Abstract
In humans, high level of collagen remodeling is seen during normal physiological events such as bone renewal, as well as in pathological conditions, such as arthritis, tumor growth and other chronic wounds. Our lab recently discovered that collagen mimetic peptide (CMP) is able to hybridize with denatured collagens at these collagen remodeling sites with high affinity. Here, we show that the CMP's high binding affinity to denatured collagens can be utilized to deliver angiogenic signals to scaffolds composed of heat-denatured collagens (gelatins). We first demonstrate hybridization between denatured collagens and QKCMP, a CMP with pro-angiogenic QK domain. We show that high levels of QKCMP can be immobilized to a new artificial matrix containing both fibrous type I collagen and heat denatured collagen through triple helix hybridization, and that the QKCMP is able to stimulate early angiogenic response of endothelial cells (ECs). We also show that the QKCMP can bind to excised tissues from burn injuries in cutaneous mouse model, suggesting its potential for promoting neovascularization of burn wounds.
Keywords: collagen, VEGF, tissue engineering, angiogenesis, microvasculature
1. Introduction
Collagen is an integral part of the extracellular matrix (ECM) with unique triple helical super-secondary protein structure. The triple helix is comprised of three individual polypeptide chains with repeating glycine-X-Y units, where X and Y are highly populated by proline and hydroxyproline, respectively [1]. Collagen fibrils are formed when rod-like collagen molecules of high triple helical content (e.g. type I and type II) self-associate with precise registry. These high order collagen structures are stabilized by intra and intermolecular covalent crosslinking between glycated lysine and hydroxylysine residues with the involvement of lysyl oxidase [2].
Although such complex and stable supramolecular structure of collagen makes the protein remarkably resistant to common proteinases, collagen remodeling takes place in normal physiological condition, and more intensely in pathological events, such as arthritis, fibrosis, and tumor growth. Turnover of extracellular collagen is mainly facilitated by two classes of enzymes: matrix metalloproteinases (MMPs) and cathepsins, which, depending on types, can cleave either fibrillar or nonfibrillar collagens through various proteolytic mechanisms. Interests in these ECM proteinases have been growing since both MMPs and cathepsins have been implicated in cancer pathology [3,4]. Cancer cells secret these proteinases to degrade collagens and other ECM components to allow for cell invasion into adjacent stroma and intravasation into surrounding vasculatures [5-8].
Upon enzymatic degradation or hydrolysis, collagen triple helix is converted into denatured gelatin strands [9-11]. Previously, we showed that collagen mimetic peptide (CMP), a small peptide (2 – 3 kDa) comprised mainly of collagen's ubiquitous Gly-Pro-Hyp repeat unit, is able to bind to type I collagen [12-14]. Recently, we found that these CMPs bind to MMP digested or heat denatured collagens with even higher affinity than to intact collagens. This led us to develop in vivo CMP-based diagnostic probes capable of targeting tumors and bone remodeling activity [15-17]. Concurrently, we developed a bifunctional peptide (QKCMP) that is comprised of two domains: a QK domain that emulates the alpha-helical signaling domain of vascular endothelial growth factor, VEGF (QK domain: KLTWQELYQLKYKGI) and the CMP domain which can bind to collagens [18]. We investigated the peptide's ability to bind to scaffolds made of pure collagen, and activate endothelial cells. In QKCMP-modified collagen substrates, endothelial cells (ECs) were seen to develop network like structures, with cells developing filopodia extensions in 2D, and in 3D, tube sprouting was observed. These results suggested that the collagen bound QKCMPs are stimulating endothelial cells with activity similar to matrix-bound VEGF [18].
In light of recent discovery that CMP targets denatured collagens more effectively than intact collagens, we wanted to test if QKCMP's affinity to denatured collagens could be explored for promoting angiogenic response in natural and artificial tissue scaffolds containing both collagen and gelatin. In our prior work involving pure collagen, high level of CMP was incorporated only when CMP was pre-mixed with collagen solution before the neutralization and fiber assembly [18]. This was not ideal because the presence of CMP can affect the fiber formation process and because it does not allow for local modification of the scaffold. Knowing that CMP hybridizes with gelatin more efficiently than with collagens, we thought that high concentration of CMP can be incorporated to collagen-gelatin mixture system by directly applying it to the neutralized scaffolds. Compared to the pure collagen system, the collagen-gelatin mixture system is unique both in terms of scaffold fabrication and biological properties. Since areas of high collagen turnover, such as wound sites, are known to contain both collagen and gelatin [19-21], the collagen and gelatin mixture system could be an interesting model for ECM of various pathologic conditions.
There have been some attempts to create collagen-gelatin hybrid systems for tissue engineering applications. [22-24]. For example, collagen-gelatin scaffolds impregnated with fibroblast growth factors showed promise as treatment for chronic skin ulcers by acting as an artificial dermis [23,25-27]. Based on these previous investigations, we decided to fabricate collagen-gelatin mixture scaffolds (Col-Gel), and study their structures as well as their ability to serve as an artificial ECM, particularly in the context of QKCMP immobilization and EC activation. We created an artificial matrix system that mimics the collagen and gelatin interplay found in nature, and also explored its potential as cell scaffold materials and as an in vitro platform to study QKCMP interaction with remodeling collagens. Additionally, we tested QKCMP's binding affinity to excised wound tissues from burn mice models to further validate our hypothesis that the CMP can bind to denatured and degraded collagens present at wound sites.
2. Results and Discussion
2.1. QKCMP Binding to Heat Denatured Type I Collagen
In our prior studies on interactions between CMPs and collagenous proteins, we demonstrated that CMPs readily interact with both intact [12,13,28] and denatured collagens [15,29], but that the level of binding is orders of magnitude higher for denatured collagens. Furthermore, even when conjugated to additional peptide sequences such as QK [18,30], CMP retains its ability to interact with collagens. Specifically, we have shown that by first heating QKCMP above its melting temperature and quickly quenching it in an ice bath, we can create thermally quenched slow-folding single strand peptides that can hybridize with natural collagens at body temperature [18]. We thought that this binding behavior can be readily applied to denatured collagens, and conducted a series experiments to explore QKCMP's ability to hybridize with heat denatured collagens.
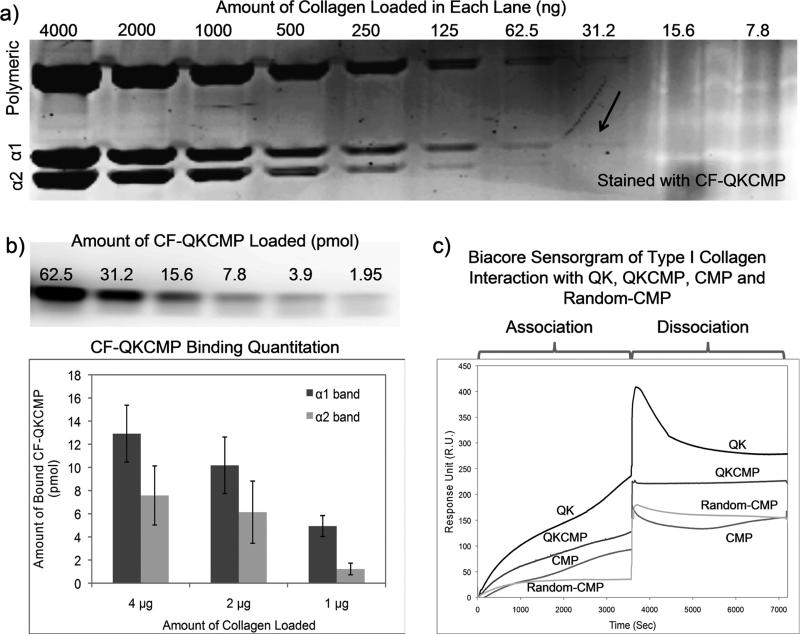
We have previously shown that carboxyfluorescein labeled CMP (CF-CMP) can be used as a staining agents for collagenous proteins in SDS-PAGE with high specificity and sensitivity [15]. Using a similar staining method, we studied the sensitivity of CF-QKCMP binding to heat denatured type I collagen bands resolved in SDS-PAGE. We first prepared serial dilutions of type I, rat collagen in 0.02 N acetic acid, heated the samples in SDS-PAGE loading buffer, and resolved the protein (7.8 ng to 4 μg) through gel electrophoresis. The resulting gel was then stained with a thermally quenched CF-QKCMP solution (6 μM). Staining was carried out over 3 hr to ensure that QKCMP binding to the collagen bands is saturated. As seen in Fig. 1a, CF-QKCMP binds to both α1 and α2 collagen bands (two lowest bands), as well as the multitude of bands containing their high molecular weight cross-linked products. The faintest but clearly detectable fluorescent band contained 31.2 ng of collagen. This level of sensitivity is comparable to the gels stained with coomassie blue, but slightly less than CF-CMP, where the lowest detectable fluorescent band contained 15.6 ng of collagen [15].
