Abstract
Background
Bofutsushosan is a well known Kampo, traditional Japanese medicine, based on ancient Chinese medicine mainly used in the treatment of hypercholesterolemia in Japan. We selected two Kampo formulas, Boiogito and Keishibukuryogan mainly used in the treatment of hypercholesterolemia in China to compare with Bofutsushosan and cholesterol absorption inhibitor ezetimibe.
Methods
Hypercholesterolemia and fatty liver were induced by high cholesterol (containing 2% cholesterol and 0.5% cholic acid) diet in male Wistar rats for 6 and 12 weeks. Kampo formulas Boiogito, Bofutsushosan, Keishibukuryogan and ezetimibe were added to the high-cholesterol diet, respectively. After 6 and 12 weeks, body and liver weights, blood chemistry, cholesterol concentrations, fat-related and inflammatory-related factors were examined.
Results
High-cholesterol diet increased body and liver weights, and serum cholesterol concentrations. Boiogito and ezetimibe improved them. Serum ICAM-1 and RBP4 were increased in the high cholesterol diet group. Boiogito and ezetimibe improved them too. In the histological examinations of liver and adipose tissues, we observed a significant improvement after treatment. Immunostaining expression of ICAM-1 in aorta was improved by Boiogito, Bofutsushosan, Keishibukuryogan and ezetimibe. The mRNA expression of RBP4, HFABP, CFABP, MCP1 and CCR2 in liver and adipose tissue were decreased by Boiogito and ezetimibe.
Conclusion
Boiogito has a protective effect on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats and more effective than Bofutsushosan and Keishibukuryogan. The lipid-lowering effect of Boiogito is not stronger than ezetimibe. But the anti-inflammatory (MCP1, CCR2) and anti-arteriosclerotic (ICAM-1) effects of Boiogito are more potent than ezetimibe.
Keywords: Bofutsushosan, Boiogito, Keishibukuryogan, fatty liver, hypercholesterolemia
Hypercholesterolemia is one of the major risk factors for many cardiovascular diseases, such as atherosclerosis, hypertension and myocardial infarction.1, 2 Non-alcoholic fatty liver disease (NAFLD) is common in the general population, and it occurs even more frequently in patients with hypercholesterolemia.3, 4 Patients with NAFLD have a high risk of cardiovascular disease (CVD).5 In addition, NAFLD is often associated with atherosclerotic signs including the presence of carotid plaques6 and coronary arterial calcification.7, 8
It is generally believed that the occurrence and development of hypercholesterolemia have significantly correlation to lipid metabolism-related genes, such as retinol-binding protein 4 (RBP4), heart fatty acid-binding protein (HFABP), cutaneous fatty acid-binding protein (CFABP). 9, 10 RBP4, a protein secreted by hepatocytes and adipose tissue, is closely related to hypercholesterolemia and NAFLD.11 HFABP and CFABP belongs to FABPs family which may play a broad role in cellular fatty acid metabolism.12 In addition to lipid metabolism-related genes, inflammatory cytokines are associated with hypercholesterolemia. Engström et al. have supported the view that inflammation could be a risk factor for developing hypercholesterolemia.13 Monocyte chemoattractant protein-1 (MCP-1) and its receptor CC chemokine receptor 2 (CCR2) are important inflammatory chemokines linked with hypercholesterolemia.14, 15 Intercellular adhesion molecule-1 (ICAM-1) can promote the development and progression of atherosclerosis.16 Therefore, it is a simple and convenient method to detect hypercholesterolemia by monitoring RBP4, C-FABP, H-FABP, MCP1, CCR2, ICAM-1 expression.
Bofutsushosan (BTS), Boiogito (BOT) and Keishibukuryogan (KBG) are well-known Japanese Kampo and Chinese traditional herbal medicines which are used to improve obesity. 17, 18 BTS has been reported to inhibit atherosclerosis,19 obesity, 17, 18, 20 hypertension 17 and hyperglycemia.21 BOT and KBG are also Kampo preparations which have been used in patients with obesity. 22, 23 However, pharmacological evidence for the effects of treatment of NAFLD of BOT and KBG still remains obscure. Using the well-established experimental model of high-cholesterol diet in rats, we compared the pharmacological efficacies of BOT, BTS and KBG.
MATERIALS AND METHODS
Rats and feeding method
Sixty male Wistar rats aged 8-weeks (purchased from Shimizu Laboratory Supplies, Kyoto, Japan) were kept in an air-conditioned room at 25 °C with 55% humidity and given standard chow. After 3 days of acclimation, the rats were divided into 6 groups: control group (C, n = 10), high-cholesterol diet group (H, n = 10), high-cholesterol diet with Kampo formula BOT group (HA, n = 10), high-cholesterol diet with Kampo formula BTS group (HB, n = 10), high-cholesterol diet with Kampo formula KBG group (HC, n = 10), high-cholesterol diet with ezetimibe group (HE, n = 10). The rats in each group were numbered from 1 to 10. Group C was fed a standard rat diet (CE-2; Japan Clea, Tokyo, Japan). A high-cholesterol diet11, 24 was supplied for Group H; it was prepared by adding 2% cholesterol and 0.5% cholic acid to the standard diet. The high-cholesterol diets with BOT, BTS or KBG were made by adding 1% extract formulations of BOT, BTS or KBG to the high-cholesterol diet, respectively. These Kampo extract formulations were generous gift from Tsumura (Tokyo, Japan). The high-cholesterol diet with ezetimibe was made by adding 0.0006% of ezetimibe (LKT laboratories, St Paul, MN). The amount of feed for each rat was regulated to 25 g/day and water was supplied ad libitum. Body weights, systolic and diastolic blood pressure and heart rate were measured weekly. Blood pressure and heart rate were measured by a noninvasive computerized tail-cuff method (BP-98A; Softron, Tokyo).
Sample collection
On days 42 and 84, 5 rats of each group in the order of how they were numbered were sacrificed by collecting blood from the heart under pentobarbital anesthesia after fasting for 12 h. Liver tissue, adipose tissue around the left kidney and abdominal aorta were removed, and then portions of the samples were stored in a 10% formalin solution for hematoxylin-eosin staining and oil red O staining.25 The remaining samples were immediately transferred into EP tubes containing 500 μL of RNA later (Ambion, Austin, TX), quickly frozen in liquid nitrogen, and stored at –80 °C. Serum levels of total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglyceride, blood sugar, creatinine, total bilirubin, alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were analyzed for rats using an auto analyzer at an accredited clinical laboratory (SRL, Tokyo).
ICAM-1 and RBP4
Serum samples were applied for an enzyme-linked immunosorbent assay (ELISA) of ICAM-1 (R&D, Minneapolis, MN) and RBP4 (Aviscera Bioscience, CA) according to the manufacturer’s instructions.
RT-PCR
Total RNA was extracted from the liver and adipose tissue around the left kidney using TRIzol reagent according to the manufacturer’s instructions (Promega, Carlsbad, CA). A semiquantitative real-time PCR (RT-PCR) was performed with Line-Gene (Toyobo, Tokyo) and SYBR Green I (Roche, Basel, Switzerland). The detection was executed at the extension reaction stage in each cycle.
