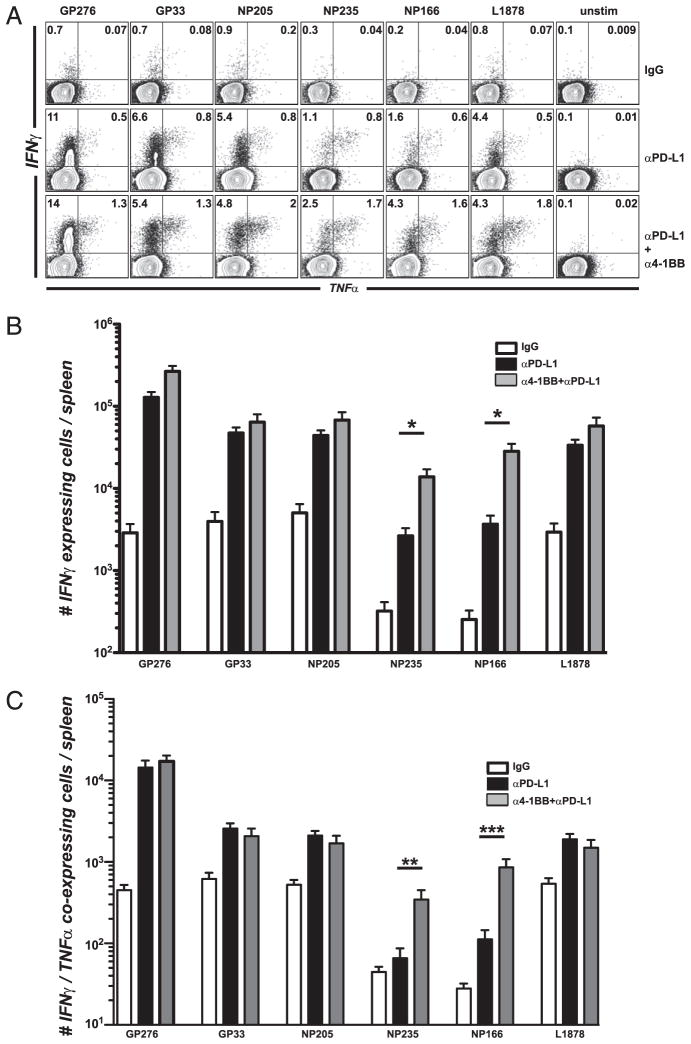
Functional changes occurring in mice treated with combined 4-1BB and PD-L1 therapy were examined to determine how this stimulation was affecting T cell physiology. We evaluated the ability of virus-specific T cells to produce cytokines by stimulating cells with different LCMV CD8 T cell epitopes. For several T cell epitopes tested, the low-dose 4-1BB stimulation with PD-L1 blockade enhanced cytokine expression of antiviral CD8 T cells above the level of rescue seen with anti–PD-L1 alone (Fig. 3A). Also, the percentage of CD8 T cells producing IFN-γ and TNF-α increased compared with control or PD-L1 blockade alone (anti–4-1BB alone was similar to IgG control animals). In most cases, the percentage of dual cytokine-producing T cells increased in the double-treated group. Therefore, a reduced agonistic anti–4-1BB regimen given concomitantly with PD-L1 blockade acts synergistically to augment CD8 T cell responsiveness and function during chronic LCMV infection. Absolute numbers of functional cells were significantly enhanced for some LCMV epitope-specific responses (Fig. 3B). The effect was more noticeable for subdominant responses (NP235 and NP166), which had significantly more coexpression of antiviral cytokines IFN-γ and TNF-α (Fig. 3C). This suggested qualitative differences between dual regimen and PD-L1 blockade alone. No generation of viral-specific CD4 T cell responses was observed with any of these regimens (data not shown). In this model of stringent chronic LCMV infection, mice are devoid of LCMV-specific CD4 T cells and cannot be rescued by any treatment.
FIGURE 3.
Low dose of 4-1BB stimulation together with PD-L1 blockade increases cytokine production from LCMV-specific CD8 T cells. Experimental set-up as in Fig. 2A. A, Percentage of CD8 T cells from spleen-producing cytokines after 5 h stimulation with several LCMV peptides. B, Total numbers of IFN-γ cytokine-producing CD8 T cells after 5 h stimulation with several LCMV peptides. C, Total numbers of dual IFN-γ and TNF-α cytokine-producing CD8 T cells after 5 h stimulation with LCMV peptides. *p = 0.05, **p = 0.0016, ***p = 0.0010. Experiments were repeated four times.
