Abstract
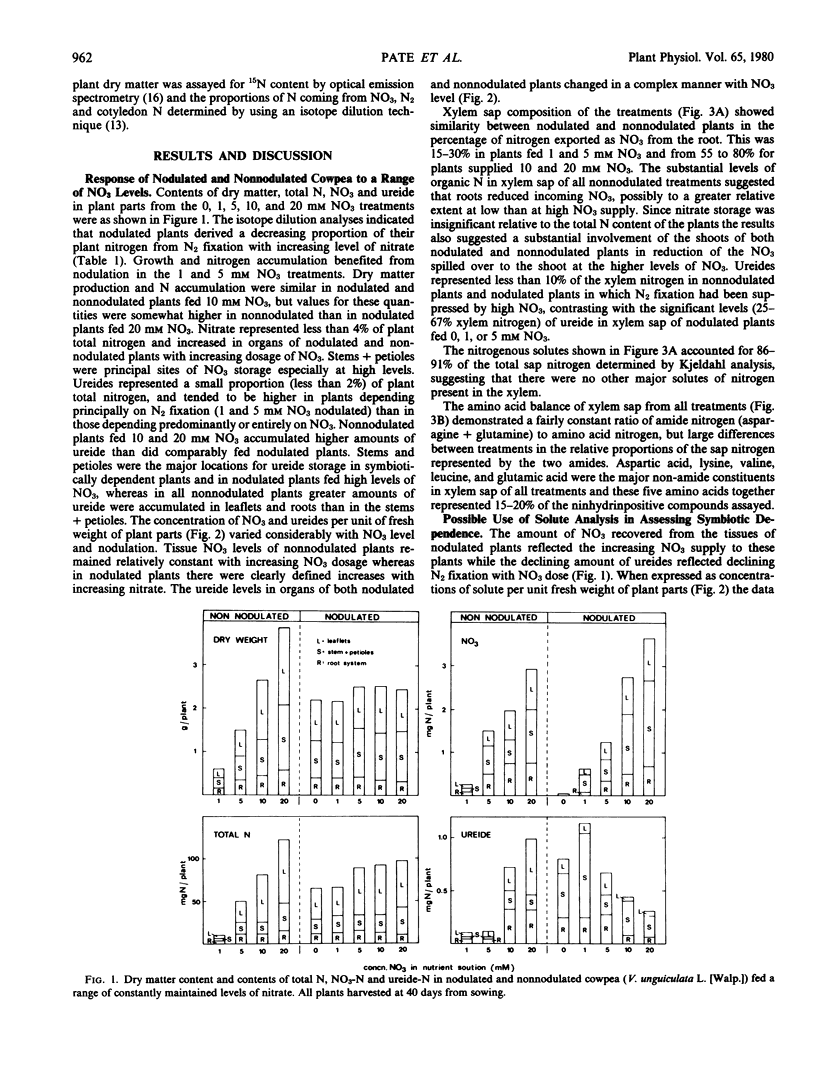
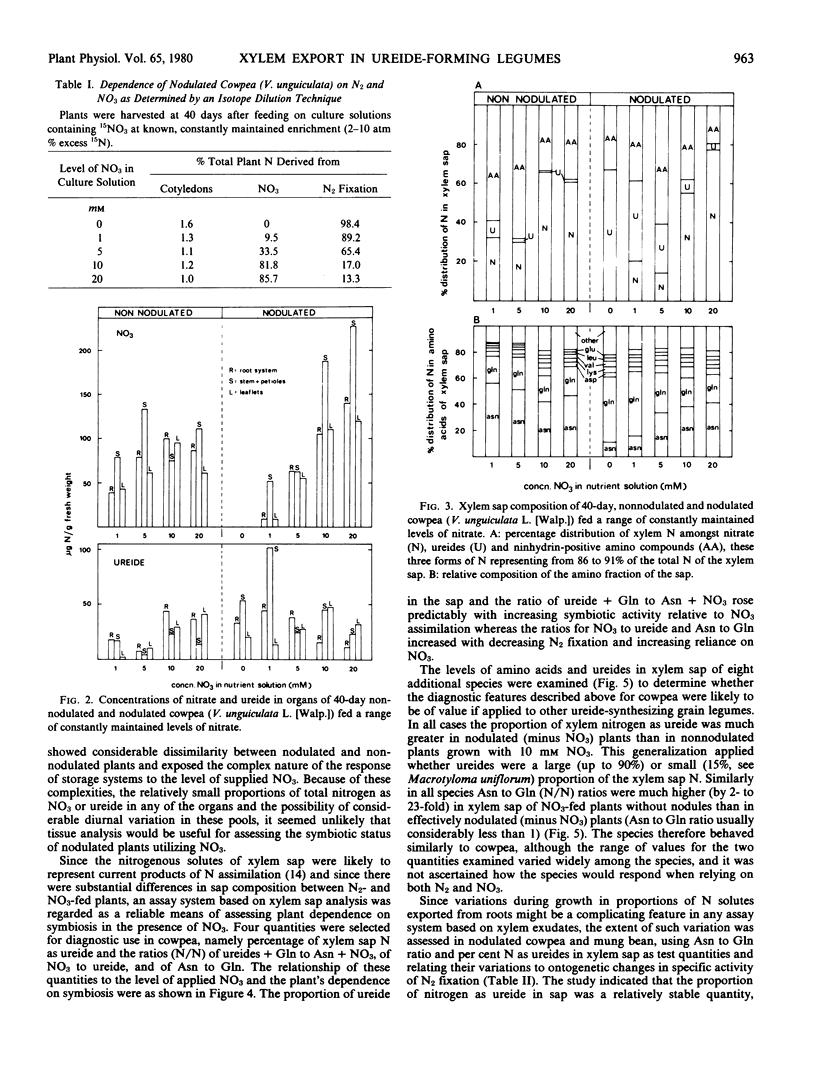
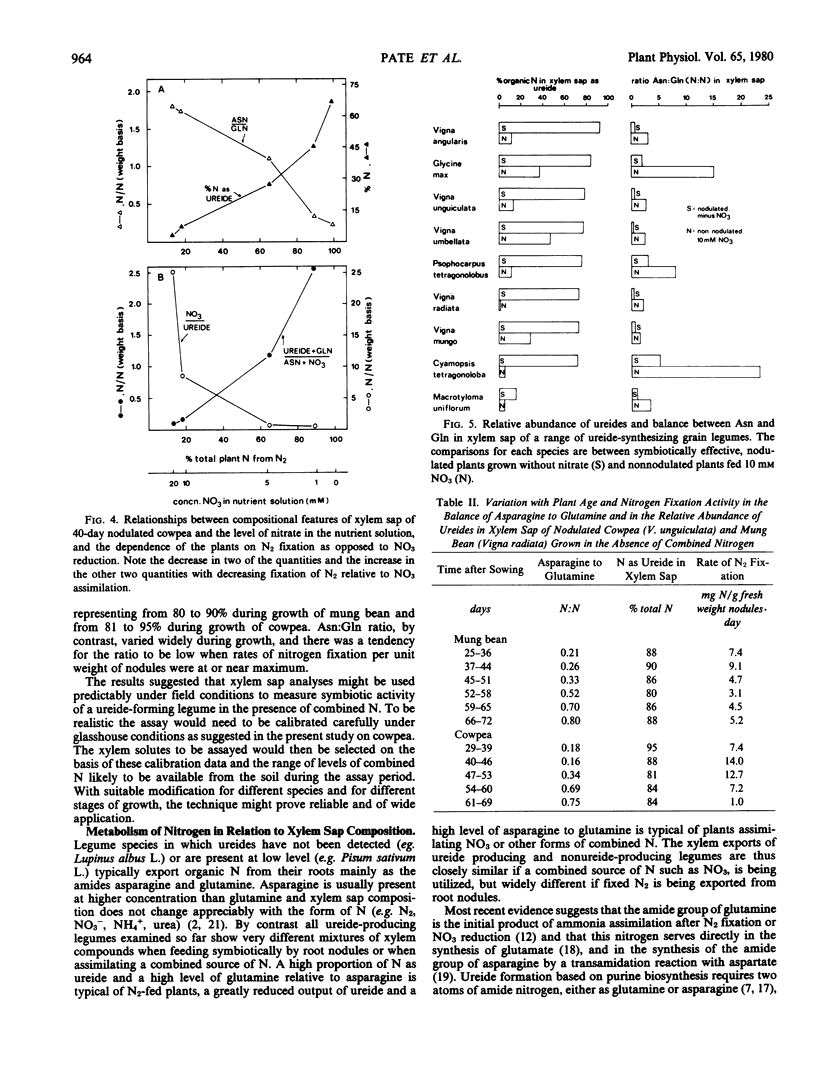
Xylem sap composition was examined in nodulated and nonnodulated cowpea (Vigna unguiculata [L.] Walp.) plants receiving a range of levels of NO3 and in eight other ureide-forming legumes utilizing NO3 or N2 as sole source of nitrogen. A 15N dilution technique determined the proportions of plant nitrogen derived from N2 in the nodulated cowpeas fed NO3. Xylem sap composition of NO3-fed, nodulated cowpea varied predictably with the relative extents to which N2 and NO3 were being utilized. The ratios of asparagine to glutamine (N/N) and of NO3 to ureide (N/N) in xylem sap increased with increasing dependence on NO3 whereas per cent of xylem nitrogen as ureide and the ratio of ureide plus glutamine to asparagine plus NO3 (N/N) in xylem sap increased with increasing dependence on N2 fixation. The amounts of NO3 and ureides stored in leaflets, stems plus petioles, and roots of cowpea varied in a complex manner with level of NO3 and the presence or absence of N2 fixation. All species showed higher proportions of organic nitrogen as ureide and several-fold lower ratios of asparagine to glutamine in their xylem sap when relying on N2 than when utilizing NO3. In nodulated (minus nitrate) cowpea and mung bean (Vigna radiata [L.] Wilczek) the percentage of xylem nitrogen as ureide remained constant during growth but the ratio of asparagine to glutamine varied considerably. The biochemical significance of the above differences in xylem sap composition was discussed.