The primer sets for RBP4, HFABP, CFABP, MCP1, CCR2 and beta-actin mRNA were all synthesized by Hokkaido System Science (Sapporo, Japan). The sequence of each primer is listed in Table 1. Using the 2–δδCT method, mRNA expression was semi-quantitatively measured as a relative amount of each target RNA to a known housekeeping gene (beta-actin) expression level.26, 27
Table 1.
The sequence of each PCR primer
| Gene | Primer | |
| RBP | Forward | 5'-gacaaggctcgtttctctgg-3' |
| Reverse | 5'-gactcgtcccttggctgtag-3' | |
| H-FABP | Forward | 5'-ctagcatgagggaagcaagg-3' |
| Reverse | 5'-tgcttcatccagacaagtgg-3' | |
| C-FABP | Forward | 5'-gggctggctcttaggaagat-3' |
| Reverse | 5'-aaaacacggtcgtcttcacc-3' | |
| MCP1 | Forward | 5'-ctgtagcatccacgtgctgt-3' |
| Reverse | 5'-tgctgctggtgattctcttg-3' | |
| CCR2 | Forward | 5'-gatcctgcccctacttgtca-3' |
| Reverse | 5'-agatgagcctcacagcccta-3' | |
| Beta-actin | Forward | 5'-gtagccatccaggctgtgtt-3' |
| Reverse | 5'-ccctcagatgggcacagt-3' | |
Immunohistochemical studies
During the immunohistochemical analyses, 4% formaldehyde-fixed aorta tissue specimens were processed. The following monoclonal antibody was used: anti-ICAM-1 (Abcam, Cambridge, United Kingdom). As a negative control, tissues were stained without the primary antibody. The optical densities were measured by Image-Pro Plus version 6.0 software (Media Cybernetics, Rockville, MD).
Statistical analyses
The data are expressed as the mean ± SEM. For the continuous variables, differences in responses among groups were compared using Mann-Whitney’s U test for non-parametric variables by SPSS 11.0 J (SPSS Japan, Tokyo). P < 0.05 was considered statistically significant.
RESULTS
lower heading
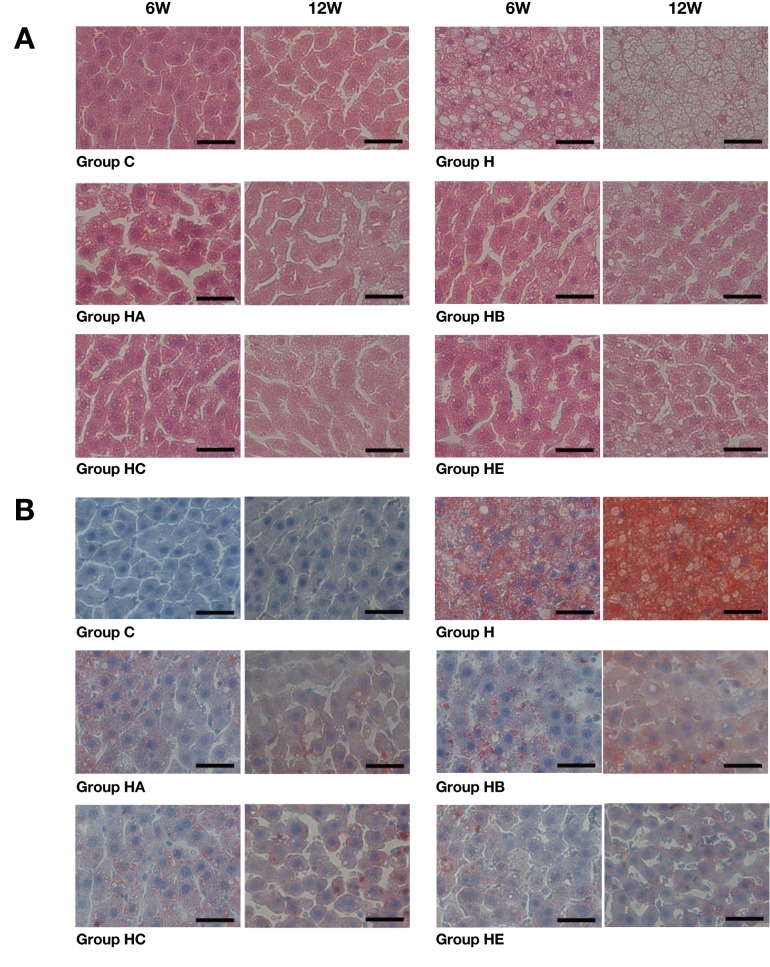
As Fig. 1 shows, the fatty degeneration (steatosis) of liver was observed in the high-cholesterol diet-fed (H, HA, HB, HC and HE) groups, but not in Group C. These changes comprising tiny and large vacuoles as well as pleomorphic nuclei were more prominent in Groups HA, HB, HC and HE than in Group H (Fig. 1A). Oil red O staining revealed that the livers in all high-cholesterol diet supplemented groups were filled with microvesicular or macrovesicular fat deposits; they were depicted as reddish deposits ( Fig. 1B). Overall, fatty liver changes were more prominent in Group H than Groups HA, HB, HC and HE (× 400).
Fig. 1.
Histopathological examination of liver.
A: Fatty degeneration (steatosis) of the liver is observed in the high-cholesterol diet-fed (H, HA, HB, HC and HE) groups, but not in Group C in hematoxylin-eosin stained tissues. Fatty changes increase with time to a greater extent in Group H than Groups HA, HB, HC and HE.
B: Oil red O staining reveals more lipid droplets (stained red) to be accumulated in vacuoles in Group H than other groups.
6W, 6 weeks; 12W, 12 weeks. C, control: standard diet for 6 and 12 weeks (n = 5). H: high-cholesterol diet for 6 and 12 weeks (n = 5). HA: high-cholesterol diet with Boiogito for 6 and 12 weeks (n = 5). HB: high-cholesterol diet with Bofutsushosan for 6 and 12 weeks (n = 5). HC: high-cholesterol diet with Keishibukuryogan for 6 and 12 weeks (n = 5). HE: high-cholesterol diet with ezetimibe for 6 and 12 weeks (n = 5). Bars express 25 μm.
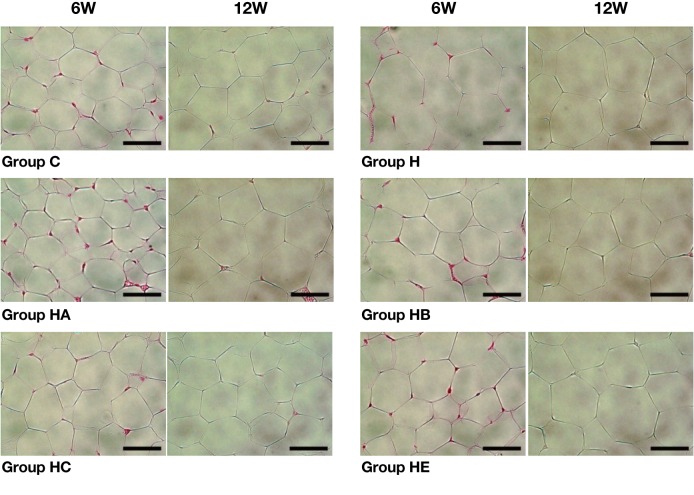
Histological examination of adipose tissue
As Fig. 2 shows, the larger fat cells were observed in the high-cholesterol diet-fed (H, HA, HB, HC and HE) groups, but not in Group C. It was more prominent in Group H than Groups HA, HB, HC and HE (× 400).
