Abstract
Proton pump inhibitors (PPIs) have been associated with diminished bone mineral density (BMD) and an increased risk of fracture; however, prior studies have not yielded consistent results, and many have suboptimal ascertainment of both PPI use and BMD. We used data from the Study of Women’s Health Across the Nation (SWAN), a multicenter, multi-ethnic, community-based longitudinal cohort study of women across the menopause transition to examine the association between annualized BMD changes and new use of PPIs. We compared changes in BMD in new PPI users with changes in BMD in new users of histamine 2 receptor antagonists (H2RAs) and with changes in BMD in subjects who did not use either class of medications. Mixed linear regression models included recognized risk factors for osteoporosis, including demographics, menopausal transition stage, body mass index (BMI), lifestyle factors, as well as comorbidities and concomitant medications. To provide further evidence for the validity of our analytic approach, we also examined the effects of hormone-replacement therapy (HT), a class of medications that should reduce bone loss, on changes in BMD as an internal positive control group. We identified 207 new users of PPIs, 185 new users of H2RAs, and 1,676 non-users. Study subjects had a mean age of 50 years and were followed for a median of 9.9 years. Adjusted models found no difference in the annualized BMD change at the lumbar spine, femoral neck, or total hip in PPI users compared with H2RA users or non-users. These results were robust to sensitivity analyses. BMD increased as expected in HT users, supporting the validity of our study design. These longitudinal analyses plus similar prior studies argue against an association between PPI use and BMD loss.
Keywords: BONE MINERAL DENSITY, PROTON PUMP INHIBITORS, HORMONE-REPLACEMENT THERAPY, HISTAMINE 2 RECEPTOR ANTAGONISTS
The musculoskeletal system is metabolically active and responsive to many stimuli, including hormonal and immunologic inputs.(1,2) The responsiveness of the skeleton has facilitated development of drugs that enhance bone mineral density (BMD) and reduce the risk of fractures.(3,4) However, it has been increasingly recognized that many commonly used medications may have deleterious effects on the skeleton. Proton pump inhibitors (PPIs) represent one such category of medication suspected of having a negative impact on the skeleton and possibly increasing the risk of osteoporosis and fractures.
There have been at least four published longitudinal epidemiologic studies examining the effects of PPIs on BMD (Table 1).(5–8) Although several studies have found baseline differences in BMD between PPI users and non-users, none has found consistent associations at most anatomic sites in longitudinal analyses. Moreover, these studies have important methodologic limitations that may hinder interpretation of their findings. First, assessment of PPI use was infrequent—once every 3 to 5 years—as was the testing of BMD. Second, three of the four studies compared PPI use with non-use, without adjusting for potential confounding. Although one study did adjust for gastrointestinal diagnoses, none used other acid-suppressive treatments, such as H2 receptor antagonists (H2RAs), as an alternative control group. Moreover, none employed a new user design, whereby a true BMD baseline (pre-PPI) can be established; this design is thought to be optimal for drug epidemiology.(9)
Table 1.
Prior Longitudinal Studies Examining the Adjusted Relationship Between Proton Pump Inhibitors (PPI) and Bone Mineral Density (BMD)
| Author (year) | Study population | PPI assessment | BMD assessment | Adjusted resultsa,b |
|---|---|---|---|---|
| Yu (2008)(5) | n = 4230 men aged ≥65 years | PPI use defined as taking medication for previous 4 weeks to clinic visit. | SOF: baseline and 4.9 years later. | PPIs had no significant effect on change in BMD at total hip. |
| n = 2856 women aged ≥65 years | SOF: baseline and 4.9 years later. MrOS: baseline and 4.6 years later. | MrOS: baseline and 4.6 years later. | Annual change in BMD: | |
| Subjects in the Osteoporotic Fractures in Men Study (MrOS) or women from the Study of Osteoporotic Fractures (SOF) | Assessed at the hip. Annualized % change. | Total hip: −0.70 (p = 0.08) (women) Total hip: −0.38 (p = 0.39) (men) | ||
| Targownik (2010)(6) | N = 2549 subjects | Drug history for 5 years before baseline. PPI user if subject with use of PPI on ≥50% of the days (standard dose). | Change assessed for the lumbar spine and the total hip. | PPIs had no significant effect on change in BMD at standard or high-intensity doses. |
| Subjects from the Manitoba Bone Mineral Density Database | Assessments separated by 1 to 3 years. | Annualized change in BMD: | ||
| Annualized % change. | Spine: 0.03%±0.22% (p > 0.2) (standard dose PPI) | |||
| Total hip: −0.17%±0.18% (p > 0.2) (standard dose PPI) | ||||
| Gray (2010)(8) | n = 6695 (hip); n = 6629 (spine) | Assessed at baseline and year 3. Duration of PPI use described as <1 year, 1 to 3 years, or >3 years. | Assessed at baseline and years 3 and 6 | PPIs had no significant effect on change in BMD when time- varying PPI use was included. |
| Women aged 50 to 79 years from Women’s Health Initiative. | Assessed for total hip and spine. | Three-year changes in BMD including women on Ca/Vit D (PPI not considered as time-varying): | ||
| Three-year % change. | Hip: 0.62 (PPI use) versus 1.36 (PPI non-use) (p = 0.05) | |||
| Spine: 2.30 (PPI use) versus 1.34 (PPI non-use) (p = 0.94) | ||||
| Targownik (2012)(7) | n = 8340 completed baseline; n = 6458 completed 5-year assessment; n = 4512 completed 10-year assessment | Assessed at baseline, year 5, and year 10. PPI user if reported PPI use at all time points included in the analysis. | Assessed at baseline and years 5 and 10. | PPIs had no significant effect on change in BMD. |
| Population-based stratified random sample of Canadian population (CaMOS) aged ≥25 years. | Assessed for femoral neck, total hip, and spine. | 10-year change in BMD: | ||
| 10-year % change. | Hip: 0.9% (−1.0 to 2.9%) (p = NS) | |||
| Femoral neck: −0.5% (−2.9 to 1.8%) (p = NS) | ||||
| Spine: −0.7% (−3.3 to 1.9%) (p = NS) |
Note: Studies without a non-user group were excluded.