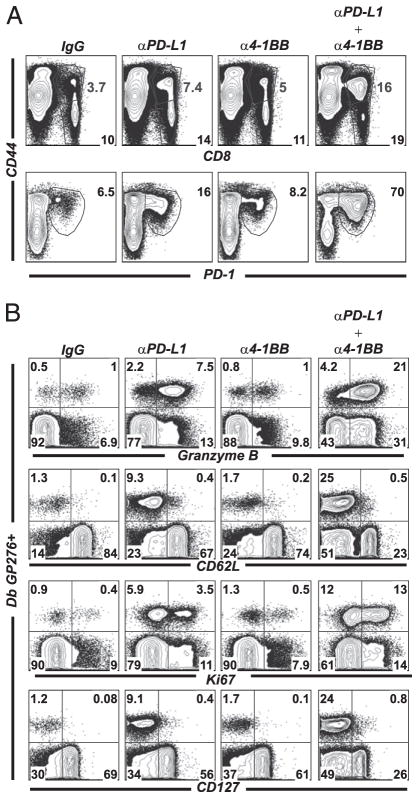
One difference between PD-L1 alone and the combined (low-dose anti–4-1BB) regimen was the large increase in total activated CD8 T cells. There is a doubling in the percentages of lymphocytes that are CD8 T cells as well as increased percentages of activated (CD44hi) CD8 T cells in the dual-treated mice, compared with control mice (Fig. 4A, first row). Additionally, the percentages of CD8 T cells that were PD-1 positive was dramatically increased in the dual treatment, suggesting enhanced expansion of exhausted CD8 T cells of several specificities (Fig. 4A, second row).
FIGURE 4.
Low dose of 4-1BB stimulation together with PD-L1 blockade results in marked phenotypic and functional differences in LCMV-specific CD8 T cells. Experimental set-up as in Fig. 2A. A, First row, Flow plots are gated on total lymphocytes in spleen. Bottom numbers represent percentage CD8+ T cells within the total lymphocyte population. Top numbers represent the percentage of CD8+ T cells that are activated (CD44hi) within the total lymphocyte population. Second row, Flow plots are gated on CD8 T cells. Numbers indicate the percentage of cells that are PD-1hiCD44hi within the CD8 population (representing total expanded CD8 T cells). B, Granzyme B, CD62L, Ki67, and CD127 expression on Db gp276–286-specific CD8 T cells in spleen. Plots are gated on CD8 T cells. Experiments were repeated at least three times.
The dual-treated group had a marked increase of granzyme B production in CD8 T cells (Fig. 4B, first row). Indeed, 50% of all CD8 T cells after dual treatment now produced granzyme B, compared with 8% in the control group or 20% in the PD-L1 alone group. Also, CD8 T cells from the dual group had lower CD62L and CD127 expression, suggesting higher overall activation compared with PD-L1 blockade alone (Fig. 4B, second and fourth rows).
The dual treatment also induced more T cell proliferation, as measured by expression of Ki67 (Fig. 4B, third row) (25). This correlates with the increase of T cell numbers seen in the dually treated mice, indicating that at least part of T cell rescue is coupled to T cell division.