Fig. 2.
Histopathological examination of adipose tissue.
The larger fat cells are observed in the high-cholesterol diet-fed (H, HA, HB, HC and HE) groups, but not in Group C (56.13 ± 11.51 and 64.88 ± 13.96 μm after 6 and 12 weeks, respectively) in hematoxylin-eosin stained tissues. Fat cells change with time to a greater extent in Group H (97.00 ± 20.14 and 104.63 ± 22.56 μm after 6 and 12 weeks, respectively) than Groups HA, HB, HC and HE (55.63 ± 5.85 and 78.88 ± 16.34 μm, 75.63 ± 11.53 and 78.13 ± 16.47 μm, 74.63 ± 13.94 and 88.13 ± 8.58 μm, 74.38 ± 13.21 and 83.13 ± 16.63 μm after 6 and 12 weeks, respectively). Bars express 50 μm.
Body and liver weights
As Table 2 shows, there were no significant differences in the baseline of body weights at the beginning of the experiment. The body weights had no significant differences during the experiment too. Liver weights were significantly lower in Group HE than Group H after 6 and 12 weeks (P < 0.05).
Table 2.
Body and liver weights
| Group C | Group H | Group HA | Group HB | Group HC | Group HE | ||
| Body weight (g) | 0W | 233.1 ± 6.6 | 233.5 ± 6.6 | 235.9 ± 6.7 | 237.2 ± 9.4 | 237.1 ± 7.8 | 236.1 ± 6.7 |
| 6W | 402.8 ± 15.9 | 398.5 ± 23.2 | 390.0 ± 21.9 | 392.4 ± 8.6 | 395.9 ± 23.9 | 385.8 ± 24.6 | |
| 12W | 448.6 ± 14.0 | 477.1 ± 15.5* | 461.6 ± 26.1 | 457.6 ± 32.7 | 470.2 ± 32.9 | 453.3 ± 29.2 | |
| Liver weight (g) | 6W | 10.1 ± 0.9 | 15.0 ± 0.8* | 15.5 ± 2.6 | 15.8 ± 1.4 | 14.9 ± 1.1 | 10.8 ± 1.0† |
| 12W | 12.5 ± 3.5 | 19.0 ± 3.1* | 18.9 ± 1.5 | 19.9 ± 2.4 | 19.9 ± 2.2 | 12.1 ± 1.1† | |
6W, 6 weeks; 12W, 12 weeks. ALP, alkaline phosphatase; ALT, alanine aminotransferase; BS, blood sugar; Cr, creatinine; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TBil, total bilirubin; TC, total cholesterol; TG, triglyceride. *P < 0.05 versus Group C. †P < 0.05 versus Group H. Data are expressed as the mean ± SEM.
Blood chemistry and cholesterol concentrations
After 6 weeks of the experiment, the concentration of LDL-C in Group H (8.4 ± 1.7 mg/dL) was significantly elevated compared to the concentration in Group C (5.6 ± 1.1 mg/dL) (P < 0.05). After 12 weeks, the concentration of TC in Group H (78.8 ± 5.4 mg/dL) was significantly elevated compared with it in Group C (56.6 ± 13.0 mg/dL) (P < 0.05). The concentration of HDL-C in Group H (22.2 ± 0.8 mg/dL) was significantly elevated compared with it in Group C (16.0 ± 2.6 mg/dL) (P < 0.05). The concentration of LDL-C elevated to a greater extent in Group H (11.8 ± 0.8 mg/dL) than that in Groups HA and HE (9.0 ± 1.7 and 8.0 ± 1.2 mg/dL, respectively) (P < 0.05). The concentration of ALT in Group H (148.7 ± 25.7 U/L) was significantly elevated compared with it in Group C (53.4 ± 3.0 U/L) (P < 0.05). It was decreased in Groups HA, HB and HC (79.0 ± 17.4, 104.3 ± 26.1, 79.2 ± 18.5 U/L, respectively) than in Group H (P < 0.05). The concentration of ALP in Group H (432.7 ± 31.7 U/L) was significantly elevated compared with it in Group C (331.0 ± 28.5 U/L) (P < 0.05). It was lower in Groups HA, HB and HC (334.3 ± 32.1, 337.0 ± 25.5, 283.3 ± 27.1 U/L, respectively) than in Group H (P < 0.05) (Table 3).
Table 3.
Blood chemistry and cholesterol concentrations
| Group C | Group H | Group HA | Group HB | Group HC | Group HE | ||
| TC (mg/dL) | 6W | 55.5 ± 15.2 | 68.2 ± 6.8 | 67.4 ± 13.7 | 65.4 ± 9.8 | 68.6 ± 13.8 | 78.5 ± 12.4 |
| 12W | 56.6 ± 13.0 | 78.8 ± 5.4* | 73.7 ± 10.4 | 75.2 ± 12.0 | 70.5 ± 12.0 | 73.2 ± 3.5 | |
| HDL-C (mg/dL) | 6W | 18.0 ± 2.6 | 21.0 ± 2.2 | 20.4 ± 4.5 | 19.4 ± 2.9 | 20.0 ± 3.2 | 17.5 ± 2.7 |
| 12W | 16.0 ± 2.6 | 22.2 ± 0.8* | 22.7 ± 3.2 | 23.8 ± 3.1 | 20.0 ± 3.4 | 19.4 ± 1.1 | |
| LDL-C (mg/dL) | 6W | 5.6 ± 1.1 | 8.4 ± 1.7* | 8.6 ± 2.3 | 8.0 ± 1.9 | 8.8 ± 2.6 | 8.8 ± 2.2 |
| 12W | 6.0 ± 1.4 | 11.8 ± 0.8* | 9.0 ± 1.7† | 9.8 ± 2.7 | 10.5 ± 4.4 | 8.0 ± 1.2† | |
| TG (mg/dL) | 6W | 31.5 ± 7.5 | 34.4 ± 11.2 | 28.0 ± 17.1 | 18.2 ± 3.9† | 21.8 ± 8.8 | 25.0 ± 8.0 |
| 12W | 29.2 ± 9.2 | 26.4 ± 4.7 | 22.7 ± 6.9 | 24.0 ± 7.9 | 19.8 ± 5.4 | 16.4 ± 7.0† | |
| BS (mg/dL) | 6W | 109.4 ± 7.7 | 99.6 ± 7.2 | 112.8 ± 8.8† | 110.8 ± 12.0 | 112.6 ± 10.5 | |
| 12W | 102.8 ± 6.1 | 112.0 ± 11.0 | 111.3 ± 20.0 | 114.8 ± 17.8 | 99.2 ± 7.6 | 101.2 ± 9.2 | |
| Cr (mg/dL) | 6W | 0.30 ± 0.03 | 0.30 ± 0.04 | 0.35 ± 0.06 | 0.35 ± 0.05 | 0.34 ± 0.04 | 0.34 ± 0.07 |
| 12W | 0.32 ± 0.04 | 0.35 ± 0.03 | 0.34 ± 0.02 | 0.36 ± 0.11 | 0.34 ± 0.03 | 0.32 ± 0.03 | |
| TBil (μmol/L) | 6W | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.02 |
| 12W | 0.04 ± 0.02 | 0.03 ± 0.02 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.04 ± 0.01 | |
| ALT (U/L) | 6W | 60.0 ± 11.7 | 72.2 ± 11.4* | 71.8 ± 18.2 | 61.3 ± 10.2 | 93.6 ± 17.1 | 111.0 ± 11.1† |
| 12W | 53.4 ± 3.0 | 148.7 ± 25.7* | 79.0 ± 17.4† | 104.3 ± 26.1† | 79.2 ± 18.5† | 109.7 ± 25.8 | |
| ALP (U/L) | 6W | 413.0 ± 22.6 | 497.8 ± 24.8 | 377.5 ± 31.2† | 399.0 ± 20.1† | 450.0 ± 35.6 | 519.0 ± 34.9 |
| 12W | 331.0 ± 28.5 | 432.7 ± 31.7* | 334.3 ± 32.1† | 337.0 ± 25.5† | 283.3 ± 27.1† | 514.8 ± 17.8† | |
6W, 6 weeks; 12W, 12 weeks. ALP, alkaline phosphatase; ALT, alanine aminotransferase; BS, blood sugar; Cr, creatinine; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TBil, total bilirubin; TC, total cholesterol; TG, triglyceride. P < 0.05 versus Group C. †P < 0.05 versus Group H. Data are expressed as the mean ± SEM.