Covariates differed by study. Yu (2008) included age, race, BMI, alcohol use, exercise, corticosteroids, NSAIDs, calcium supplements, osteoporosis medications, self-reported health, concurrent weight change, initial total hip BMD, caffeine intake, smoking, and history of stomach surgery. Targownik (2010) included age, sex, BMI, cardiovascular disease, diabetes, hypertension, COPD, renal disease, cirrhosis, thyroid disease, alcohol abuse, inflammatory bowel disease, celiac disease, oral ERT, SERMs, calcitonin, bisphosphonates, systemic corticosteroids (>5mg prednisone per day), anti-androgens, tamoxifen, anti-epileptics, SSRIs, and thiazide diuretics. Targownik (2012) included age, sex, age-sex interactions, BMI, history of minimal trauma fracture, family history of minimal trauma fracture, presence of RA, IBD, CLD, CKD, thyroid disease, smoking, heavy alcohol use (≥14 drinks per week), history of falls, use of concomitant medications that may influence BMD (corticosteroids, thiazides β-blockers, nitrates, anti-epileptics, SSRIs, tamoxifen, and osteoporosis medications, total calcium intake, and vitamin D intake). Gray (2010) included age, race/ethnicity, BMI, smoking, physical activity level, self-reported health, parental history of hip fractures, diabetes mellitus, history of fractures, corticosteroid use, physical function construct, history of myocardial infarction or angina, history of asthma or emphysema, arthritis, osteoporosis, psychoactive medications use, past or current use of hormone therapy or bisphosphonates, moderate or severe stomach ulcers, moderate or severe heartburn, WHI trial participation, and intervention arms.
Only results from adjusted longitudinal analyses are presented.
Because multiple studies have reported that fracture risk is increased in current users of PPIs, many patients are concerned that they may increase their risk of developing osteoporosis if they use PPIs.(10–12) A clear answer about whether PPIs cause reductions in BMD, possibly putting some users at risk of fracture, would have major public health implications. PPIs are among the most widely utilized medications in the United States, often with ambiguous indications and questionable evidence of efficacy.(13) Because efficacy may be unclear in many PPI users, it is particularly important to determine whether these agents are safe. If PPIs predispose patients to osteoporosis without clear evidence of benefits, changes in their indications and availability would be indicated.
To address limitations presented by prior work, we compared longitudinal BMD changes in women who initiated PPI use with BMD changes in women who initiated use of H2RAs and with BMD changes in women who did not use either class of medications. This study was conducted among women participating in the Study of Women’s Health Across the Nation (SWAN), a large multi-ethnic, community-based, longitudinal cohort of women transitioning through the menopause. To provide additional evidence for the validity of our study design, we performed similar analyses in women taking hormone-replacement therapy (HT), a class of drugs known to increase BMD and thus who could serve as a “positive control” group.
Materials and Methods
Study design
The Study of Women Across the Nation (SWAN) is a community-based, multi-ethnic longitudinal observational cohort study of the menopause transition that enrolled 3302 pre- or early perimenopoausal women between the ages of 42 and 52 years at seven clinical sites across the United States. After enrollment between 1996 to 1998, women are seen annually to monitor a wide variety of measures, with 5 of the 7 sites measuring BMD annually. Information on medication use is collected prospectively at all sites. A detailed description of the study design has been published previously.(14)
The primary question of the current study was whether use of PPIs accelerates the rate of bone loss in midlife women. To address this question, we compared the annualized rate of change in BMD among subjects in SWAN who initiated PPIs with the rates of change in BMD in users of H2RAs and in women who did not report use of either class of medications. The annualized change in BMD was calculated as the annual percent change in a linear regression model, facilitating comparison of results across study groups. The change was calculated from a baseline BMD value determined at the visit before the first use of a PPI or H2RA. For participants not reporting use of these medications, we randomly selected a frequency-matched visit to establish a comparable baseline. To provide additional evidence supporting the validity of our study methods, we employed the same methods to test whether HT initiation was associated with a reduction in rates of bone loss (a positive control exposure).
