Abstract
Background
Blood pressure (BP) is often inadequately controlled in patients with chronic kidney disease (CKD). Previous reports of the longitudinal association between achieved level of BP and end-stage renal disease (ESRD) have not incorporated time-updated BP with appropriate adjustment for known confounders.
Objective
To assess the association between baseline and time-updated systolic BP (SBP) with the progression of CKD.
Design
Observational, prospective cohort study (ClinicalTrials.gov identifier: NCT00304148)
Setting
Seven US clinical centers
Patients
Participants of the Chronic Renal Insufficiency Cohort (CRIC) Study (N=3,708) followed for a median (25th, 75th percentiles) of 5.7 (4.6, 6.7) years
Measurements
The mean of three seated SBP measurements were used as the visit-specific SBP. SBP was time-updated as the mean of that visit and all prior visits. Outcomes were ESRD and the composite renal endpoint of ESRD (dialysis or transplantation) or halving of the estimated glomerular filtration rate (eGFR). Analyses investigating baseline and time-updated SBP utilized traditional Cox proportional hazards models and marginal structural models, respectively.
Results
SBP was ≥130 mmHg at all study visits in 19.2% of participants, and ≥140 mmHg in 10.6%. The hazard ratio (95% confidence interval) for ESRD among participants with SBP 130–139 mmHg, compared to SBP <120 mmHg, was 1.46 (1.13–1.88) using only baseline data, and was 2.37 (1.48–3.80) using all available time-updated data. Among those with SBP ≥140 mmHg, corresponding hazard ratios were 1.46 (1.18–1.88) and 3.37 (2.26–5.03), respectively.
Limitations
SBP was measured once annually, and the CRIC Study cohort is not a random sample.
Conclusions
Among participants in the CRIC Study, time-updated SBP over 130 mmHg was more strongly associated with progression of CKD than analyses based on baseline SBP.
Funding
The CRIC Study is funded under cooperative agreements from the National Institute of Diabetes and Digestive and Kidney Diseases, Clinical Translational Science Awards, and other NIH grants.
INTRODUCTION
Hypertension is common in patients with chronic kidney disease (CKD (1). Observational studies (2,3) and clinical trials (4–7) provide compelling evidence of the association between elevated blood pressure (BP) and progression of CKD though clinical trial data are inconsistent and may suggest a plateau of effect once BP is lowered <140/90 mmHg.
The 2003 Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) specified a BP target of <130/80 mmHg for individuals with CKD or diabetes compared to a BP target of <140/90 mmHg in other hypertensive populations (8). However, the paucity of high quality evidence to support this lower target BP for patients with CKD, especially those without proteinuria and those with diabetes, has led the JNC 8, Kidney Disease Improving Global Outcomes (KDIGO), and American Diabetes Association to raise BP targets for patients with CKD to <140/90 mmHg (9–11).
Clinical trials and observational studies continue to inform our understanding of the association between BP level and CKD progression - each from an important vantage point. Intention-to-treat analyses from clinical trials provide evidence of the efficacy of anti-hypertensive therapies including BP targets, but in selected study populations eligible for and willing to participate in experimental research. By contrast, analyses of achieved BP from observational studies provide the unique opportunity to study associations of BP with clinical outcomes among a broader, more representative population. Further, when these latter studies take advantage of BP measured over time, they can characterize the longitudinal pattern of hypertension. Importantly, these longitudinal, observational studies also provide a more robust assessment of associations with outcomes than do analyses examining relations to a single measure of BP that may attenuate with extended follow-up.
The goal of the current study was to compare the association between BP level and CKD progression utilizing baseline and time-updated BP measurements in CRIC Study participants, independent of other important time-updated factors. We hypothesized that elevated BP would be associated with more rapid progression of CKD and that the association between baseline levels of BP and kidney disease progression would understate this relation compared to updated BP levels.
METHODS
Study Design and Population
The Chronic Renal Insufficiency Cohort (CRIC) Study enrolled a total of 3,939 men and women with mild to moderate CKD between June 2003 and August 2008 at seven clinical centers in the United States (Ann Arbor/Detroit, MI; Baltimore, MD; Chicago, IL; Cleveland, OH; New Orleans, LA; Philadelphia, PA; and Oakland, CA). Study participants (45% women; 42% black; 13% Hispanic; 48% with diabetes mellitus) were followed at annual clinic visits where data were obtained, blood pressure was measured, and blood and urine specimens were collected. Details on study design and baseline participant characteristics were previously published (12–14). Study participants provided written informed consent and the study protocol was approved by institutional review boards at each of the clinical centers.