ICAM-1 and RBP4 in serum
ICAM-1 concentrations were increased in Group H compared to Groups C, HA, HB and HE after 12 weeks (Fig. 4, P < 0.05). RBP4 concentrations were increased in Group H compared to the other groups including Groups C, H, HA and HE after 6 and 12 weeks (Fig. 3, P < 0.05).
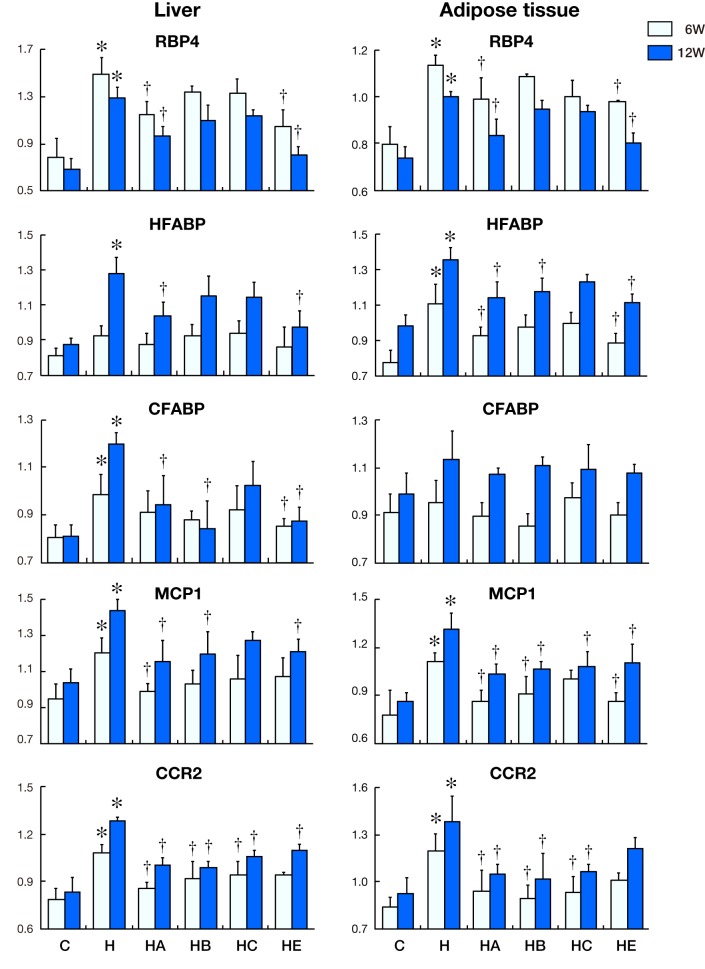
Fig. 4.
Changes in mRNA expression with time in liver and adipose tissue. Levels of RBP4, HFABP, CFABP, MCP1 and CCR2 against beta-actin mRNA expression are shown in the above histograms. The left-side graphs show the expression in liver. The right-side graphs show the expression in adipose tissue around the left kidney.
Fig. 3.
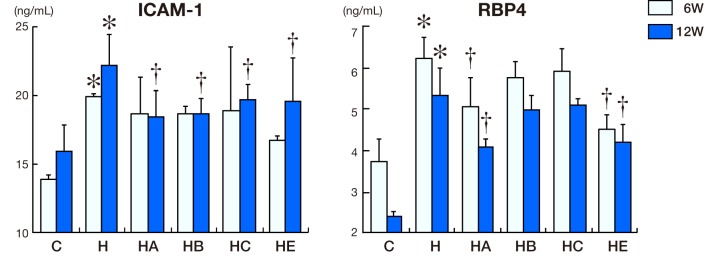
Intercellular adhesion molecule-1 (ICAM-1) and retinol-binding protein 4 (RBP4) concentration in serum. ICAM-1 and RBP4 concentrations in control, standard diet group (C, n = 5), high-cholesterol diet group (H, n = 5), high-cholesterol diet with BOT group (HA, n = 5), high-cholesterol diet with BTS group (HB, n = 5), high-cholesterol diet with KBG group (HC, n = 5) and high-cholesterol diet with ezetimibe group (HE, n = 5) for 6 and 12 weeks. *P < 0.05 versus Group C. †P < 0.05 versus Group H.
mRNA expression in liver and adipose tissue
Figure 4 demonstrates the changes of mRNA expression in liver and adipose tissue round the left kidney of rats for 6 and 12 weeks.
RBP4 mRNA expression in liver in Group H was significantly increased compared to that in Groups C, HA and HE after 6 and 12 weeks (P < 0.05). HFABP mRNA expression in Group H was significantly increased compared to that in Groups C, HA and HE after 12 weeks (P < 0.05). Compared with the other groups, the mRNA expression of CFABP in liver in Group H were higher than that in Groups C, HA, HB and HE at 12 weeks (P < 0.05), whereas they were not obvious at 6 weeks. Compared with the other groups, the mRNA expression of MCP1 in liver in Group H remained high during the entire experiment, especially after 12 weeks (P < 0.05). The expression of CCR2 in liver in Groups HA, HB, HC (at 6 and 12 weeks) and HE (at 12 weeks) were lower than that in Group H (P < 0.05).
A down-regulation of RBP4 mRNA expression in Group H appeared in adipose tissue around the left kidney after 12 weeks; even it was higher in Group H than that in Groups C, HA and HE (P < 0.05). The mRNA expression of HFABP was higher in Group H than that in Groups C, HA, HE (at 6 and 12 weeks) and HB (at 12 weeks) (P < 0.05). CFABP mRNA expression had no significant difference during the whole experiment among all the groups. The expression of MCP1 in adipose tissue in Groups HA, HB, HE (at 6 and 12 weeks) and HC (at 12 weeks) was lower than that in Group H (P < 0.05). The CCR2 mRNA expression in Group H was higher than that in Groups HA, HB and HC (P < 0.05).