CD8 T cell viability and apoptosis in either high- or low-dose 4-1BB stimulation combined with PD-L1 blockade
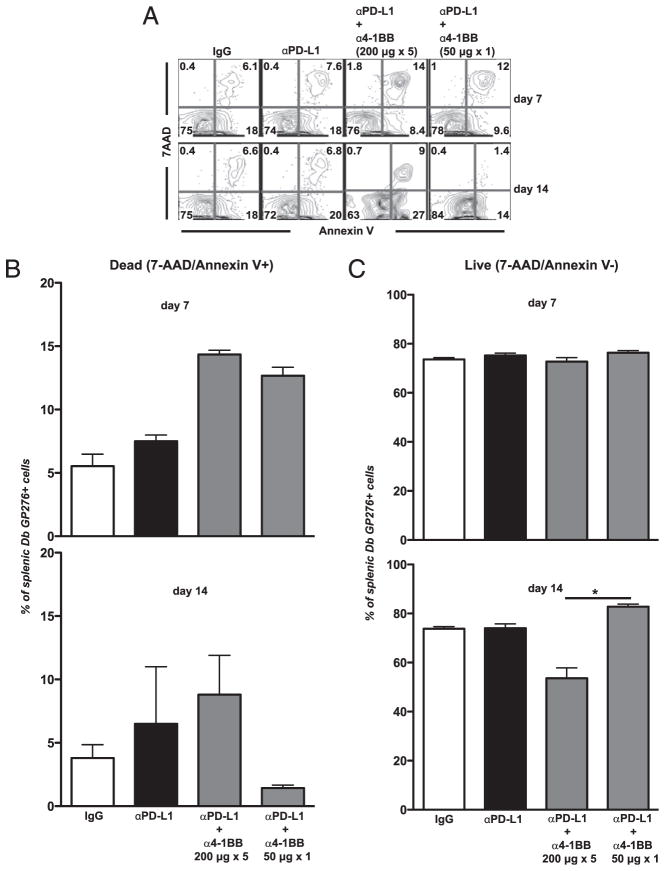
We wanted to determine how changing the amount and duration of 4-1BB stimulation during standard PD-L1 blockade affected Ag-specific CD8 T cell viability. Therefore, we examined apoptosis of LCMV-specific CD8 T cells in animals given a high (Fig. 1A) or low (Fig. 2A) dose of anti–4-1BB together with PD-L1 blockade. We measured apoptosis by annexin V/7-aminoactinomycin D (7AAD) staining at days 7 and 14 after treatment (Fig. 5). As expected, a proportion of Ag-specific CD8 cells are apoptotic in untreated animals, in agreement with previous reports (30).
FIGURE 5.
LCMV-specific CD8 T cell viability after cotreatment with high or low dose of anti–4-1BB together with PD-L1 blockade. Experimental set-up as in Figs. 1A or 2A. A, FACS plot showing the percentage of splenic Dbgp276–286-specific CD8 T cells that are apoptotic or alive by annexin V/7AAD staining in spleen at days 7 and 14 posttreatment. B, Percent of Dbgp276–286-specific CD8 T cells that are positive for both annexin V and 7AAD (apoptotic population). C, Percentage of Dbgp276–286-specific CD8 T cells that are negative for both annexin V and 7AAD (42). *p < 0.05. Experiments were repeated three times.
At day 7, there was a 2-fold increase in the percentages of viral-specific CD8 T cells that were apoptotic in either of the combined regimens (Fig. 5A, first row). This suggested that even though there is expansion of viral-specific cells, there is also apoptosis occurring at the same time in the dual-treated groups. By day 14, however, the low-dose combined regimen equilibrated and had very few percentages of apoptotic cells, whereas the high combined regimen resulted in increased percentages of apoptotic cells (Fig. 5A, second row, 5B). Our results show that the amount and duration of 4-1BB signaling is crucial, as overstimulation will lead to apoptotic death, whereas an optimal (lower) level of costimulation leads to a larger, more viable T cell population. The percentages of annexin V−/7AAD− cells, which represented viable cells, were examined (Fig. 5C). By day 7, most groups had similar percentages of live (annexin V−/7AAD−) viral-specific CD8 T cells. However, by day 14, the low-dose anti–4-1BB plus PD-L1 blockade group had a statistically higher percentage of live cells, compared with the high-dose anti–4-1BB plus PD-L1 blockade group (Fig. 5C). This suggested a survival advantage after a low dose of anti–4-1BB and anti–PD-L1. We have also looked at later time points (after day 30), and the low-dose anti–4-1BB plus PD-L1 blockade group still has increased levels of viral-specific cells after interruption of treatment (data not shown).