Study sample
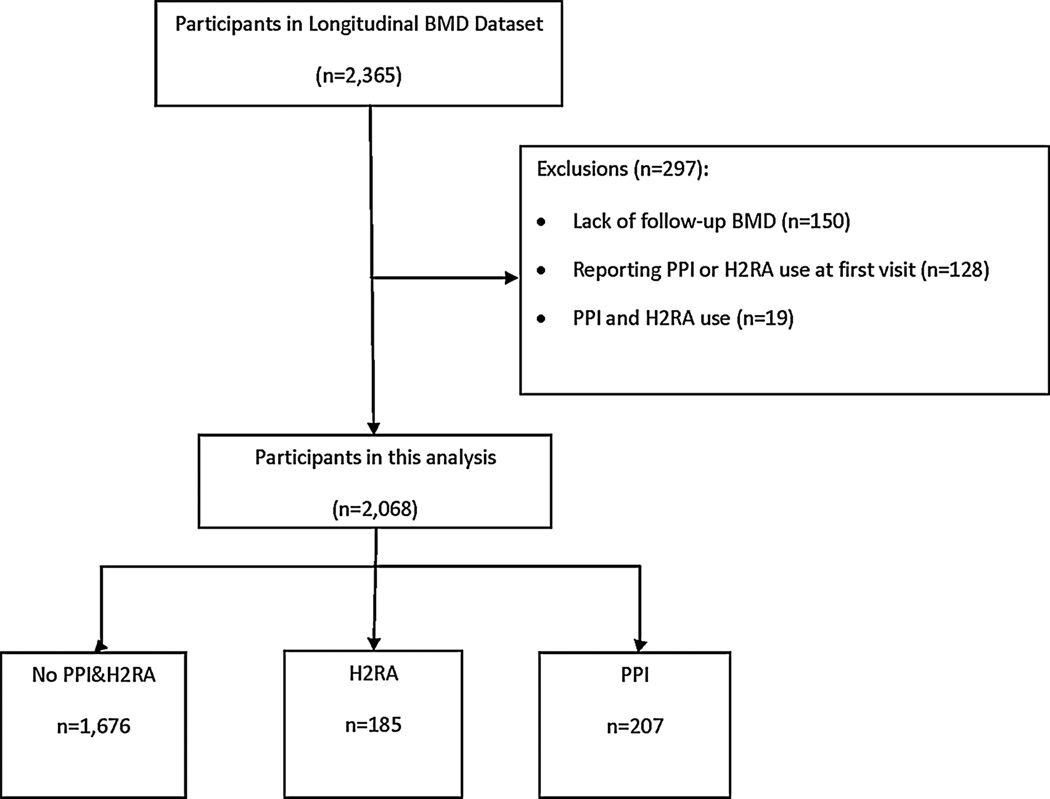
We constructed two samples, one to examine the associations between PPI or H2RA use and bone loss (cohort 1, Fig. 1) and the second to examine the relation between HT use and bone loss (cohort 2). To construct cohort 1, we first identified new users of a PPI or H2RA by excluding participants who reported use of either type of medication at the first SWAN visit. Participants who reported PPI or H2RA use at a subsequent visit were identified as new users. Additionally, subjects were required to have undergone at least two BMD measurements after the study baseline (see the section above for definition of baseline for drug users and non-users). We censored subjects reporting use of both a PPI and H2RA at the same visit. New users who discontinued use of these medications were censored at the last annual visit when usage of the drug was reported; they were also censored when a woman using a PPI added an H2RA or vice versa. Women who became pregnant were also censored at the visit before reporting pregnancy. Cohort 2 was created in an analogous fashion.
Fig. 1.
Study Cohort 1.
Written informed consent was obtained from all study participants. The informed consent procedures, study protocol, and forms were approved by all SWAN site Institutional Review Boards.
Assessment of medication use
At each visit, interviewers administered questionnaires to ascertain all medication use since the last study visit, and use was verified by inspection of medication containers. Type of medication was classified from product brand or generic names using a computerized medication dictionary (Iowa Drug Information Service [IDIS] Drug Vocabulary, College of Pharmacy, University of Iowa, Iowa City, IA, USA). PPI and H2RA use was assessed at each visit to determine ongoing use. Dosage of PPIs and H2RAs was not available in the study data set.
Measurement of bone mineral density
BMD of the lumbar spine, femoral neck, and total hip (g/cm2) was measured by dual-energy X-ray absorptiometry (DXA) using Hologic instruments (Hologic Inc., Waltham, MA, USA). Three sites used Hologic 4500A models throughout, and two sites upgraded from the 2000 to 4500A models during follow-up but cross-calibrated the machines. Each DXA laboratory measured a Hologic anthropomorphic spine phantom daily, and a phantom was circulated among laboratories for cross-calibration. Phantom measurements were analyzed by Synarc, Inc. (Waltham, MA, USA) and calibration regression coefficients used by the study’s coordinating center to adjust DXA measurements for minor temporal or geographic variations in densitometer performance. Additional quality-control measures included review of every scan image by a local site investigator and central review or a random subset of 5% of all scans and all problem scans by Synarc, Inc.(14,15) Short-term in vivo measurement variability was 0.014 g/cm2 (1.4%) for the lumbar spine and 0.016 g/cm2 (2.2%) for the femoral neck.