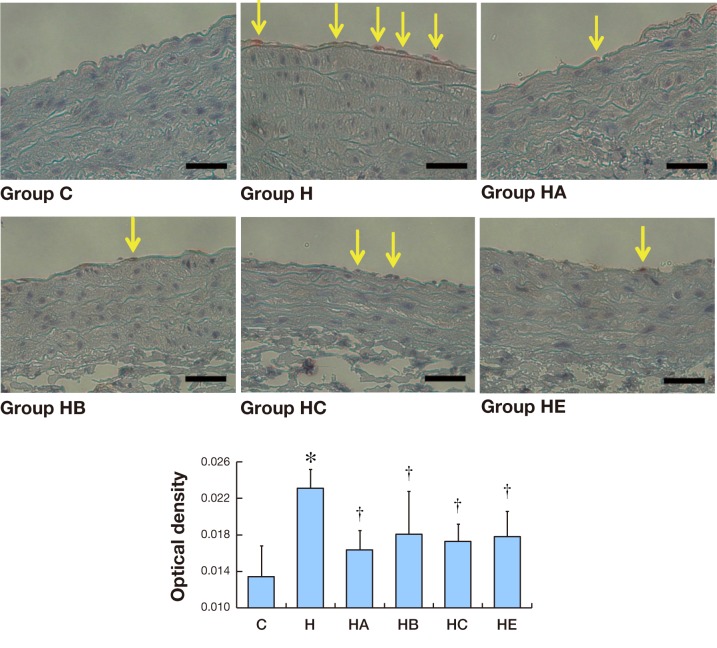
ICAM-1 immunostaining expression in aorta
As shown in Fig. 5, after 12 weeks, little ICAM-1 immunostaining (red-brown deposits indicate positive staining) was found on the whole layers of abdominal aortas in Group C. Significantly positive ICAM-1 immunostaining was observed in Group H and mainly localized on the endothelial layers. Positive ICAM-1 immunostaining was also observed in Groups HA, HB, HC and HE, but less than Group H. While ICAM-1 immunoreactivities were very weak in Groups HA, HB, HC and HE, the positive area and the strength of ICAM-1 stainings was markedly lower than those in Group H (× 400).
Fig. 5.
Immunohistochemistry expression of ICAM-1 in aorta.
A: Seldom ICAM-1 immunostaining (red-brown deposits indicate positive staining) is found on the whole layers of abdominal aortas in Group C. Bars express 25 μm.
B: Mean optical density values of ICAM-1. The photographs generated were quantitatively analyzed the optical density of ICAM-1 with Image-Pro Plus version 6.0 software. *P < 0.05 versus Group C. †P < 0.05 versus Group H.
Blood pressure and heart rate
No significant changes in systolic or diastolic blood pressure and heart rates were observed during the experiment.
DISCUSSION
Hypercholesterolemia is one of major risk factors contributing to cardiovascular diseases. 3, ,4 Patients with NAFLD have a high risk of CVD, too.5 Elevated concentrations of plasma TC and LDL-C as well as reduced concentration of HDL-C are negative risk factors for CVD is well documented. 28–30 Lipid-lowering drugs, such as statins, hydroxyl-methylglutaryl-coenzyme A reductase inhibitors can well reduce LDL-C and increase HDL-C. Adverse reactions such as rhabdomyolysis and hepatitis were sometimes caused by them. Therefore, minimizing the side effects of lipid-lowering drugs is also very important. The mechanism of Kampo formula is to restore the balance of the body. It is a safe way to achieve the balance between LDL-C and HDL-C concentrations. In this study, we observed the effect of Kampo formulas (BOT, BTS, KBG) on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. LDL-C, ALT and ALP concentrations in the serum of rats fed high-cholesterol diet with BOT decreased. ALT (at 6 weeks) and ALP (at 12 weeks) concentrations in Group HE was significantly elevated compared with that in Group H. The reason is still unknown. The steatosis of liver and hypertrophy of fat cells caused by high-cholesterol diet could be alleviated by BOT, BTS and KBG as shown by histopathological examinations.
Retinol-binding protein 4 (RBP4), a protein secreted by hepatocytes (80%) and adipose tissue (20%), is a 21-kDa protein that facilitates the transport of retinol through the circulation to peripheral tissues. 11 It plays a key role in the control of metabolic and proliferative cell functions,31 including steatogenesis. 32 Recently, the role of adipokines, specifically RBP4 in the pathogenesis of obesity-related diseases is widely being discussed. 33–36 Many studies have reported the relationship between RBP4 and obesity as well as its related complications. 35–37 Recent studies demonstrated that RBP4 levels were increased in obese and insulin-resistant humans and mouse models. 38–41 Stefan et al. found a direct relation between hepatic fat content and blood levels of RBP4 in healthy subjects, too.42 It has been reported that RBP4 mRNA expression can be up-regulated in liver and adipose tissue of rats with high cholesterol diet for 12 weeks. 11 In our experiment, RBP4 expression in liver and adipose tissue of rats fed a high-cholesterol diet as well as the expression in serum were up-regulated. BOT and ezetimibe shows greater effect to decrease the level of RBP4 mRNA expression in liver and adipose tissue, the serum expression of RBP4 can also be decreased by BOT and ezetimibe.
HFABP is a member of a family of binding proteins with distinct tissue distributions and diverse roles in fatty acid metabolism, trafficking, and signaling.43 It is a low molecular-weight cytoplasmic protein that is abundant in the myocardium 44 and produced by skeletal muscle,45 cardiomyocytes, kidney distal tubular cells46 and specific parts of the brain. 47, 48 HFABP mRNA expression has been reported to be up-regulated in livers of rats fed high-cholesterol diet for 28 days.11 In our study, this phenomenon was also demonstrated. HFABP mRNA expression in the liver and adipose tissue of high-cholesterol diet groups was significantly increased compared to the control group after 12 weeks.
CFABP was originally identified as being over-expressed in the psoriatic skin. 49, 50 It is typical of the FABP family and binds long-chain fatty acids with high affinity49, 51 and were thought to play a important role in the storage and transport of fatty acids.50, 52, 53 CFABP was reported to be up-regulated after 12 weeks in liver and adipose tissue of high-cholesterol diet rats. 11 Here, the mRNA expression of CFABP in liver of high-cholesterol diet rats were higher after 12 weeks while there were no significant differences between groups in adipose tissue. BOT, BTS and ezetimibe can down-regulate the CFABP mRNA expression in liver.
BOT, BTS and KBG are all used as anti-obesity medications in Japan. 54 BOT is used for people with a solid build and thick abdominal subcutaneous fat. 54 It could decrease the mouse body weight, fat accumulation, TC and triglyceride level, but cannot influence fasting blood glucose levels or insulin levels. 22 BTS is used for patients with flabby constitution and proneness to fatigue.54 It can decrease food intake, body weight, blood pressure, white adipose tissue weight and ameliorate the adipocytokine dysregulation in white adipose tissue.55 KBG is commonly used for women with sudden weight gain in menopause and could led to a reduction in blood cholesterol. 54, 56 According to these reports, even BOT, BTS and KBG are all used in the treatment of obesity, they are suitable for different situations. In our study, BOT has a better effect on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. Ougi and Ginger are the key components of BOT, and it has been reported that Ougi and Ginger of BOT contribute greatly to the beneficial effects on abnormal lipid metabolism. 22 Matsuda et al. also found that Ginger tends to improve lipid metabolism.24 Wang et al. reported that Ougi could significantly reduced plasma levels of TC and LDL-C and improved the atherosclerosis profile. 57 These results partly explain the present findings. We speculate that inhibition of lipid absorption might contribute to the effect of BOT. 22 However, the mechanisms of BTS and KBG are still unknown.