Figure 1.
CMP derivatives binding to heat denatured type I collagen. a) Fluorescence image of SDS-PAGE loaded with denatured type I collagen and stained with carboxyfluorescein labeled QKCMP (CF-QKCMP). Hot CF-QKCMP (80°C) was directly added to resolved SDS-PAGE gel. Each lane was loaded with serial dilution of heat denatured type I collagen. b) Fluorescence image of SDS-PAGE loaded with serial dilution of CF-QKCMP (top panel), and amount of CF-QKCMP bound to the α1 and α2 bands of the heat denatured collagen (bottom panel). c) Biacore sensorgrams showing heat denatured type I collagen interacting with QK, QKCMP, CMP, and Random-CMP. The biotin labeled peptides were immobilized on sensor chips displaying streptavidin, and denatured collagens were applied to the sensor surface during association phase, followed by elution with blank PBS during dissociation phase. Error bars represent ± SD.
In order to quantify the amount of CF-QKCMP bound to the collagen strands, a separate gel loaded with predetermined amounts of CF-QKCMP was run. In the past, we attempted to determine the level of CF-CMP binding to collagen bands in SDS-PAGE by running a standard gel loaded with calibrated amounts of CF-CMP. However, due to the lack of charge groups, the CF-CMP did not migrate during the electrophoresis which prevented us from attaining a reliable calibration curve. CF-QKCMP, in contrast, contains charge groups within the QK domain and, as a result, traveled down into the gel during electrophoresis in a form of clear band. As illustrated in the gel image of Fig. 1b, the range of detectable fluorescent intensity was from 2 pmol to 62 pmol of peptide concentration. We created a custom calibration curve by correlating the fluorescent intensity with the peptide concentration, which was then used to convert the observed fluorescent signals of CF-QKCMP bound to collagen bands in Fig. 1a to peptide concentration (Fig. 1b, lower panel). Across all three collagen concentrations tested, α1 band showed higher CF-QKCMP intensity compared to α2 band. This higher binding is likely due to the higher amount of protein present in the α1 band, since type I collagen molecule is composed of two α1 strands and one α2 strand. The amount of CF-QKCMP bound to the α1 bands in the samples with 4 μg and 2 μg of protein was roughly two-folds higher than that of the α2 bands. Furthermore, based on the intensity of the coomassie blue staining, we estimated that the α1 and α2 band accounts for about 1/3 and 1/6 of the overall protein content loaded in each lane, respectively [15]. We could therefore estimate for the first time that the ratio of peptide to collagen binding is roughly 1.8 ± 0.5 CF-QKCMP per α1 strand and 1.6 ± 0.9 CF-QKCMP per α2 strand, indicating that CF-QKCMP binds to both chains in a similar fashion.
To further verify the binding interactions between QKCMP and heat denatured collagen, and to examine how the presence of the QK domain affects CMP-gelatin binding, we conducted Biacore surface plasmon resonance experiment. Peptides—QKCMP, QK, CMP, and random-CMP (CMP with scrambled sequence [18])—were first immobilized on separate sensor chips, and a dilute solution of heat-denatured rat-tail type I collagen was passed across the chip in a flow chamber. Fig. 1c shows the time-course sensorgrams for the different peptides tested, where we divided the interactions into association and dissociation regimes. Sensorgrams show a steady increase in signal for CMP but not for random-CMP in the association regime, indicating that gelatin binding to CMP is mainly due to triple helical propensity as previously reported [15,29]. The gradual increase in signal for CMP is likely related to the slow binding between the peptide and gelatin. Unlike many other biomolecular interactions that are quick and one-toone in nature, the unique folding mechanism of CMP requires three separate strands to converge and fold into a triple helical conformation, a slow process that could take up to hours for complete folding [31]. This slow folding mechanism presumably also explains the slow rise in signal intensity seen in the later part of the dissociation regime. Gelatin which initially came loose from the chip seems to be slowly re-folding with the CMP strands, creating a dense structure at the surface of the chip, leading to a slow rise in signal intensity. Gelatin also showed strong binding affinity to QKCMP as evidenced by the signal increase during association and minimal signal loss during dissociation. In fact, QKCMP exhibited a higher signal intensity than that of the CMP. This higher affinity between QKCMP and gelatin may have been caused by the presence of the QK domain which can attract gelatin through non-specific means, such as hydrophobic and charge-charge interactions. Although these may be weak interactions, they can bring the protein close to the peptide and help facilitate the slow hybridization between CMP and gelatin. Interestingly, for the QK peptide, although the signal intensity during the association phase was highest among all peptides tested, there was also a sharp decrease in signal during the dissociation phase, which is indicative of transient and nonspecific binding. The greater signal stability of CMP and QKCMP compared to QK, and the lack of binding for scrambled CMP sequence confirmed that the gelatin binding to CMP is indeed mediated by the triple helical folding. Both the SDS-PAGE gel binding and the Biacore surface plasmon resonance studies corroborate our previous studies showing that CMPs bind readily to denatured collagens [15,29], and that the affinity interaction is preserved even in the presence of QK domain.
2.2. Production of Collagen-Gelatin Films and Immobilization of QKCMP for Endothelial Cell Activation
Having established QKCMP's binding affinity to denatured collagens, we turned our attention to creating an artificial scaffold system that mimics collagens that are undergoing remodeling. Although it is well known that collagen and gelatin fragments are present in abundance at sites of high collagen turnover [2,19-21,32], very little work has gone into studying the local architecture of the collagen and gelatin mixture system. Therefore, we created a series of collagen and gelatin mixture (Col-Gel) films to study the changes in the scaffold's morphological features as a function of collagen composition. We created these films by mixing different amounts of collagen and gelatin, followed by neutralization with PBS (pH 7) to allow formation of collagen fibers in the presence of free gelatin strands.
