Abstract
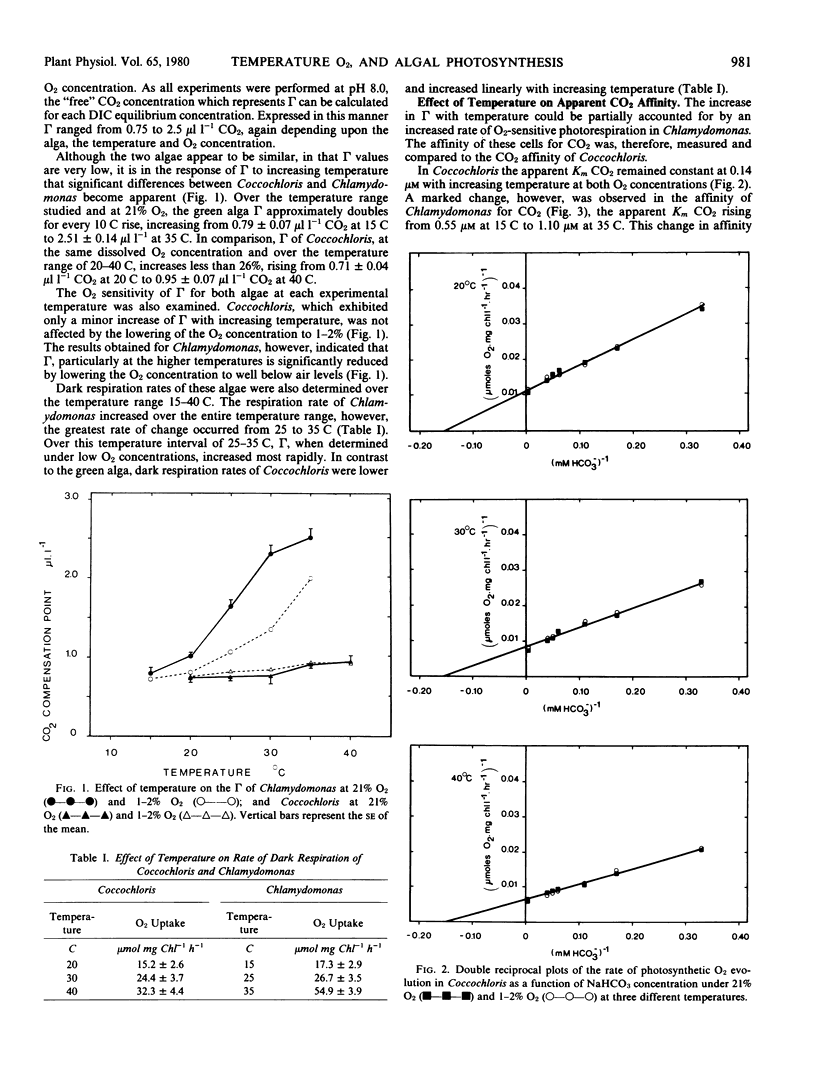
The CO2 compensation points of Coccochloris peniocystis, a blue-green alga and Chlamydomonas reinhardtii, a green alga, were determined at pH 8.0 in a closed system by a gas chromatographic technique. The compensation point of Chlamydomonas increased markedly with temperature, rising from 0.79 microliter per liter CO2 at 15 C to 2.5 microliters per liter CO2 at 35 C. In contrast, the compensation point of Coccochloris at 20 C was 0.71 microliter per liter CO2 and rose to only 0.95 microliter per liter CO2 at 40 C.
The compensation point of the green alga was significantly reduced at low O2 concentrations (1 to 2%) when measured over the temperature range of 15 to 35 C. The compensation point of the blue-green alga, over the temperature range of 20 to 40 C, was unaffected by lowering the O2 concentration.