Inflammatory factors play important roles in NAFLD and atherosclerosis progression. 8 MCP1 is an important inflammatory chemokine that can be produced by a variety of cells including vascular endothelial cells, vascular smooth muscle cells, monocytes and other cells. It is a member of the CC chemokine family.58, 59 Plasma concentration of MCP1 increases with obesity. 60, 61 CCR2 is a receptor of MCP1 and can help MCP1 to accomplish its effect. 62 Dietary cholesterol can induce the MCP1 gene expression, 14 and CCR2 expression was increased in hypercholesterolemic patients compared with normocholesterolemic controls.15, 63 In our experiment, compared with the other groups, the mRNA expression of MCP1 and CCR2 in both liver and adipose tissue in Group H remained high level during the entire experiment, especially after 12 weeks. BOT, BTS, KBG and ezetimibe can down-regulate the MCP1 and CCR2 expression. This effect of BOT, BTS and KBG is direct or indirect through inhibition of cholesterol metabolism is still unknown.
ICAM-1, a member of the immunoglobulin superfamily, is one of the markers of endothelial cell activation. It plays an important role in neutrophil migration and adhesion of endothelial cells 64 and is partly involved in the whole process of monocyte adhesion, migration and transformation.16 This migration is one of the earliest events in the atherosclerotic process. 65 ICAM-1 was up-regulated in neointimal and medial smooth muscle cells after vascular injury. 66 Sekiya et al. reported that KBG could prevent the progression of atheromatous plaque,67 but no literature could prove the effect of BOT and BTS on preventing atherosclerosis. In our experiment, serum ICAM-1 concentration and immunostaining expression were increased in Group H after 12 weeks. BOT, BTS, KBG and ezetimibe can down-regulate it. We speculate that down-regulated ICAM-1 may be one of the mechanisms of BOT, BTS and KBG to prevent atherosclerosis.
Overall, BOT has a protective effect on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats and more effective than BTS and KBG. Ezetimibe has the similar effect on lipid related factors, such as LDL-C, RBP4, HFABP and CFABP. However, the anti-inflammatory (MCP1, CCR2) and anti-arteriosclerotic (ICAM-1) effects of BOT are more potent than ezetimibe. This may be a guide on clinical use. The mechanism is still uncertain and further studies should be designed.
Acknowledgments
Acknowledgments: We would like to express our sincere appreciation for the fellowship and research grant from the Japan Research Foundation of Clinical Pharmacology (JRFCP).
A preliminary report has appeared in abstract form in Japanese. 68
The authors declare no conflict of interest.
REFERENCES
- 1.Roffi M , Brandle M , Robbins MA , Mukherjee D . Current perspectives on coronary revascularization in the diabetic patient. Indian Heart J. 2007; 59: 124-36. . [PubMed] [Google Scholar]
- 2.Mohamed AR , El-Hadidy WF , Mannaa HF . Assessment of the Prophylactic Role of Aspirin and/or Clopidogrel on Experimentally Induced Acute Myocardial Infarction in Hypercholesterolemic Rats. Drugs R D. 2014September18. [Epub ahead of print]. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angulo P , Lindor KD . Non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2002; 17Suppl: S186-90. . [DOI] [PubMed] [Google Scholar]
- 4.Marchesini G , Moscatiello S , Di Domizio S , Forlani G . Obesity-associated liver disease. J Clin Endocrinol Metab. 2008; 93 (11Suppl 1): S74-80. . [DOI] [PubMed] [Google Scholar]
- 5.Targher G , Marra F , Marchesini G . Increased risk of cardiovascular disease in non-alcoholic fatty liver disease: causal effect or epiphenomenon?. Diabetologia. 2008; 51: 1947-53. . [DOI] [PubMed] [Google Scholar]
- 6.Dick TJ , Lesser IA , Leipsic JA , Mancini GB , Lear SA . The effect of obesity on the association between liver fat and carotid atherosclerosis in a multi-ethnic cohort. Atherosclerosis. 2013; 226: 208-13. . [DOI] [PubMed] [Google Scholar]
- 7.Sung KC , Wild SH , Kwag HJ , Byrne CD . Fatty liver, insulin resistance, and features of metabolic syndrome: relationships with coronary artery calcium in 10,153 people. Diabetes Care 2012; 35: 2359-64. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim EJ , Kim BH , Seo HS , Lee YJ , Kim HH , Son HH . Cholesterol-induced non-alcoholic fatty liver disease and atherosclerosis aggravated by systemic inflammation. PLoS One. 2014; 9: e97841. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Margareto J , Gómez-Ambrosi J , Marti A , Martínez JA . Time-dependent effects of a high-energy-yielding diet on the regulation of specific white adipose tissue genes. Biochem Biophys Res Commun. 2001; 283: 6-11. . [DOI] [PubMed] [Google Scholar]
- 10.Kushiro M , Takahashi Y , Ide T . Modulation of cutaneous fatty acid-binding protein mRNA expression in rat adipose tissues by hereditary obesity and dietary fats. J Oleo Sci. 2007; 56: 533-41. . [DOI] [PubMed] [Google Scholar]
- 11.Wang X , Hasegawa J , Kitamura Y , Wang Z , Matsuda A , Shinoda W . Effects of hesperidin on the progression of hypercholesterolemia and fatty liver induced by high-cholesterol diet in rats. J Pharmacol Sci. 2011; 117: 129-38. . [DOI] [PubMed] [Google Scholar]
- 12.Bass NM . Function and regulation of hepatic and intestinal fatty acid binding proteins. Chem Phys Lipids. 1985; 38: 95-114. . [DOI] [PubMed] [Google Scholar]
- 13.Engström G , Hedblad B , Janzon L , Lindgärde F . Long-term change in cholesterol in relation to inflammation-sensitive plasma proteins: a longitudinal study. Ann Epidemiol. 2007; 17: 57-63. . [DOI] [PubMed] [Google Scholar]
- 14.Tous M , Ferré N , Rull A , Marsillach J , Coll B , Alonso-Villaverde C . Dietary cholesterol and differential monocyte chemoattractant protein-1 gene expression in aorta and liver of apo E-deficient mice. Biochem Biophys Res Commun. 2006; 340: 1078-84. . [DOI] [PubMed] [Google Scholar]
- 15.Han KH , Tangirala RK , Green SR , Quehenberger O . Chemokine receptor CCR2 expression and monocyte chemoattractant protein-1-mediated chemotaxis in human monocytes. A regulatory role for plasma LDL. Arterioscler Thromb Vasc Biol. 1998; 18: 1983-91. . [DOI] [PubMed] [Google Scholar]
- 16.Nighoghossian N , Derex L , Douek P . The vulnerable carotid artery plaque: current imaging methods and new perspectives. Stroke. 2005; 36: 2764-72. . [DOI] [PubMed] [Google Scholar]