Inclusion/Exclusion Criteria
Participants were eligible for the CRIC Study if they were between 21 and 74 years of age and met the following age-specific eGFR criteria: 20–70 mL/min/1.73m2 for individuals aged 21–44 years, 20–60 mL/min/1.73m2 for individuals aged 45–64 years, and 20–50 mL/min/1.73m2 for individuals aged 65–74 years. Individuals with prior dialysis (>1 month), NYHA Class III/IV heart failure, polycystic kidney disease, or other primary kidney diseases requiring active immunosuppression were excluded from participation. A total of 3,708 participants were included in the present analysis after excluding participants with missing baseline BP (N=1), urine protein (N=197), and other covariate data (N=33).
Data Collection
Main Predictor
At each annual in-person clinic visit, three seated BP measurements were obtained using a Tycos Classic hand aneroid cuff and sphygmomanometer following a standardized protocol. The mean of all BP measurements were used as the BP values for that visit. Time-updated mean BP measurements averaged the mean seated BP at any given visit and those from all prior visits. The current analysis examined baseline and time-updated mean SBP continuously per 10 mmHg increase, and by four SBP categories (<120 (referent), 120–129, 130–139, and ≥140 mmHg) to evaluate the association of BP with CKD progression.
Outcomes and Censoring Events
Two measures of CKD progression were studied; development of ESRD and a composite endpoint of ESRD or halving of eGFR from baseline. ESRD was defined as receipt of maintenance dialysis or a kidney transplant and was ascertained primarily through self-report. Information collected on ESRD by study investigators was supplemented by the United States Renal Data System (USRDS). Estimated GFR was calculated from serum creatinine and cystatin C using a CRIC Study equation (15). Time to eGFR halving was imputed assuming a linear decline in kidney function between in-person annual visit measures (16). Participants’ follow-up was censored at time of death (N=389 for ESRD analyses), withdrawal (N=146), loss to follow-up (N=171), or the end of the follow-up period, whichever occurred first. Deaths were ascertained from next of kin, death certificates, obituaries, reviews of hospital records, and linkage with the Social Security Death Master File. Outcomes were ascertained from study entry through March 2011.
Covariates
Participants self-reported information on socio-demographics (age, sex, race/ethnicity, education level), and history of cardiovascular disease at baseline and medication usage at baseline and follow-up study visits. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic, or other. Self-reported history of any cardiovascular disease at baseline included prior myocardial infarction, coronary revascularization, heart failure, stroke, or peripheral arterial disease. Cardiovascular events throughout follow-up were adjudicated by two physician reviewers. At each study visit, participants were queried about any medication usage in the prior 30 days. All anti-hypertensive medications were categorized into drug classes, and the total number of anti-hypertensive drug classes was calculated. Hypertension awareness was determined by a positive response to the question, “Has a doctor or other health professional ever told you / told you since your last CRIC visit that you have hypertension or high blood pressure?” Anthropometric measures were assessed using standardized protocols. Body mass index (BMI) was derived as weight in kg divided by height in meters squared. Serum creatinine was measured by an enzymatic method (www.orthoclinical.com) through October 2008 and by the Jaffe method (www.beckmancoulter.com) thereafter, and standardized to isotope dilution mass spectrometry-traceable values (17,18). Serum cystatin C was measured using a particle-enhanced immunonephelometric assay on the Siemens BN™ II System (www.siemens.com). Urine total protein and creatinine, and plasma glucose were also measured using standard assays. Protein-to-creatinine ratios from 24-hour and spot urine specimens were very highly correlated (ρ=0.96), and as such, were used interchangeably. Diabetes mellitus was defined as a fasting glucose >6.99 mmol/L (126 mg/dL), a non-fasting glucose >11.10 mmol/L (200 mg/dL), or use of insulin or other medications for glycemic control.
Statistical Analysis
Summary statistics and distributions of all BP variables were generated. Maximum differences in follow-up SBP levels from baseline were summarized using four categories of absolute differences (<10, 10–<20, 20–<30, and ≥30 mmHg). Study variables were described overall and across baseline SBP categories (<120 mmHg, 120–139 mmHg, and ≥140 mmHg) using mean and standard deviation for continuous variables, and frequency and proportion for categorical variables. Differences in characteristics across SBP categories were compared using ANOVA and chi-square tests, as appropriate. Elevated SBP for each study participant was characterized in three ways, as the percentage of study visits with a SBP level ≥120, ≥130, and ≥140 mmHg. Crude rates and 95% confidence intervals of ESRD and the composite renal endpoint were calculated overall and within levels of baseline SBP.