2.2.1. Electron Microscopy of Collagen-Gelatin Films
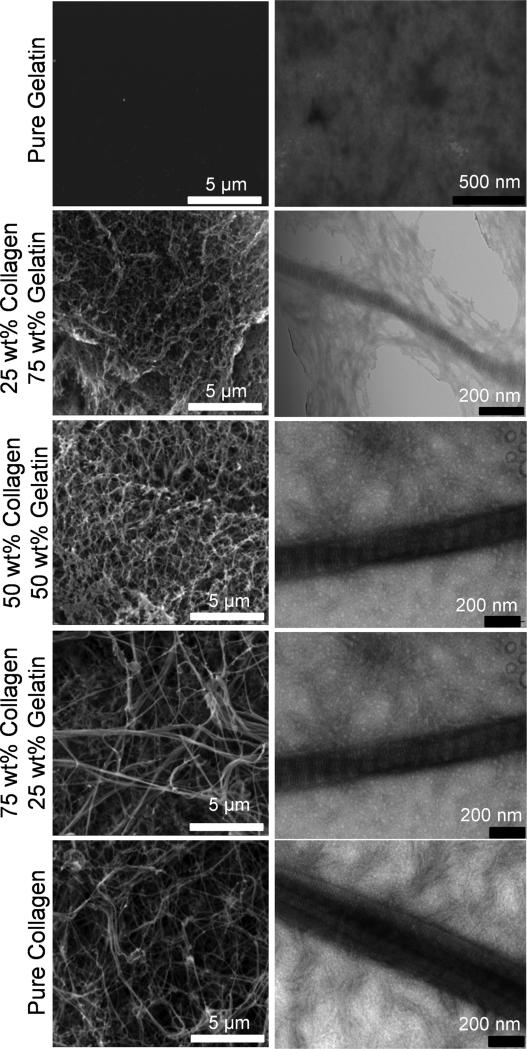
Morphology of the Col-Gel films was studied with scanning and transmission electron microscopy (SEM and TEM, respectively). Specifically, we wanted to investigate whether collagen molecules can assemble into fibers in the presence of gelatin. As seen in the SEM images in Fig. 2 (left panels), fibers in the Col-Gel films were easily discernable in all the films that contained collagen. In samples with high collagen content, the fibers were long, thick and discrete, with large spaces between the interwoven fibers. At lower collagen content, fibers appeared shorter, thinner, and less discernable while films assumed a mesh like appearance consisting of small sponge-like porous structures. Here, the thin fibers appeared to provide the template for pore formation, with glue-like substances partially attached to the fibers filling out the space between the pores. Pure gelatin sample appeared featureless, void of any pores or fibers. These results show that even amidst a high level of gelatin molecules, collagen fibers continue to persist in the Col-Gel mixture films as long as the collagen is present.
Figure 2.
SEM (left panels) and TEM (right panels) of collagen-gelatin mixture films with variation in composition. Compositions shown are before neutralization, corresponding to initial state in Figure 3a.
To verify the structure of the fibers, the Col-Gel films were stained with uranyl acetate and observed under TEM (Fig. 2, right panels). Positively stained collagen fibers are easily discernable under high resolution electron microscopy, as fibers possess a characteristic banding pattern which results from the precisely staggered arrangement of collagen molecules within the fibers [33]. In the pure collagen sample, intertwining fibers with periodic banding patterns were clearly visible. However, as gelatin was introduced to the mixture, fewer and fewer interwoven fibers were found in the samples. The individual collagen fibers in the mixture samples also showed banding patterns, but interestingly they were encased in a structure-less yet electron-dense substance. We believe that this substance, similar to the glue-like substances seen in SEM, is gelatin. As seen in the TEM micrograph of pure gelatin, gelatin appears to be electron dense after staining, although it has no defined structure on its own. Upon neutralization, collagen molecules seem to be able to assemble into fibers within the amorphous gelatin matrix. The results indicate that the pH dependent collagen fibrillogenesis can proceed even in the presence of large amounts of gelatin, and that the gelatin simply remains in the background and acts as a space filler. Therefore the Col-Gel film can be considered a simplified model of ECM with high collagen remodeling activity.
2.2.2. Stability of Collagen-Gelatin Films
Type I collagen is a fiber-forming protein with excellent mechanical properties that has been widely studied as tissue substrates [34]. Gelatin, on the other hand, does not assemble into fibers and therefore has low mechanical strength and thermal stability [35]. In fact, without auxiliary crosslinking, gelatin is soluble in water. In order to mimic the ECM and to serve as effective cell scaffolds, our Col-Gel films must have good stability to allow cell attachment and support anchorage-dependent cell growth. Thus, we investigated how variation in Col-Gel content affects film stability. We examined the stability of the films by incubating them in PBS at 37 °C and following the protein loss overtime.
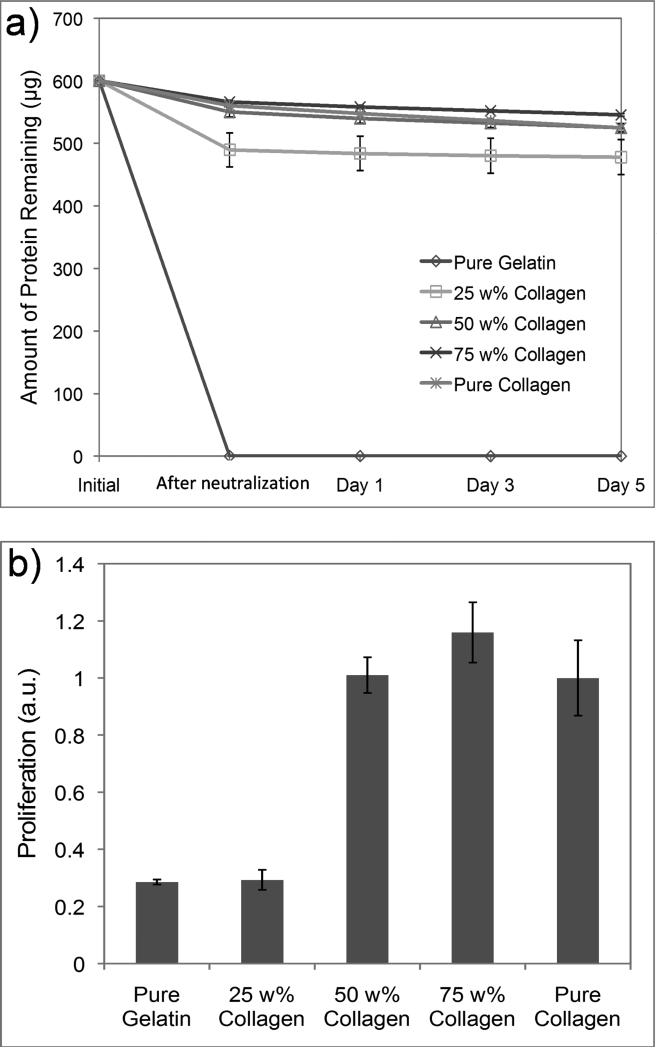
As expected, the gelatin film was very unstable, and all protein content quickly dissolved during the 37 °C incubation (Fig. 3a). With increased collagen content, however, the films became substantially more stable. In terms of protein loss, films with over 50 wt% of collagen appeared to be almost as stable as the pure collagen films. As seen in the electron microscopy study (Fig. 2), the high collagen content allowed for the formation of stable fibers, and these collagen fibers likely contributed to the stability of the film by forming mesh-like structure that prevented the dissolution of the loose gelatin chains. We speculate that the collagen fibers are acting as physical cross-links between gelatin strands which make it difficult for the highly entangled gelatins to be separated from one another and dissolve into solution.
Figure 3.
a) Amount of proteins remaining in the collagen-gelatin films during incubation at 37°C. b) HUVEC proliferation rate on collagen-gelatin films determined five day after cell seeding as measured by WST-1 assay. Error bars represent ±SD.
We then cultured human umbilical vein endothelial cells (HUVECs) on the Col-Gel films for 5 days to investigate films’ capacity to sustain long-term cell growth. As seen in Fig. 3b, on substrates with 50 wt% or higher collagen content, HUVECs proliferated at a comparable rate despite the gelatin content. On these substrates, cells proliferated overtime and eventually displayed cobblestone-like morphology. Conversely, cell proliferation rate dropped precipitously on films with less than 50% collagen content. On these films, cells displayed mostly a round morphology and never fully attached to the substrates. The difference in cell behavior is likely related to the stability of the Col-Gel films. EC survival is heavily anchorage dependent; without strong adhesion to the underlying substrates, cultured human ECs are known to quickly undergo apoptosis [36]. While the rate of protein loss in Col-Gel films with 50 wt% and 75 wt% collagen was comparable to that of pure collagen films, films with 25 wt% collagen and pure gelatin were much less stable and considerable amount of protein dissolved during incubation. These unstable films cannot provide robust surface for HUVEC attachment. Our studies indicate that the stability of Col-Gel films is highly dependent on the collagen content and that the stability of the film directly affects its effectiveness as a scaffold material, especially for culturing anchorage dependent cells. The results also suggest that Col-Gel films with over 50 wt% collagen content are well suited for long-term cell culture.