- 17.Hioki C , Yoshimoto K , Yoshida T . Efficacy of bofu-tsusho-san, an oriental herbal medicine, in obese Japanese women with impaired glucose tolerance. Clin Exp Pharmacol Physiol. 2004; 31: 614-9. . [DOI] [PubMed] [Google Scholar]
- 18.Ono M , Ogasawara M , Hirose A , Mogami S , Ootake N , Aritake K . Bofutsushosan, a Japanese herbal (Kampo) medicine, attenuates progression of nonalcoholic steatohepatitis in mice. J Gastroenterol. 2014; 49: 1065-73. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohno K , Chung HJ , Maruyama I , Tani T . Bofutsushosan, a traditional Chinese formulation, prevents intimal thickening and vascular smooth muscle cell proliferation induced by balloon endothelial denudation in rats. Biol Pharm Bull. 2005; 28: 2162-5. . [DOI] [PubMed] [Google Scholar]
- 20.Yoshida T , Sakane N , Wakabayashi Y , Umekawa T , Kondo M . Thermogenic, anti-obesity effects of bofu-tsusho-san in MSG-obese mice. Int J Obes Relat Metab Disord. 1995; 19: 717-22. . [PubMed] [Google Scholar]
- 21.Morimoto Y , Sakata M , Ohno A , Maegawa T , Tajima S . [Effects of Byakko-ka-ninjin-to, Bofu-tsusho-san and Gorei-san on blood glucose level, water intake and urine volume in KKAy mice]. Yakugaku Zasshi. 2002; 122: 163-8. Japanese. . [DOI] [PubMed] [Google Scholar]
- 22.Shimada T , Akase T , Kosugi M , Aburada M . Preventive Effect of Boiogito on Metabolic Disorders in the TSOD Mouse, a Model of Spontaneous Obese Type II Diabetes Mellitus. Evid Based Complement Alternat Med. 2011; 2011: 931073. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagata Y , Goto H , Hikiami H , Nogami T , Fujimoto M , Shibahara N . Effect of keishibukuryogan on endothelial function in patients with at least one component of the diagnostic criteria for metabolic syndrome: a controlled clinical trial with crossover design. Evid Based Complement Alternat Med. 2012; 2012: 359282. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda A , Wang Z , Takahashi S , Tokuda T , Miura N , Hasegawa J . Upregulation of mRNA of retinoid binding protein and fatty acid binding protein by cholesterol enriched-diet and effect of ginger on lipid metabolism. Life Sci. 2009; 84: 903-7. . [DOI] [PubMed] [Google Scholar]
- 25.Koopman R , Schaart G , Hesselink MK . Optimisation of oil red O staining permits combination with immunofluorescence and automated quantification of lipids. Histochem Cell Biol. 2001; 116: 63-8. . [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ , Schmittgen TD . Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25: 402-8. . [DOI] [PubMed] [Google Scholar]
- 27.Schmittgen TD , Zakrajsek BA , Mills AG , Gorn V , Singer MJ , Reed MW . Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000; 285: 194-204. . [DOI] [PubMed] [Google Scholar]
- 28.Daniels TF , Killinger KM , Michal JJ , Wright RW , Jr, Jiang Z . Lipoproteins, cholesterol homeostasis and cardiac health. Int J Biol Sci. 2009; 5: 474-88. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharrett AR , Ballantyne CM , Coady SA , Heiss G , Sorlie PD , Catellier D ; Atherosclerosis Risk in Communities Study Group Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001; 104: 1108-13. . [DOI] [PubMed] [Google Scholar]
- 30.Liu Y , Lei L , Wang X , Ma KY , Li YM , Wang L . Plasma cholesterol-raising potency of dietary free cholesterol versus cholesteryl ester and effect of β-sitosterol. Food Chem. 2015; 169: 277-82. . [DOI] [PubMed] [Google Scholar]
- 31.Desvergne B . RXR: from partnership to leadership in metabolic regulations. Vitam Horm. 2007; 75: 1-32. . [DOI] [PubMed] [Google Scholar]
- 32.Larter CZ , Farrell GC . Insulin resistance, adiponectin, cytokines in NASH: Which is the best target to treat?. J Hepatol. 2006; 44: 253-61. . [DOI] [PubMed] [Google Scholar]
- 33.Lebensztejn DM , Wojtkowska M , Skiba E , Werpachowska I , Tobolczyk J , Kaczmarski M . Serum concentration of adioponectin, leptin and resistin in obese children with non-alcoholic fatty liver disease. Adv Med Sci. 2009; 54: 177-82. . [DOI] [PubMed] [Google Scholar]
- 34.Louthan MV , Barve S , McClain CJ , Joshi-Barve S . Decreased serum adiponectin: an early event in pediatric nonalcoholic fatty liver disease. J Pediatr. 2005; 147: 835-8. . [DOI] [PubMed] [Google Scholar]
- 35.Christou GA , Tselepis AD , Kiortsis DN . The metabolic role of retinol binding protein 4: an update. Horm Metab Res. 2012; 44: 6-14. . [DOI] [PubMed] [Google Scholar]
- 36.Kotnik P , Fischer-Posovszky P , Wabitsch M . RBP4: a controversial adipokine. Eur J Endoc rinol. 2011; 165: 703-11. . [DOI] [PubMed] [Google Scholar]
- 37.Saki F , Karamizadeh Z , Honar N , Moravej H , Ashkani-Esfahani S , Namvar Shooshtarian MH . Association of Plasma Retinol Binding Protein-4 (RBP4) and Sonographic Grading of Fatty Liver in Obese Iranian Children. Hepat Mon. 2012; 12: e7103. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham TE , Yang Q , Blüher M , Hammarstedt A , Ciaraldi TP , Henry RR . Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006; 354: 2552-63. . [DOI] [PubMed] [Google Scholar]
- 39.Norseen J , Hosooka T , Hammarstedt A , Yore MM , Kant S , Aryal P . Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol. 2012;32:2010-9; : -. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klöting N , Graham TE , Berndt J , Kralisch S , Kovacs P , Wason CJ . Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007; 6: 79-87. . [DOI] [PubMed] [Google Scholar]
- 41.Liu Y , Wang D , Li D , Sun R , Xia M . Associations of retinol-binding protein 4 with oxidative stress, inflammatory markers, and metabolic syndrome in a middle-aged and elderly Chinese population. Diabetol Metab Syndr. 2014; 6: 25. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stefan N , Hennige AM , Staiger H , Machann J , Schick F , Schleicher E . High circulating retinol-binding protein 4 is associated with elevated liver fat but not with total, subcutaneous, visceral, or intramyocellular fat in humans. Diabetes Care. 2007; 30: 1173-8. . [DOI] [PubMed] [Google Scholar]
- 43.Qian Q , Kuo L , Yu YT , Rottman JN . A concise promoter region of the heart fatty acid-binding protein gene dictates tissue-appropriate expression. Circ Res. 1999; 84: 276-89. . [DOI] [PubMed] [Google Scholar]
- 44.Glatz JF , van Bilsen M , Paulussen RJ , Veerkamp JH , van der Vusse GJ , Reneman RS . Release of fatty acid-binding protein from isolated rat heart subjected to ischemia and reperfusion or to the calcium paradox. Biochim Biophys Acta. 1988; 961: 148-52. . [DOI] [PubMed] [Google Scholar]