The association of baseline SBP with renal endpoints was examined using traditional Cox proportional hazards models with adjustment for baseline age, sex, race/ethnicity, education level, history of cardiovascular disease, number of anti-hypertensive medication drug classes taken, use of an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), hypertension awareness, BMI, diabetes, level of eGFR, and urine protein-to-creatinine ratio. Adjustment for eGFR and urine protein-to-creatinine ratio included quadratic spline terms. To examine the potential impact of death as a competing risk, rather than a censoring event, competing risk sensitivity analyses of baseline SBP on renal endpoints were conducted using the STCRREG command in Stata/MP 13.1 (StataCorp LP, College Station, TX). To assess the relationship between time-updated SBP and outcomes, analytical approaches such as marginal structural models (MSMs) are needed that control for the challenges created by the fact that changing level of kidney function is potentially both a consequence and a cause of elevated BP (i.e., time-updated kidney function is a time-dependent confounder) (19–21). MSM was utilized with time-updated SBP data and adjustment for the same covariates as the baseline SBP models (see appendix). All covariates with the exception of sex, race/ethnicity, education level, and hypertension awareness were time-updated. Hazards ratios and 95% confidence intervals were reported for all models. SBP was modeled in terms of hazard ratios per 10 mmHg increase and also across discrete categories (<120, 120–129, 130–139, and ≥140 mmHg). Hazard ratio estimates depicted in Table 2 using MSM and categorical SBP should be interpreted as the risk of the renal endpoint for someone whose mean SBP across study visits was always in that BP category. Additional examples of hazard ratios associated with an SBP history that included some proportion of the study period with mean SBP across different SBP categories were also calculated by weighting the regression coefficients for each of the SBP categories according to the percentage of the study period SBP fell within each of the categories. We calculated the cumulative incidence of ESRD over follow-up in four different hypothetical scenarios using the MSM models in which we assumed all participants followed the same SBP history (i.e., SBP always <120, 120–129, 130–139, and ≥140 mmHg; see appendix) (22,23).
Table 2.
Multivariable associations of baseline and time-updated systolic blood pressure with renal endpoints in the Chronic Renal Insufficiency Cohort (CRIC) Study.
| Models of Baseline SBP Hazard Ratio (95% CI) |
Models of Time-Updated SBP* Hazard Ratio (95% CI) |
|
|---|---|---|
| Renal Endpoint: ESRD | ||
|
| ||
| Continuous SBP | ||
|
| ||
| Per 10 mmHg increase | 1.09 (1.05–1.13) | 1.26 (1.18–1.34) |
|
| ||
| Categorical SBP (Reference: SBP <120) | ||
|
| ||
| SBP 120–129 | 1.07 (0.82–1.39) | 0.92 (0.54–1.56) |
| SBP 130–139 | 1.46 (1.13–1.88) | 2.37 (1.48–3.80) |
| SBP ≥140 | 1.46 (1.18–1.88) | 3.37 (2.26–5.03) |
| Renal Endpoint: ESRD/eGFR halving | ||
|
| ||
| Continuous SBP | ||
|
| ||
| Per 10 mmHg increase | 1.11 (1.07–1.15) | 1.25 (1.18––1.32) |
|
| ||
| Categorical SBP (Reference: SBP <120) | ||
|
| ||
| SBP 120–129 | 1.04 (0.83–1.30) | 1.13 (0.72–1.76) |
| SBP 130–139 | 1.49 (1.20–1.85) | 2.63 (1.79–3.86) |
| SBP ≥140 | 1.71 (1.41–2.08) | 3.66 (2.58–5.19) |
Abbreviations: CI – confidence interval; eGFR – estimated glomerular filtration rate; ESRD – end-stage renal disease; SBP – systolic blood pressure.
All models are stratified by clinical center, and adjusted for age, sex, race/ethnicity, education, hypertension awareness, history of cardiovascular disease, body mass index, number of anti-hypertensive medication classes, ACE/ARB use, diabetes status, eGFR, and urine protein-creatinine ratio.
Using marginal structural models with adjustment for all covariates listed above and study time (all time-updated with the exception of sex, race/ethnicity, education level, and hypertension awareness); HR estimates depicted using marginal structural models and using categorical SBP reflect the risk of the renal endpoint for selected scenarios in which SBP at all study visits consistently fell into that BP category.
We explored effect modification by an a priori selected set of baseline characteristics including age (<55 and ≥55 years), sex, race/ethnicity, diabetes status, level of kidney function (eGFR <45 and ≥45 mL/min/1.73m2), urine protein-creatinine ratio (<0.25 and ≥0.25 mg/mmol), and use of ACE/ARBs and calcium channel blockers. Stratified analyses of the hazard ratio of the composite renal endpoint per 10 mmHg increase in time-updated SBP using MSM across these variables were reported. All analyses with the exception of competing risk models were performed using SAS 9.3 (SAS Institute, Cary, NC) utilizing the PHREG and GENMOD procedures for baseline and time-updated analyses, respectively.
Funding
The CRIC Study is funded under cooperative agreements from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Clinical Translational Science Awards, and other NIH grants. NIDDK partnered with the CRIC Steering Committee in the design, conduct, and analysis of the study and approved the submission of the manuscript for publication.