2.2.3. QKCMP Binding to Collagen-Gelatin Substrates
Previously, to create QKCMP modified collagen substrates, we combined the thermally quenched peptide with the pure collagen precursor solution prior to formation of collagen films or gels in order to maximize QKCMP binding [18]. Since CMPs have high binding affinity to denatured collagen [15], we hypothesized that the incorporation of gelatin in the collagen films would increase peptide binding. We applied thermally quenched QKCMP directly to the preformed Col-Gel films in anticipation that the gelatin would promote high level of peptide binding. Since our ultimate goal is to use QKCMP modified films as substrates for endothelial cells, we conducted binding studies only in Col-Gel films containing 50 w% and 75 w% collagen, which were demonstrated to have sufficient stability to sustain long term EC proliferation.
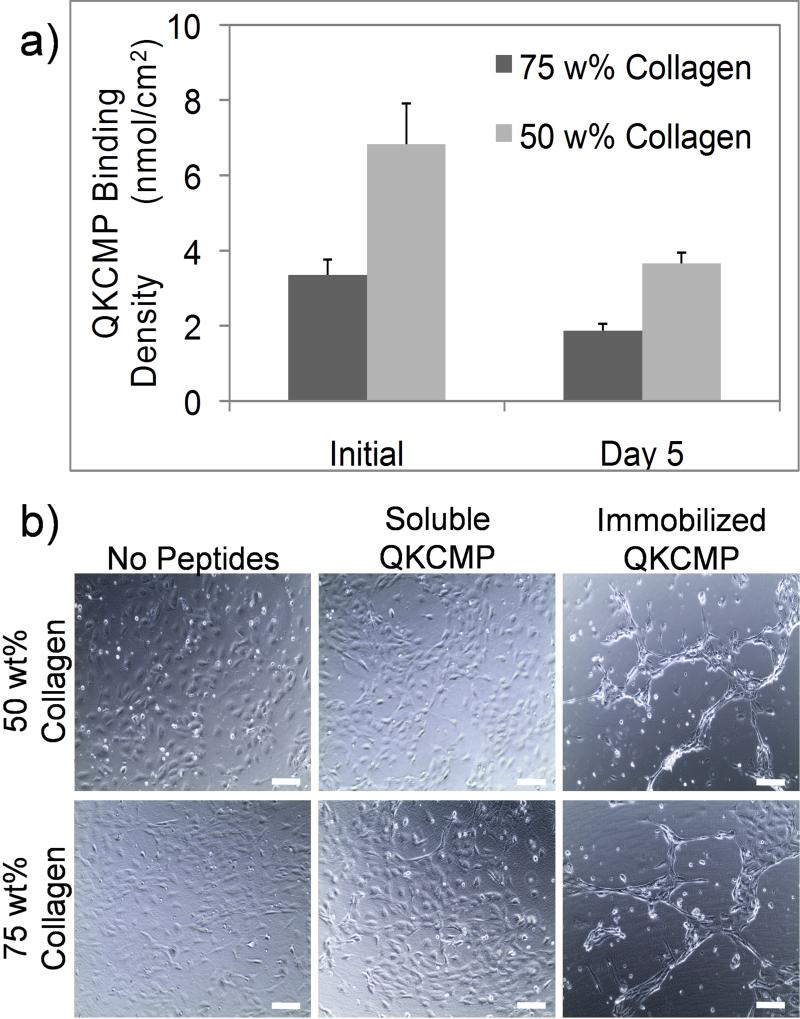
As seen in Fig. 4a, roughly 6.7 ± 0.8 nmol and 13.7 ± 2.2 nmol of QKCMP were bound to the 75 wt% and 50 wt% collagen substrates, respectively just prior to cell seeding. This corresponds respectively to ligand densities of about 3.5 nmol/cm2 and 6.5 nmol/cm2, which are comparable to our previous QKCMP-collagen binding experiment that had a binding density of 5.53 nmol/cm2. The results support our hypothesis that the presence of gelatin facilitates higher level of QKCMP binding without the need for premixing the peptides with collagen precursor solution. This is a significant improvement from the pure collagen system, since the new collagen-gelatin mixture system can be used for local modification by direct CMP application which can be explored for spatially directed cell culture and tissue growth. The level of QKCMP binding was higher for films with higher gelatin content (50 wt% collagen), which supports peptide's preferential binding to the gelatin. QKCMP bound to the Col-Gel substrates slowly released from the film on subsequent days at 37 °C. Even after 5 days of 37 °C incubation, approximately 3.7 ± 0.4 nmol (2 nmol/cm2) and 7.3 ± 0.6 nmol (3.5 nmol/cm2) of QKCMP remained on the 75 wt% and 50 wt% and films respectively. The interactions between the QKCMP and the Col-Gel matrix appear to be relatively strong and stable.
Figure 4.
a) QKCMP binding to Col-Gel films. The binding was tested on Col-Gel mixture films with 75 or 50 wt% collagen contents. Error bars represent ±SD. b) HUVEC morphology 1 day after cell seeding on Col-Gel films with no QKCMP, soluble QKCMP, and immobilized QKCMP. Bars = 100 μm.
In nature, angiogenesis is regulated by VEGF isoforms of different solubility. Among many ECM molecules, heparin serves as direct binding sites for VEGF and it also facilitates unwinding of fibronectin into extended conformation that attracts VEGF [37,38]. In our Col-Gel system, we created a similar matrix consisted of two types of collagen—the tightly assembled triple helical collagen fibers and the unwound single strand gelatin molecules. The gelatin in the Col-Gel system draws parallel to the extended fibronectin conformation found in nature, allowing the pro-angiogenic QKCMP to bind to the Col-Gel matrix.
Having successfully demonstrated QKCMP immobilization on the Col-Gel films, we tested the films’ ability to affect morphological changes in ECs. We seeded HUVECs on 50 wt% and 75 wt% Col-Gel treated with thermally quenched QKCMP. For controls, we seeded cells on Col-Gel films supplemented with equal amounts of soluble QKCMP which is in triple helical form incapable of binding to Col-Gel substrate, as well as on plain Col-Gel films without QKCMP. As seen in Fig. 4b, HUVECs showed contrasting response between immobilized and soluble QKCMP, similar to our earlier findings which also included QK-CMP with scrambled CMP sequence as a negative control [18]. While the cells remained cobblestone-like in the presence of soluble QKCMP, cells developed network-like morphology, an early indication of angiogenic response, when QKCMP was immobilized on the substrate. The QKCMP-modified Col-Gel films were able to sustain the HUVEC network morphology beyond 1 day without any additional growth factors. In our previous work, HUVECs were not able to maintain their network-like structure within this time frame when cultured on pure collagen substrate treated with QKCMP; the network formation receded into scattered cell clusters within 24 hr [18]. The extended stability of the HUVEC network morphology likely correlates to the high level of QKCMP immobilization on the surface of the Col-Gel substrate. The high amount of QKCMP binding likely created a large reservoir of immobilized pro-angiogenic cues, which were able to signal to the ECs for a longer period of time.
Our experiments demonstrated that Col-Gel mixture system is not only a viable cell scaffold material, but is also better suited for delivery of bioactive cues via CMP hybridization when compared to pure collagen scaffolds. The presence of collagen allowed for fiber formation, bestowing stability to the films and providing support for long-term EC growth, and the gelatin served as a reservoir of QKCMP binding which elicited early angiogenic response from ECs.