BMD measurements were available through the 10th annual SWAN visit for this study.
Osteoporosis risk factors (covariates)
We considered several variables at the baseline visit as potential confounders and included them in adjusted analyses. SWAN participants underwent measurement of height and weight for calculation of body mass index (BMI, weight in kilograms divided by the square of height in meters). They completed interviewer-administered or self-administered questionnaires that assessed demographic characteristics (age, race, ethnicity, income, marital status, education), lifestyle factors (tobacco use, alcohol intake), self-assessed health status, social support (4 items from the 20-item Medical Outcomes Study Social Support Survey),(16) vasomotor symptoms, medication use (bisphosphonates, hormone- replacement therapy, oral glucocorticoids, and thiazide diuretics), and self-reported comorbid conditions (osteoporosis, thyroid disease, any cancer, diabetes mellitus). In addition, physical activity was measured using a modified version of the Baecke Physical Activity Questionnaire (range 3 to 15),(17,18) but it was not measured at every visit. Menopause transition stage was assessed in SWAN based on bleeding criteria. Categories were: premenopause (no decreased regularity in menstrual bleeding during the last year), early perimenopause (decreased menstrual regularity in the past year and menstrual bleeding in the past 3 months), late perimenopause (no menses for 3 to 11 months), and postmenopause (no menses for 12 or more months). Women reporting hysterectomy or oophorectomy were classified as surgically menopausal. Menopause transition stage was updated at every study visit.
Follow-up measurements were introduced for time-varying covariates, including menopausal transition stage, vasomotor symptoms, cancer, diabetes mellitus, and the use of bone active agents.
Statistical analysis
We described the baseline subject characteristics in each of the exposure groups using descriptive statistics (mean, median, and range). Continuous variables were analyzed using ANOVA and Kruskal-Wallis tests, whereas categorical variables were analyzed using chi-square tests. Variables were transformed where necessary. The relationship between medication use and annual change in BMD (change between two subsequent annual BMD measurements) was analyzed using a mixed-effects regression modeling strategy, allowing for a random intercept and slope.(19) Factors selected a priori for inclusion in the primary models included years from medication initiation as a continuous linear covariate and several baseline covariates known to be possible correlates of BMD: study site, race/ethnicity (white, African-American, Chinese, Japanese), BMI, age, and menopause transition stage (time-varying).
We also tested covariates of interest (all those listed in Table 2) in sensitivity analyses. Only those covariates with p values < 0.10 in the unadjusted mixed model were entered into the models with the a priori variables from the primary models. Final adjusted sensitivity analysis models included only those covariates with p values < 0.05 in the multivariable mixed-effects regression models. Variables included in these sensitivity analyses differed by anatomic site of BMD outcome (spine: primary model covariates+smoking, diabetes, cancer, osteoporosis, vasomotor symptoms, hormone-replacement therapy, bisphosphonates, thiazide diuretic use, race * PPI use; femoral neck: primary model covariates+income, educational level, physical activity, cancer, diabetes, hormone-replacement therapy, bisphosphonates, thiazide diuretic use; total hip: primarymodel covariates+educational level, physical activity, cancer, diabetes, hormone-replacement therapy, bisphosphonates, thiazide diuretic use).
Table 2.
Baseline Characteristics of Three Groups Studied in Cohort 1
| Characteristic | Non users (n = 1676) | H2RA users (n = 185) | PPI users (n = 207) | p Value |
|---|---|---|---|---|
| n (%) or mean ± SD | ||||
| Age (years) | 50.2 (±3.9) | 49.6 (±4.0) | 50.7 (±4.2) | <0.0001 |
| Race/ethnicity | <0.0001 | |||
| Black | 421 (25.1) | 49 (26.5) | 79 (38.2) | |
| White | 826 (49.3) | 105 (56.8) | 99 (47.8) | |
| Chinese | 215 (12.8) | 15 (8.1) | 3 (1.5) | |
| Japanese | 214 (12.8) | 16 (8.7) | 26 (12.6) | |
| Menopausal status | <0.0001 | |||
| Surgical menopause | 67 (4.0) | 8 (4.3) | 14 (6.8) | |