RESULTS
At study entry, study participants had a mean SBP of 128.1 mmHg, a mean age of 58.4 years, a mean BMI of 32.1 kg/m2, a mean eGFR of 45.0 mL/min/1.73m2, and a mean urine protein-creatinine ratio of 1.1 mg/mmol (Table 1). In addition, most (91.8%) of the participants reported use of at least one anti-hypertensive medication, about two-thirds (68.7%) reported use of either an ACE or ARB, and on average they were prescribed 2 to 3 anti-hypertensive medication classes.
Table 1.
Baseline characteristics of participants from the Chronic Renal Insufficiency Cohort (CRIC) Study overall and by baseline level of systolic blood pressure.
| Baseline Systolic Blood Pressure (mmHg)* | |||||
|---|---|---|---|---|---|
| Overall n=3708 | <120 n=1430 | 120–<140 n=1281 | ≥140 n=997 | p | |
| Demographics | |||||
| Age, years | 58.4 (10.9) | 56.3 (11.7) | 59.2 (10.5) | 60.3 (9.7) | <.001 |
| Sex (% female) | 1682 (45.4%) | 657 (45.9%) | 560 (43.7%) | 465 (46.6%) | 0.32 |
| Race/ethnicity | <.001 | ||||
| Hispanic | 430 (11.6%) | 124 (8.7%) | 127 (9.9%) | 179 (18.0%) | . |
| Non-Hispanic Black | 1548 (41.8%) | 476 (33.3%) | 538 (42.0%) | 534 (53.6%) | . |
| Non-Hispanic White | 1585 (42.8%) | 772 (54.0%) | 565 (44.1%) | 248 (24.9%) | . |
| Other | 145 (3.91%) | 58 (4.1%) | 51 (4.0%) | 36 (3.6%) | . |
| Medical History / Lifestyle | |||||
| Diabetes mellitus | 1782 (48.4%) | 525 (36.7%) | 622 (48.6%) | 635 (63.7%) | <.001 |
| Current smoker | 481 (13.0%) | 178 (12.5%) | 156 (12.2%) | 147 (14.7%) | 0.147 |
| Family history of kidney disease | 578 (15.6%) | 189 (13.2%) | 218 (17.0%) | 171 (17.2%) | 0.007 |
| High school education | 2963 (79.9%) | 1242 (86.9%) | 1025 (80.0%) | 696 (69.8%) | <.001 |
| History of cardiovascular disease | 1239 (33.4%) | 406 (28.4%) | 434 (33.9%) | 399 (40.0%) | <.001 |
| Body mass index, kg/m2 | 32.1 (7.9) | 31.5 (7.7) | 32.5 (7.7) | 32.5 (8.2) | 0.002 |
| Blood Pressure | |||||
| Systolic blood pressure, mmHg | 128.1 (21.8) | 107.7 (8.7) | 128.87 (5.7) | 156.3 (15.3) | <.001 |
| Diastolic blood pressure, mmHg | 71.3 (12.7) | 65.2 (9.7) | 72.3 (11.2) | 78.9 (13.9) | <.001 |
| Antihypertensive medication use | |||||
| Any antihypertensive drugs | 3405 (91.8%) | 1237 (86.5%) | 1203 (93.9%) | 965 (96.8%) | <.001 |
| # antihypertensive drug classes | 2.6 (1.5) | 2.2 (1.5) | 2.7 (1.5) | 3.1 (1.5) | <.001 |
| ACE inhibitor or ARB | 2549 (68.7%) | 970 (67.8%) | 884 (69.0%) | 695 (69.7%) | 0.65 |
| Calcium channel blockers | 1505 (40.6%) | 399 (27.9%) | 575 (44.9%) | 531 (53.3%) | <.001 |
| Biochemical markers | |||||
| Estimated GFR, mL/min/1.73m2 | 45.0 (16.8) | 47.9 (18.1) | 45.4 (16.1) | 40.3 (14.7) | <.001 |
| Urine protein-creatinine ratio | 1.12 (2.64) | 0.45 (1.10) | 0.97 (2.27) | 2.29 (3.97) | <.001 |
Abbreviations: ACE – angiotensin-converting enzyme; ARB – angiotensin receptor blocker; GFR –glomerular filtration rate. P-values were generated using ANOVA and chi-square, as appropriate.
Characteristics are provided as mean (standard deviation) or N (%).
The median (25th, 75th percentiles) duration of follow-up was 5.7 (4.6, 6.7) years. The within-participant mean SBP over time ranged from 74 to 218 mmHg (mean (SD): 128.6 (19.1)). Variability of time-updated SBP from baseline was within 10, 10–<20, 20–<30, and ≥30 mmHg for 24.8%, 25.8%, 22.7%, and 26.7% of participants, respectively. A total of 33.1%, 19.2%, and 10.6% of the participants, respectively, had SBP at or above 120, 130, and 140 mmHg at all of their study visits.