2.3. Ex Vivo QKCMP Binding Studies in 3rd Degree Burn Wounds
After establishing QKCMP binding and endothelial signaling abilities in our Col-Gel mixture system, we were interested in testing the activity of our peptide in a physiologically relevant model. In search of a site with high collagen remodeling activity, we turned our attention to the cutaneous burn wound model, where collagen degradation is intensified as part of the wound healing process. Shortly after injury, epidermal cells increase collagenase production facilitating the degradation of collagens and other ECM proteins to allow for cell migration into the wound bed for re-epithelialization, granulation tissue formation, and neovascularization [39]. Proper presentation of angiogenic factors is essential for vascularization of the granulation tissue, which is needed to transport additional immune cells and nutrients necessary for tissue repair [40]. Recently, our lab has demonstrated that QKCMP immobilized to PEGDA scaffolds can enhance neovascularization of burn wounds in mice [41]. With its ability to bind to denatured collagens and signal to endothelial cells, we thought that even without a delivery vehicle like PEGDA, QKCMP could bind directly to the wound site.
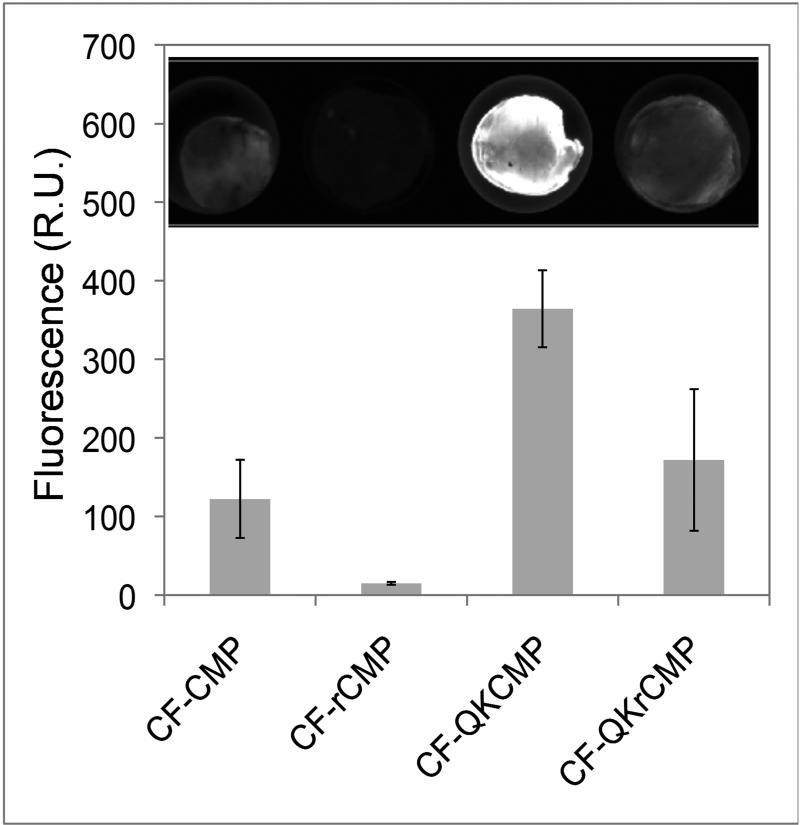
We examined the ability of QKCMP to bind to 3rd degree burn wounds by conducting ex vivo binding study. Previous ex vivo work showed that CMP can bind to cutaneous wounds harvested from animal pelts [42], and we believed that QKCMP could similarly bind to skin excised from burn wounds in mice. Mouse skin samples were harvested 48 hr after the third degree burn and treated with CF labeled CMP, random-CMP (rCMP), QKCMP, and QKrandom-CMP (QKrCMP). Fluorescence microscopy of these tissues (Fig. 5) indicated that the collagens denatured by the burn and further degraded by the healing response seem to bind readily with CMPs but not with scrambled-CMPs, confirming the triple helical propensity-driven nature of the CMP binding. Interestingly, we observed higher overall binding of QKCMP compared to CMP, which is believed to be due to nonspecific interactions mediated by the QK domain. This was also observed in the Biacore surface plasmon resonance study described above (Fig. 1c). Although the ex vivo binding experiments remain exploratory, the results corroborate the overall trends observed in our other binding studies. Furthermore, this experiment served as an important first step in translating our QKCMP immobilized artificial Col-Gel system into a mammalian tissue model by showing that the CMP and QKCMP can bind to cutaneous wounds which contain remodeling collagens. We are currently investigating the topical delivery of QKCMP and its therapeutic benefits in live animal models involving both acute and chronic wounds.
Figure 5.
Quantification of fluorescence intensities and representative fluorescence images (inset) of CMP treated mice skin excised from wound site 48 hr after controlled third degree burn injury. Error bars represent ±SD.
3. Conclusions
Elevated levels of collagen remodeling are found in sites of high MMP and cathepsin K activity, where collagens locally exist in both native and denatured gelatin-like states. Recognition of this dynamic interplay between the two forms of collagens prompted us to develop Col-Gel films that could emulate the duality of intact and denatured collagen in pathologic tissues. In addition, the mixture system also resembles the ECM conditions created when wound dressing is applied to wounds, because many wound dressings are supplemented with gelatin to subdue MMP activity [43]. Electron microscopy showed fibrous microstructure within the Col-Gel films, which were found to be crucial for film stability. We demonstrated QKCMP's binding affinity to heat denatured collagens through SDS-PAGE staining and Biacore SPR studies, and subsequently modified the Col-Gel films with high levels of the QKCMP (up to 6.5 nmol/cm2). These QKCMP modified Col-Gel films were able to support network formation of HUVECs, which is an early sign of angiogenesis. Finally, we demonstrated high levels of CMP and QKCMP binding to mouse skin excised from the burn wound site. These results suggest that QKCMP, with its high affinity for denatured collagens and ability to induce endothelial cell morphogenesis, could be used as a new therapy in wound healing [44,45].
4. Materials and methods
Materials
All amino acids and peptide synthesis reagents including N-methylpyrrolidone (NMP), 2-(1H-benzo-triazole-1-yl)-1,1,3,3-tetra-methyluronium hexafluorophosphate (HBTU) and trifluoroacetic acid (TFA) were purchased from Advanced ChemTech (Louisville, KY). Rink-type TentaGel R Ram resin was purchased from Peptides International (Louisville, KY). Carboxyfluorescein (CF), piperidine, and triisopropylsilane (TIS) were purchased from Sigma-Aldrich (St. Louis, MO). All electron microscopy supplies were obtained from Electron Microscopy Sciences (Hatfield, PA). HUVECs and all cell culture media were obtained from Lonza (Walkersville, MD). WST-1 cell proliferation assay was obtained from Roche Applied Sciences (Indianapolis, IN).
Peptide Synthesis
All peptides were synthesized by conventional solid phase peptide synthesis methods on TentaGel R Ram resin in NMP using 4 molar excess of Fmoc protected amino acids activated with HBTU. Fmoc protection groups were removed with 20% piperidine in NMP. The efficacy of the coupling and deprotection reactions was monitored with ninhydrin and chloranil tests. For non-fluorescently labeled QKCMP, the N-terminus of the peptide was capped with acetyl group by treatment with acetic anhydride. After completing the synthesis of the target sequence, the peptide as well as all side-chain protection groups were cleaved by treating the resin with a cleavage cocktail (95% TFA, 2.5% deionized water and 2.5% TIS) for 3 hr. The crude peptides were isolated by precipitation in cold diethyl ether, and dried in a vacuum.
The crude peptides were purified using high performance liquid chromatography (HPLC; Varian Polaris 210 series; Agilent Technologies, Santa Clara, CA) equipped with a semi-preparative Vydac C-4 reverse-phase column from Grace Davison Discovery Sciences (Deerfield, IL). UV detection was made at 275 nm (for general peptides) or 493 nm (for CF containing peptides). The HPLC was run at a flow rate of 4 mL/min using a solvent gradient comprised of deionized water with 0.1% TFA and acetonitrile with 0.1% TFA. The elutions corresponding to the target peak in the chromatogram were combined and lyophilized. The sequences and molecular weights of all the peptides synthesized are listed in Table 1.
Table 1.