Synergism of 4-1BB signaling and PD-L1 blockade is optimal during an infection resulting in chronic viremia
All experiments presented so far are with mice that were depleted of CD4 T cells prior to infection with LCMV clone 13 (we waited >45 d to allow for full CD8 T cell exhaustion). This infection protocol provides a stringent infection, which results in lifelong viremia (31). We were interested in understanding how CD8 T cells in a less stringent chronic infection model would respond to 4-1BB stimulation with PD-L1 blockade. To test this, mice were not CD4 T cell depleted before infecting with LCMV clone 13, and they were then treated with the low-dose 4-1BB stimulation/PD-L1 blockade starting anytime between day 15 or 21 postinfection (Fig. 6A). In this less stringent model of chronic infection, this is the optimal time window to start PD-L1 blockade.
FIGURE 6.
Low dose of 4-1BB stimulation together with PD-L1 blockade during a milder chronic infection does not result in enhanced CD8 T cell rescue compared with PD-L1 blockade alone. A, Experimental set-up. Mice were infected with LCMV clone 13 (without prior CD4 depletion). Around days 15–21 postinfection, mice received a single dose of anti–4-1BB agonistic Abs (50 μg) together with PD-L1 blockade on day 1. PD-L1 blockade alone was continued every 3 d (five times). B, Percentage of CD8 T cells from spleen producing cytokines after 5 h stimulation with several LCMV peptides. C, Total numbers of cytokine producing CD8+ splenocytes after 5 h stimulation with LCMV peptides. D, LCMV serum titers. IgG treatment was similar to anti–4-1BB alone. Experiment was repeated twice; n = 12.
The percentage (Fig. 6B) and total numbers (Fig. 6C) of cytokine-expressing cells were similar by day 14 posttreatment. Viral control was moderately impaired in the dual-treated group compared with PD-L1 blockade alone (Fig. 6D) and was similar in tissues (Supplemental Fig. 2). Thus, during a less stringent chronic LCMV infection, additional 4-1BB costimulation during PD-L1 blockade does not result in additional increase of viral-specific CD8 T cell responses or viral control.
Discussion
We hypothesized that the rescue of exhausted CD8 T cells achieved with PD-L1 blockade could be enhanced by additional costimulation. So far, the combinatorial effects of blocking Abs against multiple inhibitory receptors during chronic infection have been explored (13, 14). However, to our knowledge, no one has tested the effect of blocking the PD-1 pathway and inducing additional T cell costimulation (in the form of 4-1BB) at the same time. We have tested the effect of dual treatment with an agonistic anti–4-1BB Ab 200 μg every 3 d, along with anti–PD-L1 Ab in mice chronically infected with LCMV.
Our initial experiments showed that the inclusion of 4-1BB stimulation boosted numbers of LCMV-specific CD8 T cells 8-fold at a time point when PD-L1 blockade alone only increased cell numbers <3-fold. This also resulted in a drop in viral titers in dual-treated mice at an early time point, indicating the usefulness of this therapeutic protocol to quickly control virus. However, the large numbers of LCMV-specific CD8 T cells that were seen at day 7 were not maintained to day 14 when excessive costimulation was provided (Fig. 1), indicating that this extra push of costimulation through 4-1BB was acting to overstimulate CD8 T cells, leading to apoptosis (Fig. 5). No significant differences in viral-specific CD8 T cell frequencies or viral control were observed between the single treatments with the high-dose anti–4-1BB alone and IgG-treated animals. A slight increase in total numbers was seen by day 7, but it was not statistically significant.
We reasoned that lowering the dose of 4-1BB costimulation may not induce deletion of expanded LCMV-specific CD8 T cells by day 14 posttreatment. Thus, we adapted our protocol by decreasing the amount and duration of anti–4-1BB treatments to a single dose of 50 μg given together only with the first day of our standard PD-L1 blockade. This combination modality with a low dose of anti–4-1BB resulted in significant expansion of LCMV-specific CD8 T cells at day 7 (similar to mice receiving combination modality with high-dose 4-1BB signaling). In contrast to the high dose of anti–4-1BB combined regimen, antiviral CD8 T cell numbers were stable with the reduced costimulatory regimen (Fig. 2). No significant differences were observed between the low-dose anti–4-1BB alone and IgG-treated animals.
Therefore, there is a delicate balance between a sufficient level of costimulation to potentiate T cell rescue and overstimulation leading to cell death. The connection between excessive immune activation and exhaustion has been widely documented (32–34). Caution should be taken with therapies based on costimulatory regimens to ensure optimal cell longevity and function.