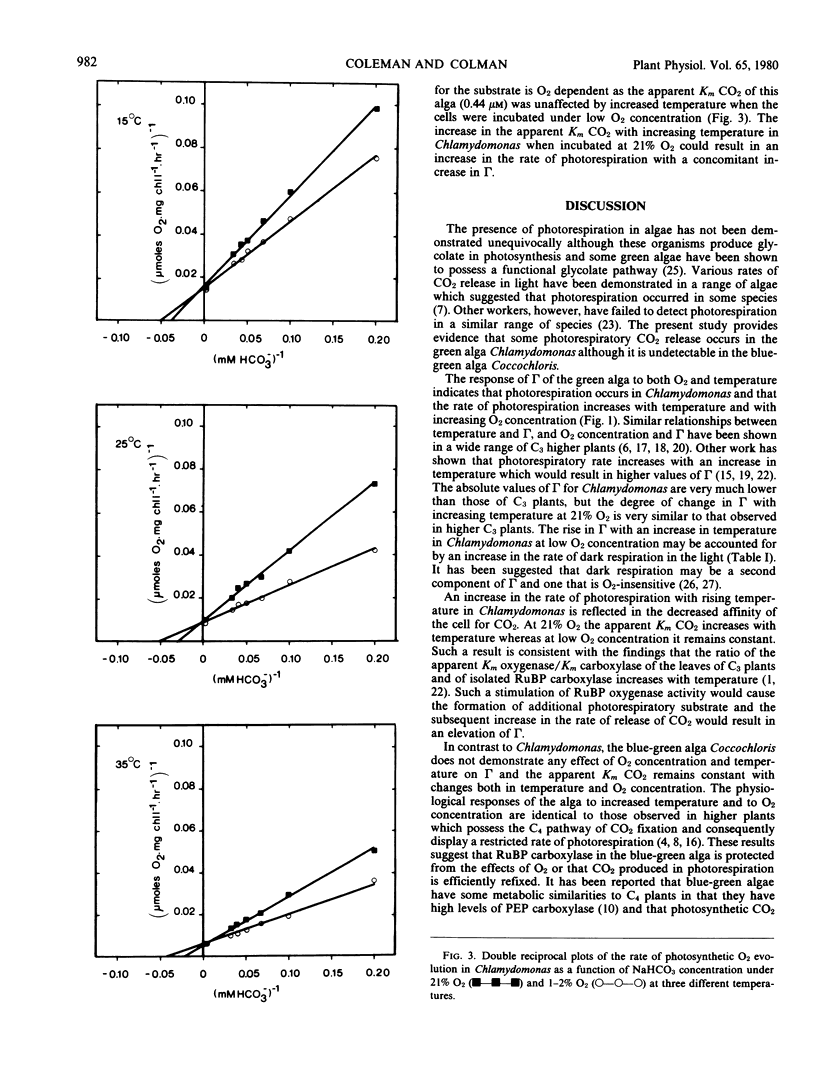
The whole cell CO2 affinity of Chlamydomonas decreased substantially with increasing temperature at 21% O2 whereas little change was observed over the same temperature regime when the CO2 affinity was determined at O2 concentrations of 1 to 2%. The CO2 affinity of Coccochloris did not decrease significantly with either increasing temperature or O2 concentration.
These results suggest that while photorespiration is undetectable in Coccochloris some photorespiratory CO2 release occurs in Chlamydomonas.