Amino acid sequences and molecular weights of CMP derivatives.
| Peptide | Sequence | Molecular Weight [g/mol] |
|---|---|---|
| QKCMP | Ac-KLTWQELYQLKYKGIGGG(POG)9a | 4526 |
| CF-CMP | CF-GGG(POG)9b | 2968 |
| CF-QKCMP | CF-GGGKLTWQELYQLKYKGIGGG(POG)9 | 5073 |
| CF-rCMP | CF-PGOGPGPOPOGOGPOPGOOPGGOOPPG | 2968 |
| CF-QKrCMP | CF-GGGKLTWQELYQLKYKGIGGGPGOGPGPOPOGOGPOPGOOPGGOOPPG | 5073 |
| Biotinylated-CMP | Biotin-GGG(POG)9 | 2817 |
| Biotinylated-rCMP | Biotin-GGGPGOGPGPOPOGOGPOPGOOPGGOOPPG | 2817 |
| Biotinylated-QK | Biotin-GGGKLTWQELYQLKYKGI | 2307 |
| Biotinylated-QKCMP | Biotin-GGGKLTWQELYQLKYKGIGGG(POG)9 | 4899 |
O: 4-hydroxyproline (Hyp); Ac: acetyl
CF: carboxyfluorescein
CF-QKCMP Binding to SDS-PAGE Resolved Type I Collagen
Rat tail type I collagen (4.73 mg/mL) (BD Bioscience, San Jose, CA) was diluted with 0.02 N acetic acid (Thermo Fisher Scientific, Rockford, IL) to predetermined concentrations (4 μg, 2 μg, 1 μg, 500 ng, 250 ng, 125 ng, 62.5 ng, 31.2 ng, 15.6 ng, and 7.8 ng), and the resulting solutions were mixed with LDS Sample Running Buffer (Life Technologies) and heated at 70°C for 10 min. The solutions were loaded into a NuPAGE Novex 4-12% Bis-Tris Gel for separation by electrophoresis. The gels were run at 200 V for 60 min in NuPAGE SDS MOPS buffer. After electrophoresis, the gels were briefly rinsed with deionized water and transferred into a plastic container. Simultaneously, 8 mL of 6 μM CF-QKCMP was heated at 80°C for at least 20 min. The peptide solutions were then quickly quenched to 37°C in an ice bath and added to the plastic container containing the resolved gel. The container was then shielded from light and left to shake for 3 hr at room temperature to allow for CF-QKCMP to bind to the resolved collagen bands in the gel. After the binding, samples were washed with approximately 15 mL of deionized water twice within 1 hr, followed by overnight washing.
To quantify the amount of peptide bound to the collagen bands, a separate gel was run with known amounts of CF-QKCMP (250 pmol, 125 pmol, 62.5 pmol, 31.3 pmol, 15.6 pmol, 7.8 pmol, 3.9 pmol, 1.95 pmol) which served as standards for the calibration curve. CF-QKCMP solution (5 μL) with known amount of peptide was mixed with 10 μL of LDS Sample Running Buffer and heated at 70°C for 10 min. The samples were then loaded into a NuPAGE Novex 4-12% Bis-Tris Gel and run at 200 V for 12 min in NuPAGE SDS MOPS buffer.
Fluorescence of the gel was imaged using Typhoon 9410 Variable Mode Imager (GE Healthcare), with 488 nm blue laser excitation, and 520 nm band-pass filter. For the collagen detection limit experiment, gels were imaged at 50 μm resolution and 600 V PMT to maximize fluorescence detection sensitivity. For the peptide binding quantification experiment, gels were imaged at 25 μm resolution at 450 V PMT to minimize background signals. The intensities of the observed fluorescence were quantified using Amersham ImageQuant TL (GE Healthcare Life Sciences). After fluorescence imaging, the gels were stained with Bio-Safe Coomassie Blue Stain (Bio-Rad Laboratories, Hercules, CA), and imaged by Gel Doc EZ System (Bio-Rad Laboratories) under epi white light illumination.
Biacore Surface Plasmon Resonance
Surface plasmon resonance experiments were conducted in a Biacore 1000 (GE Healthcare) optical biosensor with a flowcell using streptavidin treated Sensor Chip SA (GE Healthcare) and PBS buffer system. Biotinylated peptides (0.2 μM) were first heated at 80°C for 20 min and quickly quenched to room temperature. The quenched peptide (20 μL) was immediately injected into the flowcell at 5 μL/min. The peptides were immobilized onto the surface of strepavidin-coated Sensor Chip through biotin-streptavidin interaction. After peptide immobilization, 300 μL of gelatin solution (2 μM) was injected into the flowcell at 5 μL/min to initiate gelatin-peptide association. The flowcell was then washed with 300 μL of PBS at 5 μL/min. Changes in response units were calculated as follows: for association, the response unit value at 0 sec was subtracted from the end value at 3,600 sec. For dissociation, the response unit value at 3,660 sec was subtracted from the final value at 7,200 sec. The 60 sec of response data in between the association and dissociation were disregarded to exclude sudden spike in signal, which is an artifact resulting from buffer change.
Fabrication of Collagen-Gelatin Films
Rat tail type I collagen solution (3.81 mg/mL in acetic acid) was purchased from BD Biosciences. Gelatin solution (4 mg/mL) was created by dissolving type B bovine skin gelatin (Sigma Aldrich) in 0.02 N acetic acid (Thermo Fisher Scientific, Waltham, MA). Protein solutions (600 μg) at predetermined collagen-gelatin ratios (pure collagen, 75 wt% collagen-25 wt% gelatin, 50 wt% collagen-50 wt% gelatin, 25 wt% collagen-75 wt% gelatin, pure gelatin) were created by mixing the collagen and gelatin solutions. The protein solution (600 μg) was then added to the surface of 15 mm circular cover glass (Electron Microscopy Science), and allowed to dry in air overnight. The dried protein films were transferred to the wells of a 12-well tissue culture plate. PBS (750 μL) was added to the sample and left at room temperature for at least 5 min for neutralization. The neutralized films were air dried and transferred to 24 well plates for subsequent peptide binding or cell experiments.
Electron Microscopy
For SEM, collagen-gelatin films were first created on a circular cover glass as described above. The films were then treated with 1.5% glutaraldehyde in 0.1 M sodium cacodylate for one hour, and stained with 1% osmium tetraoxide for 30 min, followed by overnight staining with Kellenberger uranyl acetate solution. The samples were dehydrated with ethanol and dried with a supercritical point dryer (Tousimis 795, Rockville, MD). The dried samples were sputter-coated to 4 nm of platinum (Anatech Sputter Coater, Union City, CA), and observed under high vacuum in FEI Quanta ESEM 200 (Hillsboro, OR). For TEM, 600 μg of collagen and gelatin mixture solution created as described above was diluted 10-folds in PBS to create a final solution of 500 μL. The solution was thoroughly mixed and left at room temperature overnight for neutralization. Subsequently, holey carbon TEM grids were floated on 50 μL droplets of the protein solutions for 5 min. The grids were then washed and stained with 2% uranyl acetate. The samples were observed with Tecnai 12 TWIN transmission electron microscopy (FEI, Hillsboro, OR).
Stability Study of Collagen-Gelatin Films
To examine the stability of the collagen-gelatin mixture films, we determined the amount of protein released from the films over time. After creating the films, we neutralized the films with PBS, and collected each PBS wash solution. Next, films were transferred to 24-well tissue culture plate where 500 μL of deionized water was added to each film and incubated at 37°C overnight. The next day, water in each well was collected separately and replaced with 500 μL of PBS, followed by incubation for additional 30 min at 37°C. This process was designed to mimic the steps used in QKCMP binding experiment. Subsequently, the PBS solution in each well was collected and replaced with fresh PBS every other day. The amount of protein in each collected solution was quantified using CBQCA Protein Quantitation Kit (Life Technologies, Carlsbad, CA).