Over follow-up, 699 participants developed ESRD and 921 reached the composite renal endpoint (event rates: 38.0 and 59.9 per 1,000 person-years, respectively; Figure 1). Event rates were substantially higher at higher levels of baseline SBP. Figure 2 depicts the estimated cumulative incidence of ESRD across time-updated SBP categories. After five years of follow-up the estimated cumulative incidence of ESRD among those with time-updated SBP between 130–139 and ≥140 mmHg was 28.3% and 35.4% compared to 15.0% and 14.0% among those with SBP <120 and 120–129 mmHg.
Figure 1.
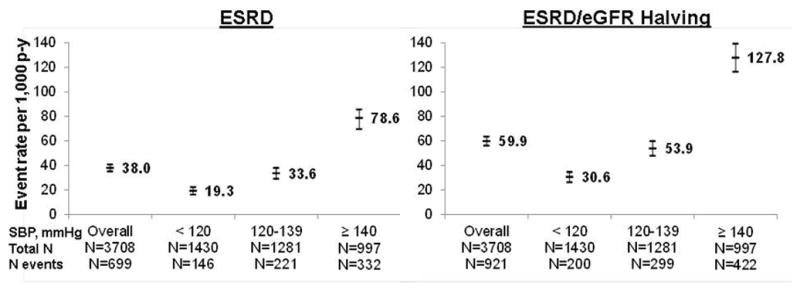
Crude event rates (95% confidence intervals) of ESRD and the renal composite endpoint of ESRD or halving of eGFR from baseline, overall and by level of SBP at baseline in the Chronic Renal Insufficiency Cohort (CRIC) Study.
Abbreviations: eGFR – estimated glomerular filtration rate; ESRD – end-stage renal disease; p-y – person-years; SBP – systolic blood pressure.
Figure 2.
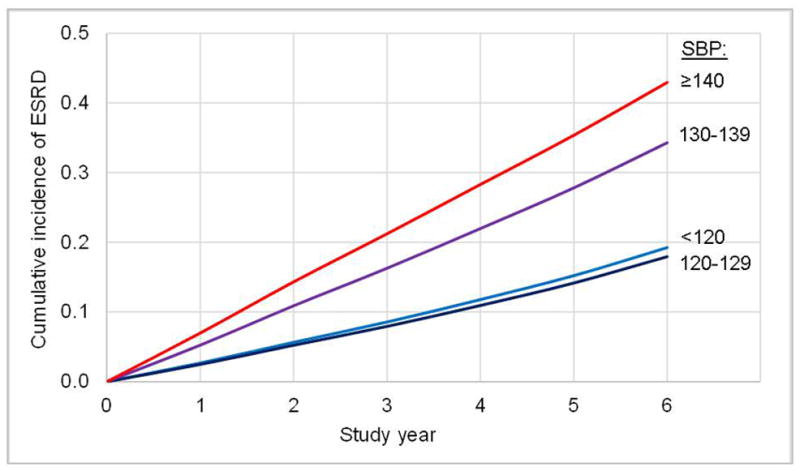
Estimated cumulative incidence of ESRD across categories of time-updated SBP among participants of the Chronic Renal Insufficiency Cohort (CRIC) Study.
Abbreviations: ESRD – end-stage renal disease; SBP – systolic blood pressure.
After multivariable adjustment, each 10 mmHg increase in baseline compared to time-updated SBP was significantly associated with a 9% compared to a 26% higher rate of ESRD, and an 11% compared to 25% higher rate of the composite renal endpoint, respectively (Table 2). Analyses of baseline and time-updated mean BP using diastolic BP, mean arterial pressure, and pulse pressure yielded similar results (data not shown). Participants with baseline SBP >130 mmHg had significantly increased risk for renal endpoints compared to those whose baseline SBP was below 120 mmHg. Hazard ratios from sensitivity analyses of baseline SBP treating death as a competing risk, rather than a censoring event, were only slightly attenuated and demonstrated similar patterns to the primary analyses. Participants always having a mean time-updated SBP 130–139 or ≥140 mmHg had a 2.4- to nearly 4-fold higher rate of the renal endpoints compared to those with mean SBP <120 mmHg (reference group; Table 2). Participants with mean time-updated SBP falling within 130–139 mmHg for half of the study period and ≥140 mmHg for the remaining half of the study period had a 2.8-fold higher rate of ESRD compared to those mean SBP was always <120 mmHg (data not shown). Additionally, those with mean SBP between 120–129, 130–139, and ≥140 mmHg, each for one-third of their study period, had 1.9-fold increased rates of ESRD compared to the reference group.
The strength of the association between SBP and the composite renal endpoint using time-updated SBP did not differ significantly across subgroups stratified on age, sex, race/ethnicity, diabetes, baseline eGFR, proteinuria, or anti-hypertensive medication usage (Figure 3).