The dual low-dose 4-1BB combined therapy also potentiated the function of LCMV-specific CD8 T cells in the face of a stringent chronic infection. Both the numbers of LCMV-specific CD8 T cells producing IFN-γ (single producers) and both IFN-γ and TNF-α (dual producers) were increased compared with PD-L1 blockade alone (Fig. 3). This was statistically significant for subdominant responses. Even though we observed a permanent increase in the viral-specific CD8 T cell response in the low anti–4-1BB plus anti–PD-L1 blockade group, viral control was only accelerated by day 7 (after day 14, viral titers were similar to anti–PD-L1 alone). It is possible that LCMV variants may appear as a result of increased T cell-mediated cytotoxic control, resulting in similar viral loads despite increased rescue of T cell responses. Another possibility may be that increased T cell rescue above a threshold (dictated by just PD-1 blockade) may not directly correlate to enhanced viral control. It is possible that viral control is a measurement determined not only by the magnitude of viral-specific T cell responses, but also by immunoregulatory pathways that act concertedly with PD-1/PD-L1 to modulate target cell killing. We are currently investigating the reasons for these results. Our findings, however, may be important in therapeutic vaccination regimens that require fast clinical intervention resulting in accelerated decline in viral titers.
There were also significant phenotypic changes after co-administration of anti–4-1BB (low dose) and anti–PD-L1 Abs. CD8 T cells downregulated CD62L and CD127, which may correlate with their overall activation status, and immediate cytotoxic capability (35–37). This phenotype may be a result of an expanded effector cell population (35). Anti–4-1BB treatment also resulted in an increase of Ki67+ antiviral CD8 T cells, as well as a large change in the frequency of CD8 T cells producing granzyme B. There were also increased percentages of PD-1+CD44+ CD8 T cells after dual treatment. This last subset seems to represent the total increase in Ag-specific cells after treatment, showing expansion of exhausted cells from different specificities that are contained within the PD-1+CD44+ subset. This cell population contains exhausted, Ag-specific T cells with different proliferative capacities after PD-1 blockade, with PD-1intCD44hi having a proliferative advantage over PD-1hiCD44hi (38).
We then wanted to know what would be the outcome of the immune response after dual treatment in the less stringent model of T cell exhaustion (LCMV clone 13 infection without CD4 depletion). In this less stringent model of chronic infection, viremia lasts only 2–3 mo and CD8 T cell exhaustion is less pronounced compared with the CD4-depleted clone 13 infection model. Dual treatment with PD-L1 blockade and a low dose of 4-1BB agonistic signaling resulted in rescue of Ag-specific T cell responses similar to that seen with PD-L1 alone (Fig. 6). However, viral control was slightly delayed compared with anti–PD-L1 alone. Hence, under less stringent conditions, the threshold of T cell rescue and viral control achieved by PD-L1 blockade alone may obviate the need for any additional costimulation, and additional activating signals may hamper antiviral control. Additionally, it is possible that lower viral Ag loads in this less stringent clone 13 infection model may be responsible for the reduced expansion of exhausted CD8 T cells after PD-L1 blockade (compared with the expansion by PD-L1 blockade in the more stringent infection model). This could be due to reduced TCR signaling in the less stringent infection, highlighting the importance of “Ag sensing” as a driver for T cell proliferation during T cell rescue regimens.
One lesson from these studies is that excessive T cell responses produced by providing additional costimulation do not always translate into enhanced viral control above a given threshold (in this case determined by just PD-L1 blockade). Administration of additional activating signals may hamper antiviral function and overall T cell fitness if not carefully controlled and tailored to a particular infection status.
The dual nature of 4-1BB costimulatory pathway is well documented. If agonistic Abs to 4-1BB are given after Ag priming, this results in augmentation of T cell responses (21, 39). Conversely, administration of Abs agonistic for 4-1BB before or during priming results in suppression of Ag-specific responses (21, 40, 41). This blunting of pathogen-specific responses has been reported to be TNF-α dependent (21). Even though our treatment started late after Ag challenge, and after chronic infection has ensued, we hypothesize that a similar mechanism of TNF-α–dependent suppression may be occurring between day 7 and day 14 posttreatment in the mice that received the high-dose anti–4-1BB plus PD-L1 blockade. One of our future directions is to test anti–TNF-α therapy to see whether it prevents the drastic decline of the expanded cells after day 7 with the high-dose dual regimen in the stringent infection LCMV model.