| Postmenopausal | 392 (23.4) | 35 (18.9) | 66 (32.2) | |
| Late perimenopausal | 109 (6.5) | 13 (7.0) | 23 (11.2) | |
| Early perimenopausal | 724 (43.2) | 73 (39.5) | 67 (32.7) | |
| Premenopausal | 278 (16.6) | 32 (17.3) | 22 (10.7) | |
| Hormone therapy | 100 (6.0) | 24 (13.0) | 13 (6.3) | |
| Self-reported health status, n (%) | 0.0003 | |||
| Excellent or good | 1444 (86.2) | 143 (79.4) | 167 (81.5) | |
| Fair | 192 (11.5) | 28 (15.6) | 32 (15.6) | |
| Poor or very poor | 23 (1.4) | 9 (5.0) | 6 (2.9) | |
| Current smoker | 212 (12.7) | 30 (16.5) | 28 (13.7) | 0.29 |
| Calcium supplement use | 997 (59.5) | 99 (53.5) | 129 (62.3) | 0.21 |
| Vitamin D supplement use | 987 (58.9) | 95 (51.4) | 129 (62.3) | 0.09 |
| BMI (kg/m2) | 27.6 (±6.7) | 30.4 (±7.4) | 29.9 (±7.5) | <0.0001 |
| Physical activity | 7.83 (±1.61) | 7.56 (±1.53) | 7.35 (±1.63) | <0.0001 |
| Bone mineral density (g/cm2) | ||||
| Lumbar spine | 1.16 (±0.24) | 1.07 (±0.14) | 1.07 (±0.16) | 0.11 |
| Femoral neck | 0.78 (±0.12) | 0.83 (±0.13) | 0.83 (±0.13) | 0.43 |
| Total hip | 0.94 (±0.20) | 0.96 (±0.14) | 0.97 (±0.14) | 0.03 |
| Comorbidities | ||||
| Heart attack/stroke/angina | 5 (0.3) | 0 (0.0) | 3 (1.5) | 0.16 |
| Thyroid disease | 138 (8.2) | 18 (9.7) | 29 (14.1) | 0.04 |
| Cancer | 21 (1.3) | 5 (2.7) | 4 (2.0) | 0.29 |
| Diabetes | 78 (4.7) | 13 (7.0) | 16 (7.8) | 0.003 |
| Osteoporosis | 30 (1.8) | 3 (1.6) | 7 (3.4) | 0.21 |
| Vasomotor symptoms | 802 (47.9) | 105 (56.8) | 131 (63.3) | <0.0001 |
| Medication use at baseline | ||||
| Hormone therapy | 200 (11.9) | 36 (19.5) | 30 (14.5) | 0.0002 |
| Bisphosphonate | 22 (1.3) | 2 (1.1) | 6 (2.9) | 0.15 |
| Oral glucocorticoids | 112 (6.7) | 31 (16.8) | 32 (15.5) | <0.0001 |
| Thiazide diuretics | 117 (7.0) | 14 (7.6) | 21 (10.1) | 0.17 |
We examined for differential BMD effects between PPI and H2RA use and menopausal transition stage by introducing interaction terms (between drug and menopausal status) in the multivariable mixed-effects regression models. Because of marginal significance of interaction terms, we performed a secondary analysis focusing on the period of stable BMD; thus, the analysis examined women’s BMD up until 1 year before the final menstrual period. In other secondary analyses, subjects were censored when they reported on the medication questionnaire use of HT, steroids, bisphosphonates, or thiazide diuretics. Two-tailed p values < 0.05 were considered statistically significant for main and interaction effects.
Positive control analyses were conducted in a parallel fashion as the primary analyses. In brief, the positive control exposure of interest was new use of HT compared with non-users (secondary cohort). Baseline for users of HT was chosen as the visit before the start of HT. Non-user baseline visits were frequency matched with the HT users. The BMD outcome was annualized change measured at the lumbar spine, femoral neck, and total hip. Covariates and regression model construction matched the primary analysis.
SAS version 9.2 (SAS Institute, Inc., Cary, NC, USA) was used for the analyses.
Results
The baseline characteristics of the three exposure groups in cohort 1 (PPI users, H2RA users, and non-users of PPIs or H2RAs) are shown in Table 2. Women from the three exposure groups were similar in age at cohort entry and were followed for a median of 9.9 years. About half were white in each group with similar distributions of other race/ethnicities represented. The menopausal transition state was different across the three exposure groups with more women in the PPI group being perimenopausal or postmenopausal at cohort entry. The mean BMI in the PPI and H2RA groups were similar but higher than the non-user group. Comorbidities and comedications of interest were more commonly noted in the PPI and H2RA groups. The BMDs at baseline for the three exposure groups were similar across the three anatomic sites. The PPIs used are described in Supplemental Table S1.
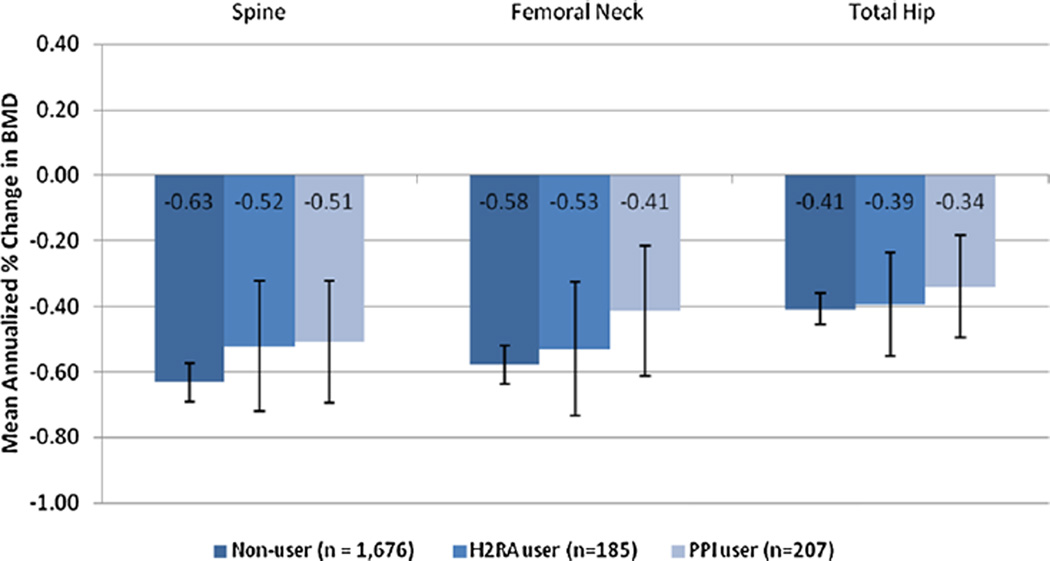
The annualized change in BMD for each group was compared in adjusted linear mixed models. The results of the primary models are illustrated in Fig. 2. No differences between exposure groups were observed at any of the anatomic sites. The estimated adjusted changes in BMD by exposure group are shown in Table 3, as are adjusted sensitivity analyses. The sensitivity analysis models did not change the estimated annual changes in BMD in any of the exposure groups.