Full text
PDF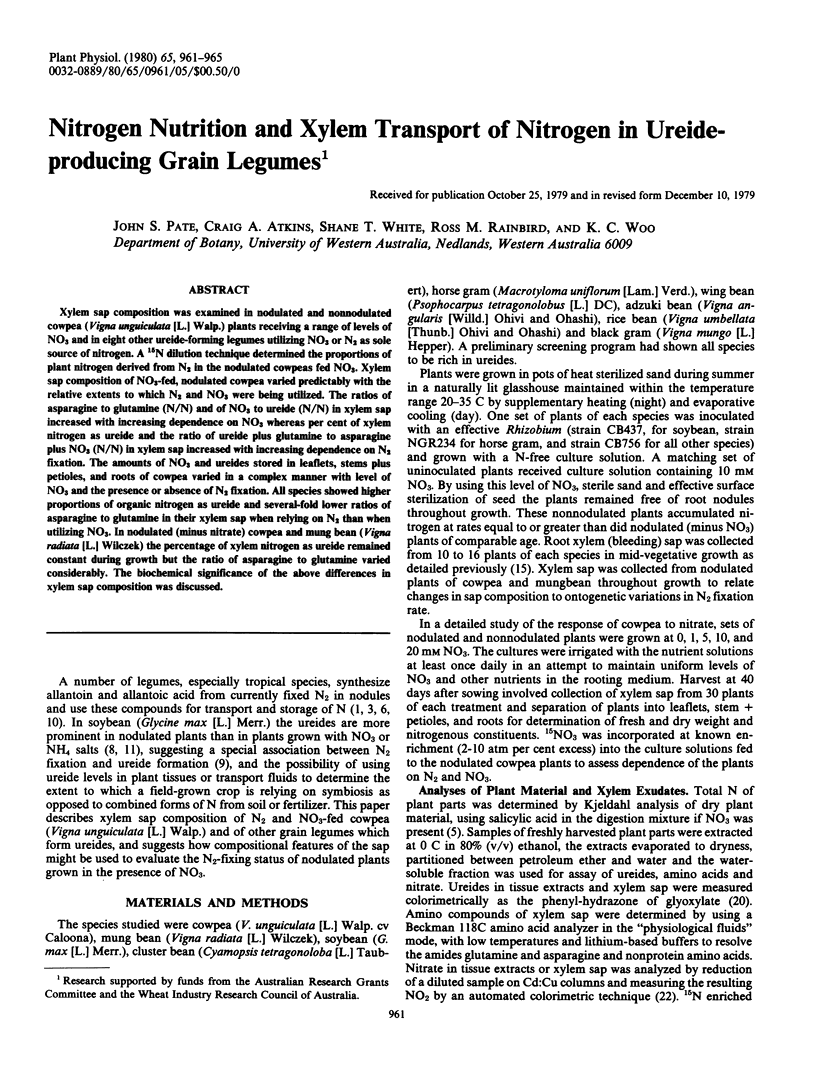

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins C. A., Pate J. S., Layzell D. B. Assimilation and Transport of Nitrogen in Nonnodulated (NO(3)-grown) Lupinus albus L. Plant Physiol. 1979 Dec;64(6):1078–1082. doi: 10.1104/pp.64.6.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastin E. F. Total nitrogen determining for plant material containing nitrate. Anal Biochem. 1978 Apr;85(2):591–594. doi: 10.1016/0003-2697(78)90259-2. [DOI] [PubMed] [Google Scholar]
- Herridge D. F., Atkins C. A., Pate J. S., Rainbird R. M. Allantoin and Allantoic Acid in the Nitrogen Economy of the Cowpea (Vigna unguiculata [L.] Walp.). Plant Physiol. 1978 Oct;62(4):495–498. doi: 10.1104/pp.62.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPOOR M., WAYGOOD E. R. Initial steps of purine biosynthesis in wheat germ. Biochem Biophys Res Commun. 1962 Sep 25;9:7–10. doi: 10.1016/0006-291x(62)90078-5. [DOI] [PubMed] [Google Scholar]
- McClure P. R., Israel D. W. Transport of nitrogen in the xylem of soybean plants. Plant Physiol. 1979 Sep;64(3):411–416. doi: 10.1104/pp.64.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERN H., WANG D., WAYGOOD E. R. BIOSYNTHESIS OF NUCLEOTIDES IN WHEAT. I. PURINES FROM C14-LABELLED COMPOUNDS. Can J Biochem. 1965 Feb;43:225–235. doi: 10.1139/o65-029. [DOI] [PubMed] [Google Scholar]
- Robertson J. G., Warburton M. P., Farnden K. J. Induction of glutamate synthase during nodule development in lupin. FEBS Lett. 1975 Jul 15;55(1):33–37. doi: 10.1016/0014-5793(75)80950-1. [DOI] [PubMed] [Google Scholar]
- Trijbels F., Vogels G. D. Degradation of allantoin by Pseudomonas acidovorans. Biochim Biophys Acta. 1966 Feb 14;113(2):292–301. doi: 10.1016/s0926-6593(66)80068-1. [DOI] [PubMed] [Google Scholar]