Cell Culture
All HUVECs were cultured in endothelial-cell complete growth media (EGM-2) at 37°C with a fully humidified atmosphere and 5% CO2. The media were replaced every other day. The cells were harvested at 70-80% confluency using 0.05% trypsin/0.53 mM ethylenediaminetetraacetic acid (EDTA) in HBSS (Mediatech, Manassas, VA). Cells after passage 2-7 were used in the experiments.
Peptide Binding and Release Study
QKCMP and CF-QKCMP were separately dissolved in deionized water to create 100 μM peptide stock solutions. Peptide solutions were heated at 80°C for at least 30 min, and quenched to 37°C in an ice bath. The cooled peptide solution (500 μL per well) was immediately added to collagen-gelatin films placed in 24-well plates. The samples were incubated at 37 °C overnight to allow for peptide binding. The next day, the excess peptide solution was removed and 500 μL of PBS was added to each well followed by incubation for 30 min at 37°C to remove unbound peptides. After the wash, each well was replenished with 500 μL of PBS and incubated at 37°C. The PBS solution was replaced every other day.
To determine the amount of peptide bound to the collagen-gelatin substrates in the binding and release study, the fluorescence intensity of the CF-QKCMP bound to the protein substrate was measured with a Gemini EM Fluorescence Microplate reader (Molecular Devices, Sunnyvale, CA). At each time point of the study, after replacing the PBS solution in each well, the samples were heated at 80°C for 30 min to denature the collagen substrates and release the peptides. The solutions were then immediately collected from each well and stored at 4°C. The fluorescence intensities of the solutions were measured at 533 nm emission and 489 nm excitation wavelengths. The fluorescence readings were converted to concentrations of CF-labeled peptides using a standard curve. For cell study, after the QKCMP binding, collagen-gelatin films were seeded with HUVECs (50,000 cells/well) in 500 μL of endothelial basal media (EBM) supplemented with 2% fetal bovine serum (FBS).
Cell Viability and Proliferation Study
Collagen-gelatin films of varying composition were created as described above and transferred to 24-well cell culture plates. To test HUVEC viability and proliferation, HUVECs (50,000 cells/well) were seeded on each collagen-gelatin substrate and cultured in 500 μL of EGM-2. The growth media was replaced every other day. On the fifth day after initial seeding, 50 μL of WST-1 solution was added to each well, and incubated for at least 2 hr. The absorbance of each sample at 450 nm was measured with a microplate reader (Molecular Devices, Sunnyvale, CA).
CMP Binding Studies on Mouse Skin Wounds
All surgical procedures were approved by the Johns Hopkins University Animal Care and Use Committee. Skin specimens harvested from mice with 3rd degree burn were gifts from Sharon Gerecht's laboratory at the Johns Hopkins University. The samples were produced according to procedures previously described by Sun and coworkers.[46,47] Briefly, mice were anesthetized and shaved. Aluminum rod (220 gauge) was heated for 5 min in 100°C water bath and placed on the posterior-dorsum of each mouse for 4 sec. Full-thick skin samples, 8 mm in diameter, were collected from the burn wound 48 hr after the injury. Since the excised skin specimens were not treated with any fixatives, binding studies were conducted within the same day of skin specimen collection. We first rinsed the skin specimen with PBS and transferred them to wells of 48 well cell culture plate. Separately, 100 μM solutions of CMP derivatives were heated to 80°C for at least 30 min to melt the triple helix. The solutions were quickly quenched to 37°C using ice bath and 200 μL of quenched solution was added to the skin specimen ensuring that the samples were completely submerged. The samples were protected from light and incubated at room temperate for 1 hr to allow for peptide binding. The samples were then rinsed for 1 hr in PBS twice, and once overnight. The samples were imaged with Typhoon 9410 Variable Mode Imager using 488 nm excitation and 520 nm band-pass filters. The intensities of the observed fluorescence were quantified using Amersham ImageQuant TL.
Acknowledgements
The authors thank Scheherazade Sadegh-Nasseri and Yuri Poluektov for help with Biacore Surface Plasmon Resonance, J. Michael McCaffery and Erin Pryce for help with electron microscopy, and Sharon Gerecht, Guoming Sun and Yu-I (Tom) Shen for mice tissue acquisition. This work was support by grants from NIAMS/NIH (R01AR060484, R21AR065124) and DOD (W81XWH-12-1-0555) awarded to S.M.Y., by NSF IGERT fellowship awarded to T.R.C., and by HHMI Graduate Training Program (NB Med) and NDSEG (23 CFR 168a) fellowships awarded to P.J.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. J Cell Sci. 2007;120:1955–8. doi: 10.1242/jcs.03453. [DOI] [PubMed] [Google Scholar]
- 2.Bugge T, Behrendt N. Cooperation between proteolysis and endocytosis in collagen turnover. In: Parks WC, Mecham RP, editors. Extracellular Matrix Degradation. Springer; Berlin Heidelberg: 2011. pp. 53–74. [Google Scholar]
- 3.Brubaker KD, Vessella RL, True LD, Thomas R, Corey E. Cathepsin K mRNA and protein expression in prostate cancer progression. J Bone Miner Res. 2003;18:222–30. doi: 10.1359/jbmr.2003.18.2.222. [DOI] [PubMed] [Google Scholar]
- 4.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 5.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–81. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnsen M, Lund LR, Rømer J, Almholt K, Danø K. Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr Opin Cell Biol. 1998;10:667–71. doi: 10.1016/s0955-0674(98)80044-6. [DOI] [PubMed] [Google Scholar]
- 7.Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit Rev Biochem Mol Biol. 48:222–72. doi: 10.3109/10409238.2013.770819. [DOI] [PubMed] [Google Scholar]
- 8.Bossard MJ, Tomaszek TA, Thompson SK, Amegadzie BY, Hanning CR, Jones C, et al. Proteolytic activity of human osteoclast cathepsin K: expression, purification, activation, and substrate identification. J Biol Chem. 1996;271:12517–24. doi: 10.1074/jbc.271.21.12517. [DOI] [PubMed] [Google Scholar]
- 9.Salo TP. On the degradation of collagen into a “parent gelatin”. J Am Chem Soc. 1949;71:2276. [Google Scholar]
- 10.Scatchard G, Oncley JL, Williams JW, Brown A. Size distribution in gelatin solutions.1 preliminary report. J Am Chem Soc. 1944;66:1980–1. [Google Scholar]
- 11.Harrington WF, Von Hippel PH. The Structure Of collagen And gelatin. In: Anfinsen MLAKB CB, John TE, editors. Adv Protein Chem. Academic Press; 1962. pp. 1–138. [DOI] [PubMed] [Google Scholar]
- 12.Wang AY, Foss CA, Leong S, Mo X, Pomper MG, Yu SM. Spatio-temporal modification of collagen scaffolds mediated by triple helical propensity. Biomacromolecules. 2008;9:1755–63. doi: 10.1021/bm701378k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang AY, Mo X, Chen CS, Yu SM. Facile modification of collagen directed by collagen mimetic peptides. J Am Chem Soc. 2005;127:4130–1. doi: 10.1021/ja0431915. [DOI] [PubMed] [Google Scholar]