- 45.Zschiesche W , Kleine AH , Spitzer E , Veerkamp JH , Glatz JF . Histochemical localization of heart-type fatty-acid binding protein in human and murine tissues. Histochem Cell Biol. 1995; 103: 147-56. . [DOI] [PubMed] [Google Scholar]
- 46.Maatman RG , van de Westerlo EM , van Kuppevelt TH , Veerkamp JH . Molecular identification of the liver- and the heart-type fatty acid-binding proteins in human and rat kidney. Use of the reverse transcriptase polymerase chain reaction. Biochem J. 1992; 288 (Pt 1): 285-90. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelsers MM , Hanhoff T , Van der Voort D , Arts B , Peters M , Ponds R . Brain- and heart-type fatty acid-binding proteins in the brain: tissue distribution and clinical utility. Clin Chem. 2004; 50: 1568-75. . [DOI] [PubMed] [Google Scholar]
- 48.Cakir E , Ozbek M , Sahin M , Cakal E , Gungunes A , Ginis Z . Heart type fatty acid binding protein response and subsequent development of atherosclerosis in insulin resistant polycystic ovary syndrome patients. J Ovarian Res. 2012; 5: 45. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madsen P , Rasmussen HH , Leffers H , Honoré B , Celis JE . Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein [PA-FABP]) that is highly up-regulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J Invest Dermatol. 1992; 99: 299-305. . [DOI] [PubMed] [Google Scholar]
- 50.Jing C , Beesley C , Foster CS , Chen H , Rudland PS , West DC . Human cutaneous fatty acid-binding protein induces metastasis by up-regulating the expression of vascular endothelial growth factor gene in rat Rama 37 model cells. Cancer Res. 2001; 61: 4357-64. . [PubMed] [Google Scholar]
- 51.Adamson J , Morgan EA , Beesley C , Mei Y , Foster CS , Fujii H . High-level expression of cutaneous fatty acid-binding protein in prostatic carcinomas and its effect on tumorigenicity. Oncogene. 2003; 22: 2739-49. . [DOI] [PubMed] [Google Scholar]
- 52.Bass NM . The cellular fatty acid binding proteins: aspects of structure, regulation, and function. Int Rev Cytol. 1988; 111: 143-84. . [DOI] [PubMed] [Google Scholar]
- 53.Horrobin DF . Essential fatty acids in clinical dermatology. J Am Acad Dermatol. 1989; 20: 1045-53. . [DOI] [PubMed] [Google Scholar]
- 54.Yamakawa J , Moriya J , Takeuchi K , Nakatou M , Motoo Y , Kobayashi J . Significance of Kampo, Japanese traditional medicine, in the treatment of obesity: basic and clinical evidence. Evid Based Complement Alternat Med. 2013; 2013: 943075. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Azushima K , Tamura K , Wakui H , Maeda A , Ohsawa M , Uneda K . Bofu-tsu-shosan, an oriental herbal medicine, exerts a combinatorial favorable metabolic modulation including antihypertensive effect on a mouse model of human metabolic disorders with visceral obesity. PLoS One. 2013; 8: e75560. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujimoto M , Tsuneyama K , Kinoshita H , Goto H , Takano Y , Selmi C .The traditional Japanese formula keishibukuryogan reduces liver injury and inflammation in patients with nonalcoholic fatty liver disease. Ann N Y Acad Sci. 2010; 1190: 151-8. . [DOI] [PubMed] [Google Scholar]
- 57.Wang D , Zhuang Y , Tian Y , Thomas GN , Ying M , Tomlinson B . Study of the effects of total flavonoids of Astragalus on atherosclerosis formation and potential mechanisms. Oxid Med Cell Longev. 2012; 2012: 282383. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X , Coriolan D , Murthy V , Schultz K , Golenbock DT , Beasley D . Proinflammatory phenotype of vascular smooth muscle cells: role of efficient Toll-like receptor 4 signaling. Am J Physiol Heart Circ Physiol. 2005; 289: H1069-76. . [DOI] [PubMed] [Google Scholar]
- 59.Anand AR , Bradley R , Ganju RK . LPS-induced MCP-1 expression in human microvascular endothelial cells is mediated by the tyrosine kinase, Pyk2 via the p38 MAPK/NF-kappaB-dependent pathway. Mol Immunol. 2009; 46: 962-8. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Panee J . Monocyte Chemoattractant Protein 1 (MCP-1) in obesity and diabetes. Cytokine. 2012; 60: 1-12. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heber D , Zhang Y , Yang J , Ma JE , Henning SM , Li Z . Green tea, black tea, and oolong tea polyphenols reduce visceral fat and inflammation in mice fed high-fat, high-sucrose obesogenic diets. J Nutr. 2014; 144: 1385-93. . [DOI] [PubMed] [Google Scholar]
- 62.Siebert H , Sachse A , Kuziel WA , Maeda N , Brück W . The chemokine receptor CCR2 is involved in macrophage recruitment to the injured peripheral nervous system. J Neuroimmunol. 2000; 110: 177-85. . [DOI] [PubMed] [Google Scholar]
- 63.Kamada Y , Kiso S , Yoshida Y , Chatani N , Kizu T , Hamano M .Estrogen deficiency worsens steatohepatitis in mice fed high-fat and high-cholesterol diet. Am J Physiol Gastrointest Liver Physiol. 2011; 301: G1031-43. . [DOI] [PubMed] [Google Scholar]
- 64.Shimizu N , Suzuki H , Wakabayashi K , Iso Y , Shibata M , Yorozuya M . [Expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in the pig coronary artery injury model: comparison of plain old balloon angioplasty and stent implantation]. J Cardiol. 2004; 43: 131-9. Japanese. . [PubMed] [Google Scholar]
- 65.Amran AA , Zakaria Z , Othman F , Das S , Al-Mekhlafi HM , Nordin NA . Changes in the vascular cell adhesion molecule-1, intercellular adhesion molecule-1 and c-reactive protein following administration of aqueous extract of piper sarmentosum on experimental rabbits fed with cholesterol diet. Lipids Health Dis. 2011; 10: 2. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yao EH , Wang HJ , Xu CS . Effects of tongxinluo on the neointima formation and expression of inflammatory cytokines in rats after carotid artery balloon injury. Indian J Pharmacol. 2014; 46: 510-4. ; . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sekiya N , Kainuma M , Hikiami H , Nakagawa T , Kouta K , Shibahara N .Oren-gedoku-to and Keishi-bukuryo-gan-ryo inhibit the progression of atherosclerosis in diet-induced hypercholesterolemic rabbits. Biol Pharm Bull. 2005; 28: 294-8. . [DOI] [PubMed] [Google Scholar]
- 68.Qian W , Tsuno S , Endo Y , Matsuda A , Miura N , Hasegawa J . [Effects of Kampo recipes on the progression of fatty liver induced by high-cholesterol diet in rats]. Jpn J Clin Pharmacol Ther. 2013; 44 (Suppl): S308. Japanese. . [Google Scholar]