Figure 3.
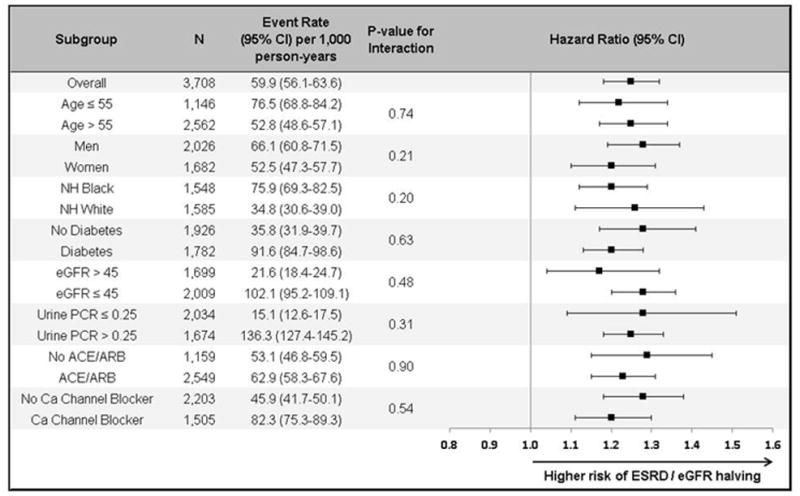
Forest plot of crude event rates (95% confidence intervals) and multivariable-adjusted hazard ratios per 10 mmHg increase in mean SBP over time on development of ESRD or halving of eGFR overall and by subgroups using marginal structural models.
Stratified by clinical center, and adjusted for age, gender, race/ethnicity, education level, history of cardiovascular disease, number of anti-hypertensive medication drug classes taken, use of an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, hypertension awareness, BMI, diabetes, level of eGFR, urine protein-to-creatinine ratio and study time;
Abbreviations: ACE – angiotensin converting enzyme inhibitor; ARB – angiotensin receptor blocker; Ca – calcium; CI – confidence interval; eGFR – estimated glomerular filtration rate; ESRD – end-stage renal disease; NH – non-Hispanic; PCR – protein-to-creatinine ratio; SBP – systolic blood pressure.
Age units: years; eGFR units: mL/min/1.73m2; PCR units: mg/mmol.
DISCUSSION
We investigated and compared the association between elevated BP and CKD progression utilizing baseline and time-updated SBP with appropriate adjustment for time-updated covariates in a well-characterized cohort with mild to moderate CKD. Our analyses of baseline BP on renal endpoints were similar in magnitude to prior reports (2,3). As we had hypothesized, we demonstrated a stronger association between achieved BP and renal endpoints using time-updated SBP and marginal structural analysis with appropriate handling of time-dependent confounding. In particular, we observed a 2.6-fold increased risk of the composite renal endpoint for those whose mean SBP across study visits was always 130–139 mmHg compared to <120 mmHg. Our findings underscore that prolonged exposure to SBP ≥130 mmHg among those with and without proteinuria and with and without diabetes is associated with important increases in the risk of CKD progression.
Achieved BP analyses to-date are highly consistent in demonstrating a graded increase in renal events associated with higher BP (2,3,24,25). Indeed, an achieved BP analysis of data from the African-American Study of Kidney Disease and Hypertension (AASK), but not its original intention-to-treat analysis, recapitulated previous achieved BP findings (26). However, these achieved BP analyses typically report associations substantially lower in magnitude compared to our MSM findings with time-updated SBP. We postulate that the disparate findings may reflect methodological shortcomings of previous work. First, most earlier studies relied on BP measurements from a single time point. This is an important limitation given the variability of BP over time, especially in the setting of CKD. These analyses investigated risk based on snapshots of BP exposure and are unable to relate prolonged BP history to outcomes. Second, reports utilizing time-updated BP used traditional statistical modeling techniques (i.e., time-updated Cox regression) that inadequately adjusted for eGFR over time given its role in this setting as a time-dependent confounder (24–26). Lastly, several key confounders including proteinuria were often not adjusted for or were inadequately characterized, thus potentially introducing meaningful residual confounding. The current study is the first to our knowledge to report, in an unbiased fashion, the impact of a history of elevated, achieved BP on CKD progression.