- 14.Yu SM, Li Y, Kim D. Collagen mimetic peptides: progress towards functional applications. Soft Matter. 2011;7:7927–38. doi: 10.1039/C1SM05329A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Foss CA, Summerfield DD, Doyle JJ, Torok CM, Dietz HC, et al. Targeting collagen strands by photo-triggered triple-helix hybridization. Proc Natl Acad Sci U S A. 2012;109:14767–72. doi: 10.1073/pnas.1209721109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Yu SM. Targeting and mimicking collagens via triple helical peptide assembly. Curr Opin Chem Biol. 2013;17:968–75. doi: 10.1016/j.cbpa.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Foss CA, Pomper MG, Yu SM. Imaging denatured collagen strands in vivo and ex vivo via photo-triggered hybridization of caged collagen mimetic peptides. J Vis Exp. 2014:e51052. doi: 10.3791/51052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan TR, Stahl PJ, Yu SM. Matrix-bound VEGF mimetic peptides: design and endothelial-cell activation in collagen scaffolds. Adv Funct Mater. 2011;21:4252–62. doi: 10.1002/adfm.201101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon A, Pettinari L, Martinelli C, Colombo G, Portinaro N, Dalle-Donne I, et al. New insights in extracellular matrix remodeling and collagen turnover related pathways in cultured human tenocytes after ciprofloxacin administration. Muscles Ligaments Tendons J. 2013;3:122–31. [PMC free article] [PubMed] [Google Scholar]
- 20.McAnulty RJ, Laurent GJ. Collagen synthesis and degradation in vivo. Evidence for rapid rates of collagen turnover with extensive degradation of newly synthesized collagen in tissues of the adult rat. Coll Relat Res. 1987;7:93–104. doi: 10.1016/s0174-173x(87)80001-8. [DOI] [PubMed] [Google Scholar]
- 21.Everts V, van der Zee E, Creemers L, Beertsen W. Phagocytosis and intracellular digestion of collagen, its role in turnover and remodelling. Histochem J. 1996;28:229–45. doi: 10.1007/BF02409011. [DOI] [PubMed] [Google Scholar]
- 22.Grover CN, Cameron RE, Best SM. Investigating the morphological, mechanical and degradation properties of scaffolds comprising collagen, gelatin and elastin for use in soft tissue engineering. J Mech Behav Biomed Mater. 2012;10:62–74. doi: 10.1016/j.jmbbm.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Takemoto S, Morimoto N, Kimura Y, Taira T, Kitagawa T, Tomihata K, et al. Preparation of collagen/gelatin sponge scaffold for sustained release of bFGF. Tissue Eng Part A. 2008;14:1629–38. doi: 10.1089/ten.tea.2007.0215. [DOI] [PubMed] [Google Scholar]
- 24.Hiraoka Y, Yamashiro H, Yasuda K, Kimura Y, Inamoto T, Tabata Y. In situ regeneration of adipose tissue in rat fat pad by combining a collagen scaffold with gelatin microspheres containing basic fibroblast growth factor. Tissue Eng. 2006;12:1475–87. doi: 10.1089/ten.2006.12.1475. [DOI] [PubMed] [Google Scholar]
- 25.Ayvazyan A, Morimoto N, Kanda N, Takemoto S, Kawai K, Sakamoto Y, et al. Collagen-gelatin scaffold impregnated with bFGF accelerates palatal wound healing of palatal mucosa in dogs. J Surg Res. 2011;171:e247–57. doi: 10.1016/j.jss.2011.06.059. [DOI] [PubMed] [Google Scholar]
- 26.Kanda N, Morimoto N, Takemoto S, Ayvazyan AA, Kawai K, Sakamoto Y, et al. Efficacy of novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor for dermis-like tissue regeneration. Ann Plast Surg. 2012;69:569–74. doi: 10.1097/SAP.0b013e318222832f. [DOI] [PubMed] [Google Scholar]
- 27.Morimoto N, Yoshimura K, Niimi M, Ito T, Aya R, Fujitaka J, et al. Novel collagen/gelatin scaffold with sustained release of basic fibroblast growth factor: clinical trial for chronic skin ulcers. Tissue Eng Part A. 2013;19:1931–40. doi: 10.1089/ten.tea.2012.0634. [DOI] [PubMed] [Google Scholar]
- 28.Mo X, An Y, Yun C-S, Yu SM. Nanoparticle-assisted visualization of binding interactions between collagen mimetic peptide and collagen fibers. Angew Chem Int Ed. 2006;45:2267–70. doi: 10.1002/anie.200504529. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Ho D, Meng H, Chan TR, An B, Yu H, et al. Direct detection of collagenous proteins by fluorescently labeled collagen mimetic peptides. Bioconj Chem. 2013;24:9–16. doi: 10.1021/bc3005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang AY, Leong S, Liang Y-C, Huang RCC, Chen CS, Yu SM. Immobilization of growth factors on collagen scaffolds mediated by polyanionic collagen mimetic peptides and its effect on endothelial cell morphogenesis. Biomacromolecules. 2008;9:2929–36. doi: 10.1021/bm800727z. [DOI] [PubMed] [Google Scholar]
- 31.Ackerman MS, Bhate M, Shenoy N, Beck K, Ramshaw JA, Brodsky B. Sequence dependence of the folding of collagen-like peptides. Single amino acids affect the rate of triple-helix nucleation. J Biol Chem. 1999;274:7668–73. doi: 10.1074/jbc.274.12.7668. [DOI] [PubMed] [Google Scholar]
- 32.Laurent GJ. Dynamic state of collagen: pathways of collagen degradation in vivo and their possible role in regulation of collagen mass. Am J Physiol. 1987;252:C1–9. doi: 10.1152/ajpcell.1987.252.1.C1. [DOI] [PubMed] [Google Scholar]
- 33.Ross MH, Kaye GI, Pawlina W. Histology a text and atlas. 4 ed Lippincott Williams & Wilkins; 2003. [Google Scholar]
- 34.Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78:929–58. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bigi A, Panzavolta S, Rubini K. Relationship between triple-helix content and mechanical properties of gelatin films. Biomaterials. 2004;25:5675–80. doi: 10.1016/j.biomaterials.2004.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Re F, Zanetti A, Sironi M, Polentarutti N, Lanfrancone L, Dejana E, et al. Inhibition of anchorage- dependent cell spreading triggers apoptosis in cultured human endothelial cells. J Cell Biol. 1994;127:537–46. doi: 10.1083/jcb.127.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitsi M, Forsten-Williams K, Gopalakrishnan M, Nugent MA. A catalytic role of heparin within the extracellular matrix. J Biol Chem. 2008;283:34796–807. doi: 10.1074/jbc.M806692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitsi M, Hong Z, Costello CE, Nugent MA. Heparin-mediated conformational changes in fibronectin expose vascular endothelial growth factor binding sites. Biochemistry. 2006;45:10319–28. doi: 10.1021/bi060974p. [DOI] [PubMed] [Google Scholar]
- 39.Singer AJ, Clark RA. Cutaneous wound healing. New Engl J Med. 1999;341:738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 40.Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc. 2000;5:40–6. doi: 10.1046/j.1087-0024.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 41.Stahl PJ, Chan TR, Shen Y-I, Sun G, Gerecht S, Yu SM. Capillary network-like organization of endothelial cells in PEGDA scaffolds encoded with angiogenic signals via triple helical hybridization. Adv Funct Mater. 2014;24:3213–25. doi: 10.1002/adfm.201303217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chattopadhyay S, Murphy CJ, McAnulty JF, Raines RT. Peptides that anneal to natural collagen in vitro and ex vivo. Org Biomol Chem. 2012;10:5892–7. doi: 10.1039/c2ob25190f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey A. Perspective article: the fate of collagen implants in tissue defects. Wound Repair Regen. 2000;8:5–12. doi: 10.1046/j.1524-475x.2000.00005.x. [DOI] [PubMed] [Google Scholar]
- 44.Chen R, Mooney D. Polymeric growth factor delivery strategies for tissue engineering. Pharm Res. 2003;20:1103–12. doi: 10.1023/a:1025034925152. [DOI] [PubMed] [Google Scholar]
- 45.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–70. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun G, Shen YI, Kusuma S, Fox-Talbot K, Steenbergen CJ, Gerecht S. Functional neovascularization of biodegradable dextran hydrogels with multiple angiogenic growth factors. Biomaterials. 2011;32:95–106. doi: 10.1016/j.biomaterials.2010.08.091. [DOI] [PubMed] [Google Scholar]
- 47.Sun G, Zhang X, Shen Y-I, Sebastian R, Dickinson LE, Fox-Talbot K, et al. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc Natl Acad Sci U S A. 2011;108:20976–81. doi: 10.1073/pnas.1115973108. [DOI] [PMC free article] [PubMed] [Google Scholar]