Fig. 2.
Mean annualized change in BMD in PPl users, H2RA users, and non-users in SWAN.
Table 3.
Mean Annualized Percent Change in Bone Mineral Density by Anatomic Site in Primary and Sensitivity Analysis Models
| Spine | Femoral neck | Total hip | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Primary modela |
Sensitivity analysis modelb |
Primary modela |
Sensitivity analysis modelc |
Primary modela |
Sensitivity analysis modeld |
|||||||
| Estimate | (95% CI) | Estimate | (95% CI) | Estimate | (95% CI) | Estimate | (95% CI) | Estimate | (95% CI) | Estimate | (95% CI) | |
| Non-user (n = 1605) | −0.61 | (−0.67, −0.55) | −0.58 | (−0.63, −0.52) | −0.46 | (−0.51, 0.42) | −0.45 | (−0.50, −0.40) | −0.32 | (−0.36, 0.27) | −0.31 | (−0.35, −0.26) |
| H2RA user (n = 185) | −0.54 | (−0.68, −0.39) | −0.51 | (−0.65, −0.37) | −0.44 | (−0.55,−0.33) | −0.45 | (−0.56, −0.34) | −0.37 | (−0.47, −0.26) | −0.36 | (−0.47, −0.25) |
| PPI user (n = 207) | −0.53 | (−0.68, −0.38) | −0.48 | (−0.62, −0.33) | −0.43 | (−0.54,−0.32) | −0.41 | (−0.53, −0.29) | −0.41 | (−0.52, −0.29) | −0.38 | (−0.49, −0.27) |
Main model adjusted for age, menopausal status, site, race, and BMI.
Spine MV model = primary model variables, smoking, diabetes, cancer, osteoporosis, vasomotor symptoms, hormone therapy, and bisphosphonate and thiazide diuretic use.
Femoral neck MV model = primary model variables, income, education level, physical activity, cancer, diabetes, hormone therapy, and bisphosphonate and thiazide diuretic use.
Total hip MV model = primary model variables, education level, physical activity, cancer, diabetes, hormone therapy, and bisphosphonate and thiazide diuretic use.
To examine whether slight imbalances in menopausal status may have confounded the relationship between PPI and H2RA use and BMD, interaction terms were tested. The interaction terms assess the possibility that the effect of medication class on BMD may vary by menopause status. Because one of the interaction terms was borderline significant, we performed an additional analysis censoring subjects 1 year before their final menstrual period, a stage where BMD is generally quite stable.(15) Consistent with the main analysis, neither PPI nor H2RA use was associated with greater loss in BMD compared with non-users in the period from the baseline visit until 1 year before the final menstrual period.
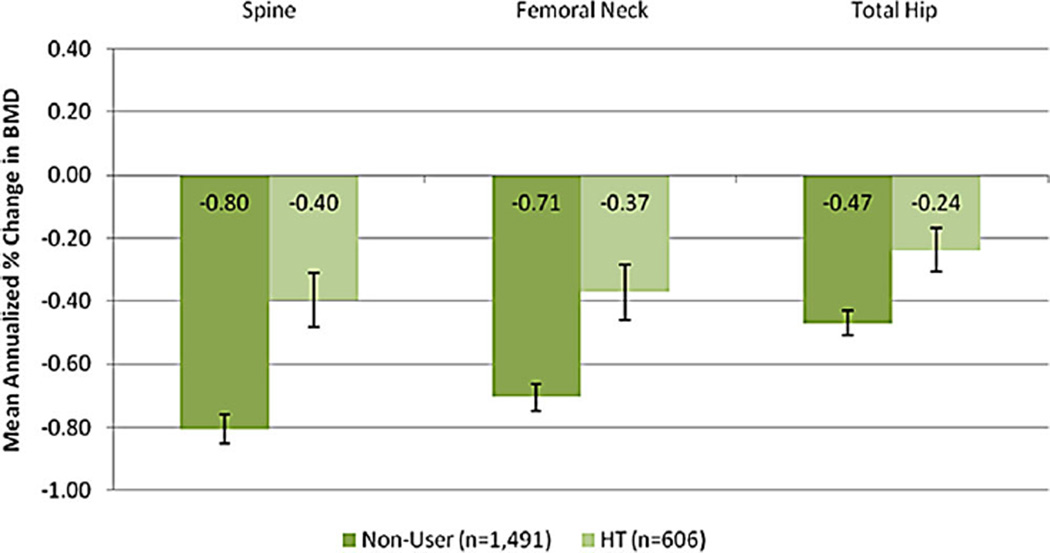
Finally, we examined the HT users, cohort 2, as a positive control exposure. New users of HT (n = 606) differed in many baseline characteristics from non-users (n = 1491) (Supplemental Table S2). We compared annualized change in BMD across these two groups using the same main model as for the primary analysis. As expected, the annualized rate of BMD decline was significantly lower in the HT users than in the non-user group at all three anatomic sites (Fig. 3).