Within clinical trials, varying targets for optimal BP control have been used and inconsistent findings have been reported (4–7,29–35). Additionally, these studies have reported differential effects of BP lowering across subgroups with and without diabetes, and with and without proteinuria. In an attempt to address the variability in findings from clinical trials, recent meta-analyses of pharmacologic clinical trials of intensive BP lowering have been performed. The first, primarily in persons with non-diabetic CKD, concluded that intensive BP lowering significantly reduced risk of CKD progression, but only among those with proteinuria (27). The second meta-analysis among persons with diabetes demonstrated reductions in the level of proteinuria, but not in the rate of CKD progression (28). These analyses of intention-to-treat data from clinical trials conflict with many of the findings from the current achieved BP analysis – namely that “lower” levels of SBP beginning at 130 mmHg are associated with increased risk for CKD progression among all subgroups. These differences likely arise from several factors including the fundamental differences in questions addressed by intention-to-treat versus achieved BP analyses, differing study populations, varying success across subgroups in reaching lower BP goals in clinical trials, and potentially residual confounding in our achieved BP analyses. Additional large clinical trials such as the Systolic Blood Pressure Intervention Trial (SPRINT) should further elucidate the impact of BP lowering on important outcomes including kidney outcomes, especially if analyses include both intention-to-treat and achieved BP analyses using MSM or similar methods.
Our study had a number of positive features. The CRIC Study is a large, multi-center, prospective study of mild to moderate CKD including similar proportions of those with and without diabetes. The study population is comprised of a diverse population of men and women, non-Hispanic whites, non-Hispanic blacks and Hispanics, with a wide age range, and broad set of underlying causes of CKD. CRIC participant retention is excellent (90% retained and actively under study as of the Year 5 visit), and linkage with USRDS and national death databases maximizes capture of primary study endpoints. Extensive annual data collection included standardized measurement of BP, kidney function, proteinuria, and numerous other relevant factors. BP measurements were performed by highly trained research personnel in triplicate. As such, CRIC BP data are likely more accurate than regularly acquired office measurements. There are also important limitations. First, we measured BP only once each year which may not accurately reflect BP levels over the entire year and may have resulted in misclassification of our key exposure. Second, we lacked data on some potentially important unmeasured confounders such as duration of hypertension or BP levels prior to enrollment into the study, and adherence to anti-hypertensive therapies. Third, data on anti-hypertensive medication use were self-reported and reflected only the 30 days preceding any study visit, which could have led to misclassification of this important time-varying exposure. Fourth, we may have lacked power to detect significant effect modification by level of proteinuria because of the relatively low levels of protein excretion among the majority of CRIC Study participants. Finally, the observational (non-randomized) design of our study precludes definitive determination of optimal target level of BP for CKD patients.
The current study confirmed previous reports from observational studies of the relationship between a single (baseline) elevated measure of BP and a higher rate of renal endpoints. However, utilization of time-updated BP with appropriate adjustment for updated covariates revealed a considerably larger magnitude of association between elevated SBP and CKD progression – a previously unreported finding. This study also suggests that prolonged exposure to SBP over 130 mmHg may portend increased risk for progressive loss of kidney function among persons with CKD regardless of diabetes or proteinuria status. The relevance of these findings for clinical practice guidelines must be assessed within the context of existing and emerging evidence from other observational and interventional studies.
Supplementary Material
Acknowledgments
The authors would like to acknowledge and thank the CRIC Study participants, study coordinators, and investigators for their efforts. Additionally, the authors thank Valerie Teal for analytical support.
Role of the Funding Source
Funding under cooperative agreements from NIH/NIDDK (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902) and supported in part by the following institutional Clinical Translational Science Awards (CTSA) and other National Institutes of Health grants: University of Pennsylvania NIH/NCATS UL1TR000003, K01DK092353, and K24DK002651, Johns Hopkins University UL1 TR-000424, University of Maryland General Clinical Research Center (GCRC) M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. Dr. Kusek (NIDDK) contributed to and approved submission of this manuscript. Dr. Anderson had full access to all the study data and has final responsibility for this submission. The views expressed in this article do not necessarily reflect those of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
Author Contributions
Author contributions to this work included literature search (AHA), generation of figures (AHA), study/analytical design (AHA, WY, RRT, GMC, JWK, JH, JPL, ERM, MR, SS, MW, JTW, HIF), data collection (AHA, RRT, JC, JH, RK, JPL, MR, SS), data analysis (WY, QP), data interpretation (AHA, WY, RRT, QP, GMC, JWK, JC, JH, RK, JPL, ERM, MR, SS, MW, JTW, HIF), and writing (AHA, WY, RRT, GMC, JWK, MR, HIF).
This abstract was presented at the American Society of Nephrology Kidney Week 2012 under the title, “Is Time-Updated Blood Pressure Related to End-Stage Renal Disease in the Setting of Chronic Kidney Disease after Accounting for Kidney Function over Time? Findings from the Chronic Renal Insufficiency Cohort (CRIC) Study.”
Conflicts of Interest
Dr. Chertow serves on the Board of Directors for Satellite Healthcare and PuraCath. He has received research support from Amgen, Keryx and Reata. He has served as an advisor to Allocure, Amgen, Ardelyx, Astra Zeneca, Gilead, Hemodialysis Plus, Keryx and Thrasos.