Full text
PDF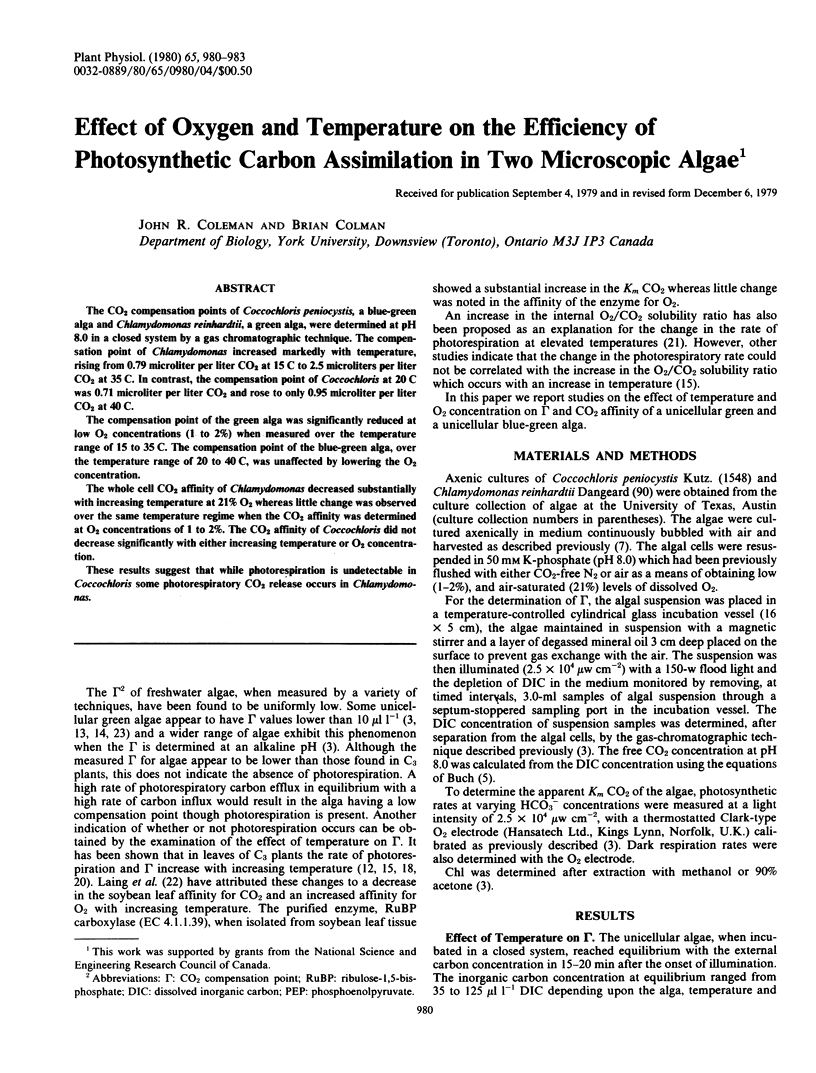

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J. Effects of CO2, O2 and temperature on a high-affinity form of ribulose diphosphate carboxylase-oxygenase from spinach. Biochem Biophys Res Commun. 1974 Sep 9;60(1):204–210. doi: 10.1016/0006-291x(74)90192-2. [DOI] [PubMed] [Google Scholar]
- Birmingham B. C., Colman B. Measurement of carbon dioxide compensation points of freshwater algae. Plant Physiol. 1979 Nov;64(5):892–895. doi: 10.1104/pp.64.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downton J., Slatyer R. O. Temperature dependence of photosynthesis in cotton. Plant Physiol. 1972 Oct;50(4):518–522. doi: 10.1104/pp.50.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hew C. S., Krotkov G., Canvin D. T. Effects of Temperature on Photosynthesis and CO(2) Evolution in Light and Darkness by Green Leaves. Plant Physiol. 1969 May;44(5):671–677. doi: 10.1104/pp.44.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krenzer E. G., Moss D. N., Crookston R. K. Carbon dioxide compensation points of flowering plants. Plant Physiol. 1975 Aug;56(2):194–206. doi: 10.1104/pp.56.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku S. B., Edwards G. E. Oxygen Inhibition of Photosynthesis: II. Kinetic Characteristics as Affected by Temperature. Plant Physiol. 1977 May;59(5):991–999. doi: 10.1104/pp.59.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing W. A. Regulation of Soybean Net Photosynthetic CO(2) Fixation by the Interaction of CO(2), O(2), and Ribulose 1,5-Diphosphate Carboxylase. Plant Physiol. 1974 Nov;54(5):678–685. doi: 10.1104/pp.54.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd N. D., Canvin D. T., Culver D. A. Photosynthesis and photorespiration in algae. Plant Physiol. 1977 May;59(5):936–940. doi: 10.1104/pp.59.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Evidence for HCO(3) Transport by the Blue-Green Alga (Cyanobacterium) Coccochloris peniocystis. Plant Physiol. 1980 Feb;65(2):397–402. doi: 10.1104/pp.65.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]