Fig. 3.
Positive Control Analyses of Hormone Therapy Use Among Women in SWAN (cohort 2).
Discussion
In this study, we failed to find evidence of accelerated bone loss from the hip or spine in midlife women who initiated use of PPIs. Rates of bone loss in the PPI users were similar to rates of bone loss in women who initiated use of H2RA blockers and women who did not use these classes of medications. Rates of bone loss were attenuated as expected in women who initiated use of HT, a finding that provides supportive evidence that our analyses did not generate spurious results.
Previously published studies examining rates of bone loss and/or the risk of fracture in people who use PPIs have generated mixed results, with some studies suggesting significant associations and others not. For example, several cross-sectional analyses have reported a relationship between PPI use and reduced BMD, and a meta-analysis reported a significant association between PPI use and fractures.(10–12,20,21) In contrast, most prior longitudinal studies have failed to find a significant association between PPIs and BMD,(5–8) as in our current study. Interpretation of prior studies is compromised, however, by a number of methodological limitations. First, none employed a new-user design in which only subjects initiating drug would be included. This is a preferred method in drug epidemiology and limits the risks of time-varying associations between drug and outcome.(9) Second, most prior studies assessed drug use and BMD relatively infrequently; some only assessed BMD and PPI use at 5-year intervals. A drug-association study needs frequent assessments of drug exposure, especially in the instance of a PPI that may be used on an as-needed basis. Third, most prior analyses compare users to non-users, without respect to potential confounding.(22) If there is no accurate assessment of symptoms that trigger a treatment (ie, gastrointestinal symptoms and PPI use), then it is always preferred to use an active comparator with similar indications (ie, H2RA). Finally, unlike prior studies, this analysis also included a positive control (HT), providing important supporting evidence that our study design yields valid results.(23)
Our study has potential limitations. As an observational study, this study could suffer from potential misclassification of exposure, ie, subjects who mistakenly reported use or nonuse of PPIs. Subjects did bring in medication containers, which should limit misclassification errors. It is also possible that some participants who had used PPIs before SWAN were misclassified as “new users.” It is also possible that some PPI and H2RA users were only taking the medication intermittently, thereby diminishing the intensity of the exposure and reducing any potential harm of these medications on BMD. However, the medication questionnaire in SWAN asks for drugs taken at least twice per week. Unmeasured confounding bias is possible; that is where the use of PPIs is associated with another variable that may affect BMD. We included a robust set of covariates to limit this possibility. Even including other bone-active agents, such as HT and bisphosphonates, did not alter the results (results not shown). We did not assess the association between PPI use and fractures. It is possible that these agents may be associated with fractures independent of any effect on BMD. For example, PPIs could increase the risk of falls, though a recent study found no relationship between PPI use and falls.(24) This study only included women going through the menopause transition. Although this is a very important population to study, these results may not generalize to other cohorts. Moreover, because bone loss during the menopause transition is so rapid,(25) it is possible that a small effect of PPIs on rates of bone loss may have been obscured by the rapid bone loss during the menopause transition. However, when the analysis was restricted to premenopausal women, in whom the rate of endogenous bone loss is negligible, we also failed to find evidence of accelerated bone loss in PPI users.(15)
In conclusion, we failed to find a significant association between PPI use and BMD loss in women participating in SWAN, a large, multi-ethnic, longitudinal study of midlife women as they transition across the menopause. These findings may have important clinical and methodologic implications. Clinically, the findings add to the body of evidence that PPIs do not affect bone adversely. Although PPIs are likely overused and may present other toxicities, the concern regarding an association with osteoporosis, including the appropriateness of the FDA-required label on PPIs warning consumers about a risk for bone loss, should be reassessed. Methodologically, this study highlights several important approaches that reduce the change of reporting spurious associations. Several of these issues may also impact observational studies assessing the relationship between PPI use and fracture risk. It is important for fracture studies also to pay careful attention to new user designs with active comparators and frequent reassessment of drug use. As future work examines the potential positive and negative effects of medications on bone health, use of techniques employed in this study—a new-user design, improved and more frequent ascertainment of medication use, active measures to address confounding, and the use of a positive control—will be important to improve the consistency and validity of results.
Acknowledgments
Clinical centers: University of Michigan, Ann Arbor—Siobán Harlow, PI, 2011–present, Mary Fran Sowers, PI, 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI, 1999–present, Robert Neer, PI, 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI, 2009–present, Lynda Powell, PI, 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI, 2011–present, Rachel Wildman, PI, 2010–2011; Nanette Santoro, PI, 2004–2010; University of Medicine and Dentistry–New Jersey Medical School, Newark—Gerson Weiss, PI, 1994–2004; University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD—Winifred Rossi 2012–present, Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—program officers.