Dr. Townsend receives grant support from NIH, is a consultant for Janssen, Merck, GSK, Novartis, receives royalties from UpToDate, Jones & Bartlett, and has received honoraria or travel stipends from the American Society of Nephrology, American Society of Hypertension, and National Kidney Foundation.
Dr. Steigerwalt receives grant support from Medtronic, is a consultant for ATCOR, and has received honoraria from Takeda.
The remaining authors have no relevant conflicts to disclose.
REFERENCE LIST
- 1.United States Renal Data System (USRDS) USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2013. [Accessed last on June 17, 2014]. http://www.usrds.org/2013/pdf/v1_ch2_13.pdf. [Google Scholar]
- 2.Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 3.Tozawa M, Iseki K, Iseki C, Kinjo K, Ikemiya Y, Takishita S. Blood pressure predicts risk of developing end-stage renal disease in men and women. Hypertension. 2003;41:1341–1345. doi: 10.1161/01.HYP.0000069699.92349.8C. [DOI] [PubMed] [Google Scholar]
- 4.Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. Modification of Diet in Renal Disease Study Group. N Engl J Med. 1994;330:877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 5.Wuhl E, Trivelli A, Picca S, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361:1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 6.Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 7.Appel LJ, Wright JT, Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363:918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 9.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 10.Kidney Disease Improving Global Outcomes. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3 doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 11.Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 13.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer MJ, Go AS, Lora CM, et al. CKD in Hispanics: Baseline characteristics from the CRIC (Chronic Renal Insufficiency Cohort) and Hispanic-CRIC Studies. Am J Kidney Dis. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson AH, Yang W, Hsu CY, et al. Estimating GFR among participants in the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2012;60:250–261. doi: 10.1053/j.ajkd.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang W, Xie D, Anderson AH, et al. Association of Kidney Disease Outcomes With Risk Factors for CKD: Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2013 doi: 10.1053/j.ajkd.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joffe M, Hsu CY, Feldman HI, Weir M, Landis JR, Hamm LL. Variability of creatinine measurements in clinical laboratories: results from the CRIC study. Am J Nephrol. 2010;31:426–434. doi: 10.1159/000296250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 19.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Yang W, Joffe MM. Subtle issues in model specification and estimation of marginal structural models. Pharmacoepidemiol Drug Saf. 2012;21:241–245. doi: 10.1002/pds.2306. [DOI] [PubMed] [Google Scholar]
- 22.Joffe MM, Greenland S. Standardized estimates from categorical regression models. Stat Med. 1995;14:2131–41. doi: 10.1002/sim.4780141907. [DOI] [PubMed] [Google Scholar]
- 23.Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: An application to post-menopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19:766–79. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition. A patient-level meta-analysis. Ann Intern Med. 2003;139:244–52. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 25.Sim JJ, Shi J, Kovesdy CP, Kalantar-Zadeh K, Jacobsen SJ. Impact of achieved blood pressures on mortality risk and end-stage renal disease among a large, diverse hypertension population. J Am Coll Cardiol. 2014;64:588–97. doi: 10.1016/j.jacc.2014.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis EM, Appel LJ, Wang X, et al. Limitations of analyses based on achieved blood pressure: Lessons from the African American Study of Kidney Disease and Hypertension Trial. Hypertension. 2011;57:1061–8. doi: 10.1161/HYPERTENSIONAHA.111.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lv J, Ehteshami P, Sarnak MJ, et al. Effects of intensive blood pressure lowering on the progression of chronic kidney disease: a systematic review and meta-analysis. CMAJ. 2013;185:949–957. doi: 10.1503/cmaj.121468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation. 2011;123:2799–810. 9. doi: 10.1161/CIRCULATIONAHA.110.016337. [DOI] [PubMed] [Google Scholar]
- 29.Wright JT, Jr, Bakris G, Greene T, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 30.Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142:342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 31.Ruggenenti P, Perna A, Loriga G, et al. Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicentre, randomised controlled trial. Lancet. 2005;365:939–946. doi: 10.1016/S0140-6736(05)71082-5. [DOI] [PubMed] [Google Scholar]
- 32.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–1462. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 33.Lewis JB, Berl T, Bain RP, Rohde RD, Lewis EJ. Effect of intensive blood pressure control on the course of type 1 diabetic nephropathy. Collaborative Study Group. Am J Kidney Dis. 1999;34:809–817. doi: 10.1016/s0272-6386(99)70036-3. [DOI] [PubMed] [Google Scholar]
- 34.Estacio RO, Jeffers BW, Gifford N, Schrier RW. Effect of blood pressure control on diabetic microvascular complications in patients with hypertension and type 2 diabetes. Diabetes Care. 2000;23(Suppl 2):B54–B64. [PubMed] [Google Scholar]
- 35.Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