By further manipulating the kinetics of T cell responses following blockade of the inhibitory PD-1 pathway, it may be possible to accelerate viral clearance during established chronic infections. However, careful tailoring of the amount of activating signals and the status of infection should be taken into consideration to prevent overt stimulation above a physiologically accepted level. In conclusion, in a more stringent model of chronic infection, PD-1 blockade together with a low dose of 4-1BB costimulation accelerates T cell restoration and viral control compared with PD-1 blockade alone. This may be important in clinical settings where immediate T cell restoration is wanted.
Acknowledgments
This work was supported by National Institutes of Health Grant AI3004 (to R.A.).
We thank Hong Wu for technical assistance and Hyun-Tak Jin for discussions.
Abbreviations used in this article
- 7AAD
7-aminoactinomycin D
- LCMV
lymphocytic choriomeningitis virus
- PD-1
programmed death 1
- PD-L1
programmed death ligand 1
Footnotes
The online version of this article contains supplemental material.
Disclosures
R.A., G.J.F., D.L.B., and S.-J.H. have patents and receive patent royalties related to the PD-1 pathway. The other authors have no financial conflicts of interest.
References
- 1.Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology. 2010;129:474–481. doi: 10.1111/j.1365-2567.2010.03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DJ, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 5.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 6.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, Ahmed R, Amara RR. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Up-regulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 8.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wherry EJ, Blattman JN, Ahmed R. Low CD8 T-cell proliferative potential and high viral load limit the effectiveness of therapeutic vaccination. J Virol. 2005;79:8960–8968. doi: 10.1128/JVI.79.14.8960-8968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–555. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 12.Blattman JN, Greenberg PD. PD-1 blockade: rescue from a near-death experience. Nat Immunol. 2006;7:227–228. doi: 10.1038/ni0306-227. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci USA. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 16.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9:271–285. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilcox RA, Chapoval AI, Gorski KS, Otsuji M, Shin T, Flies DB, Tamada K, Mittler RS, Tsuchiya H, Pardoll DM, Chen L. Cutting edge: Expression of functional CD137 receptor by dendritic cells. J Immunol. 2002;168:4262–4267. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 18.Shuford WW, Klussman K, Tritchler DD, Loo DT, Chalupny J, Siadak AW, Brown TJ, Emswiler J, Raecho H, Larsen CP, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munks MW, Mourich DV, Mittler RS, Weinberg AD, Hill AB. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology. 2004;112:559–566. doi: 10.1111/j.1365-2567.2004.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers L, Lee SW, Rossi RJ, Lefrancois L, Kwon BS, Mittler RS, Croft M, Vella AT. Combined CD137 (4-1BB) and adjuvant therapy generates a developing pool of peptide-specific CD8 memory T cells. Int Immunol. 2006;18:325–333. doi: 10.1093/intimm/dxh371. [DOI] [PubMed] [Google Scholar]
- 21.Zhang B, Maris CH, Foell J, Whitmire J, Niu L, Song J, Kwon BS, Vella AT, Ahmed R, Jacob J, Mittler RS. Immune suppression or enhancement by CD137 T cell costimulation during acute viral infection is time dependent. J Clin Invest. 2007;117:3029–3041. doi: 10.1172/JCI32426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robertson SJ, Messer RJ, Carmody AB, Mittler RS, Burlak C, Hasenkrug KJ. CD137 costimulation of CD8+ T cells confers resistance to suppression by virus-induced regulatory T cells. J Immunol. 2008;180:5267–5274. doi: 10.4049/jimmunol.180.8.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waller ECP, McKinney N, Hicks R, Carmichael AJ, Sissons JGP, Wills MR. Differential costimulation through CD137 (4-1BB) restores proliferation of human virus-specific “effector memory” (CD28(−) CD45RA(HI)) CD8(+) T cells. Blood. 2007;110:4360–4366. doi: 10.1182/blood-2007-07-104604. [DOI] [PubMed] [Google Scholar]