Coordinating center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI, 2012–present, Kim Sutton-Tyrrell, PI, 2001– 2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI, 1995–2001.
Steering committee: Susan Johnson (current chair), Chris Gallagher (former chair).
We thank the study staff at each site and all the women who participated in SWAN.
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women’s Health (ORWH) (grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The funding agencies had no direct role in the design or conduct of the study; the collection, management, analyses, and interpretation of the data; or preparation or approval of the manuscript.
Authors’ roles: DHS developed the analysis plan, interpreted analyses, drafted the manuscript, and approved it. SJD interpreted analyses, revised the manuscript, and approved it. KR developed the analysis plan, supervised and interpreted analyses, drafted parts of the manuscript, and approved it. YJL ran analyses and approved the manuscript. CCL ran analyses and approved the manuscript. AW helped draft the manuscript and approved it. GAG interpreted the analyses, revised the manuscript, and approved it. JSF interpreted the analyses, revised the manuscript, and approved it. KR and CCL performed the statistical analyses and are independent of any commercial funder. They had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
DHS receives salary support through grants to his institution from Amgen, Lilly, Pfizer, and CORRONA. He also serves in unpaid roles on trials sponsored by Lilly, Pfizer, Novartis, and Bristol Myers Squibb and as an unpaid member of the Governing Board of the National Bone Health Alliance.
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosures
All other authors state that they have no conflicts of interest.
References
- 1.Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocr Rev. 2008;29(4):403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. 2005;353(6):595–603. doi: 10.1056/NEJMcp043801. [DOI] [PubMed] [Google Scholar]
- 3.Cranney A, Guyatt G, Griffith L, et al. Meta-analyses of therapies for postmenopausal osteoporosis. IX: summary of meta-analyses of therapies for postmenopausal osteoporosis. Endocrine Rev. 2002;23(4):570–578. doi: 10.1210/er.2001-9002. [DOI] [PubMed] [Google Scholar]
- 4.Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 5.Yu EW, Blackwell T, Ensrud KE, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcif Tissue Int. 2008;83(4):251–259. doi: 10.1007/s00223-008-9170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Targownik LE, Lix LM, Leung S, Leslie WD. Proton-pump inhibitor use is not associated with osteoporosis or accelerated bone mineral density loss. Gastroenterology. 2010;138(3):896–904. doi: 10.1053/j.gastro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Targownik LE, Leslie WD, Davison KS, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study [corrected] from the Canadian Multicentre Osteoporosis Study (CaMos) Am J Gastroenterol. 2012;107(9):1361–1369. doi: 10.1038/ajg.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray SL, LaCroix AZ, Larson J, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Arch Intern Med. 2010;170(9):765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 10.Corley DA, Kubo A, Zhao W, Quesenberry C. Proton pump inhibitors and histamine-2 receptor antagonists are associated with hip fractures among at-risk patients. Gastroenterology. 2009;139(1):93–101. doi: 10.1053/j.gastro.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA. 2006;296(24):2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 12.Targownik LE, Lix LM, Metge CJ, Prior HJ, Leung S, Leslie WD. Use of proton pump inhibitors and risk of osteoporosis-related fractures. CMAJ. 2008;179(4):319–326. doi: 10.1503/cmaj.071330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidelbaugh JJ, Goldberg KL, Inadomi JM. Magnitude and economic effect of overuse of antisecretory therapy in the ambulatory care setting. Am J Manag Care. 2010;16(9):e228–e234. [PubMed] [Google Scholar]
- 14.Sowers MR, Jannausch M, McConnell D, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91(4):1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93(3):861–868. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 17.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 18.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28(3):313–323. doi: 10.1006/pmed.1998.0470. [DOI] [PubMed] [Google Scholar]
- 19.Brown H, Prescott R. Applied mixed models in medicine. Hoboken, NJ: John Wiley and Sons; 2006. [Google Scholar]
- 20.Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications and the risk of fracture. Calcif Tissue Int. 2006;79(2):76–83. doi: 10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 21.Yu EW, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: a meta-analysis of 11 international studies. Am J Med. 2011;124(6):519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker AM. Confounding by indication [comment] Epidemiology. 1996;7(4):335–336. [PubMed] [Google Scholar]
- 23.Hernan MA, Alonso A, Logan R, et al. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(6):766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cea-Soriano L, Johansson S, Garcia Rodriguez LA. Risk factors for falls with use of acid-suppressive drugs. Epidemiology. 2013;24(4):600–607. doi: 10.1097/EDE.0b013e318294bec6. [DOI] [PubMed] [Google Scholar]
- 25.Greendale GA, Sowers M, Han W, et al. Bone mineral density loss in relation to the final menstrual period in a multiethnic cohort: results from the Study of Women’s Health Across the Nation (SWAN) J Bone Miner Res. 2012;27(1):111–118. doi: 10.1002/jbmr.534. [DOI] [PMC free article] [PubMed] [Google Scholar]