- 24.Ito F, Li Q, Shreiner AB, Okuyama R, Jure-Kunkel MN, Teitz-Tennenbaum S, Chang AE. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer Res. 2004;64:8411–8419. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
- 25.Choi BK, Kim YH, Kang WJ, Lee SK, Kim KH, Shin SM, Yokoyama WM, Kim TY, Kwon BS. Mechanisms involved in synergistic anticancer immunity of anti-4-1BB and anti-CD4 therapy. Cancer Res. 2007;67:8891–8899. doi: 10.1158/0008-5472.CAN-07-1056. [DOI] [PubMed] [Google Scholar]
- 26.Kim YH, Choi BK, Oh HS, Kang WJ, Mittler RS, Kwon BS. Mechanisms involved in synergistic anticancer effects of anti-4-1BB and cyclophosphamide therapy. Mol Cancer Ther. 2009;8:469–478. doi: 10.1158/1535-7163.MCT-08-0993. [DOI] [PubMed] [Google Scholar]
- 27.Ko E, Luo W, Peng L, Wang X, Ferrone S. Mouse dendritic-endothelial cell hybrids and 4-1BB costimulation elicit antitumor effects mediated by broad antiangiogenic immunity. Cancer Res. 2007;67:7875–7884. doi: 10.1158/0008-5472.CAN-06-1744. [DOI] [PubMed] [Google Scholar]
- 28.McNamara JO, Kolonias D, Pastor F, Mittler RS, Chen L, Giangrande PH, Sullenger B, Gilboa E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J Clin Invest. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humphreys IR, Lee SW, Jones M, Loewendorf A, Gostick E, Price DA, Benedict CA, Ware CF, Croft M. Biphasic role of 4-1BB in the regulation of mouse cytomegalovirus-specific CD8(+) T cells. Eur J Immunol. 2010;40:2762–2768. doi: 10.1002/eji.200940256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsh RM, Bahl K, Wang XZ. Apoptosis and loss of virus-specific CD8+ T-cell memory. Curr Opin Immunol. 2004;16:271–276. doi: 10.1016/j.coi.2004.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matloubian M, Concepcion RJ, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sachdeva M, Fischl MA, Pahwa R, Sachdeva N, Pahwa S. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr. 2010;54:447–454. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tesselaar K, Arens R, van Schijndel GM, Baars PA, van der Valk MA, Borst J, van Oers MH, van Lier RA. Lethal T cell immunodeficiency induced by chronic costimulation via CD27–CD70 interactions. Nat Immunol. 2003;4:49–54. doi: 10.1038/ni869. [DOI] [PubMed] [Google Scholar]
- 34.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor alpha and CD62L. J Immunol. 2005;175:4686–4696. doi: 10.4049/jimmunol.175.7.4686. [DOI] [PubMed] [Google Scholar]
- 36.Hofmeister R, Khaled AR, Benbernou N, Rajnavolgyi E, Muegge K, Durum SK. Interleukin-7: physiological roles and mechanisms of action. Cytokine Growth Factor Rev. 1999;10:41–60. doi: 10.1016/s1359-6101(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 37.Appasamy PM. Biological and clinical implications of interleukin-7 and lymphopoiesis. Cytokines Cell Mol Ther. 1999;5:25–39. [PubMed] [Google Scholar]
- 38.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci USA. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laderach D, Movassagh M, Johnson A, Mittler RS, Galy A. 4-1BB co-stimulation enhances human CD8(+) T cell priming by augmenting the proliferation and survival of effector CD8(+) T cells. Int Immunol. 2002;14:1155–1167. doi: 10.1093/intimm/dxf080. [DOI] [PubMed] [Google Scholar]
- 40.Myers L, Takahashi C, Mittler RS, Rossi RJ, Vella AT. Effector CD8 T cells possess suppressor function after 4-1BB and Toll-like receptor triggering. Proc Natl Acad Sci USA. 2003;100:5348–5353. doi: 10.1073/pnas.0837611100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sumi T, Ishida W, Mittler RS, Yagita H, Taguchi O, Fukushima A. Regulatory T cells participate in 4-1BB-mediated suppression of experimental allergic conjunctivitis. Int Arch Allergy Immunol. 2009;148:305–310. doi: 10.1159/000170384. [DOI] [PubMed] [Google Scholar]
- 42.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]