Abstract
Gestational diabetes mellitus (GDM) represents glucose levels in the high end of the population distribution during pregnancy. GDM carries a small but potentially important risk of adverse perinatal outcomes and a longer-term risk of obesity and glucose intolerance in offspring. Mothers with GDM have an excess of hypertensive disorders during pregnancy and a high risk of diabetes mellitus thereafter. Diagnosing and treating GDM can reduce perinatal complications, but only a small fraction of pregnancies benefit. Nutritional management is the cornerstone of treatment; insulin, glyburide and metformin can be used to intensify treatment. Fetal measurements compliment maternal glucose measurements in identifying pregnancies that need such intensification.
Glucose testing shortly after pregnancy can stratify the near-term diabetes risk in mothers, Thereafter, annual glucose and HbA1C testing can detect deteriorating glycaemic control, a harbinger of future diabetes, usually type 2. Interventions that mitigate obesity or its metabolic effects are most potent in preventing or delaying diabetes. Lifestyle modification is the primary approach; use of medications for diabetes prevention after GDM remains controversial. Family planning allows optimization of health in subsequent pregnancies. Breastfeeding may reduce obesity in children and is recommended. Families should be encouraged to help children adopt lifestyles that reduce the risk of obesity.
INTRODUCTION
Gestational diabetes mellitus (GDM) is one of the most common medical complications of pregnancy. The disease has important health implications for mother and child. This Review discusses current evidence for the importance of GDM, opportunities to reduce risk to mother and child and recommendations for clinical care.
WHAT IS GESTATIONAL DIABETES MELLITUS?
Definition
GDM is defined as glucose intolerance with onset or first recognition during pregnancy.1 The definition does not require any return to normal glucose levels following delivery. Thus, GDM simply represents relatively high glucose levels at one point in the life of a young woman.
Detection
Outside of pregnancy, screening for clinically important levels of hyperglycaemia is generally recommended only for individuals with specific risk profiles.1 By contrast, screening for abnormal glucose levels is generally recommended as a routine component of care for pregnant women.1 Traditionally, screening during pregnancy has involved two steps. The first is a simple 1 h glucose challenge test to identify a large number of women at very low risk of clinically important hyperglycaemia; they do not need additional testing. The second step is a more complex 2 h or 3 h oral glucose tolerance test applied to the ‘at risk’ women to define the subset who have GDM. Specific cut-off points used in this detection process have varied widely. Relatively low cut-off points result in relatively high incidence rates of GDM, including many women with relatively mild hyperglycaemia. Relatively high cut-off points result in the converse.
For the purposes of this discussion, the specific cut-off points are less important than the general concept that GDM is diagnosed following a form of population screening for hyperglycaemia in young women.2 That screening occurs at a time when the women are generally quite insulin resistant, although, as discussed below, the acquired insulin resistance of late pregnancy might not be a dominant feature of the pathogenesis of GDM. As explained below, the hyperglycaemia of GDM appears to have a small but demonstrable effect on perinatal outcomes, and is also associated with important long-term health problems in affected mothers and their children.
In an effort to define uniform diagnostic criteria for GDM, Metzger and colleagues conducted the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study.3 Unlike prior efforts, the HAPO study focused on perinatal outcomes rather than future diabetes mellitus in the mother to set criteria for GDM. Approximately 25,500 women from nine countries had 75 g oral glucose tolerance tests (OGTTs) in the third trimester of pregnancy. Unless glucose levels were dangerously high, the women’s providers were blinded to the OGTT results so that patients received standard antenatal care. Rates of perinatal complications were examined in relation to the OGTT results to determine whether there was a threshold for maternal glucose levels above which perinatal risks rose abruptly. No threshold was found. Instead, risks of adverse perinatal outcomes increased relatively smoothly in association with rising maternal glucose levels, as measured by the OGTT.
In the absence of a biological threshold for GDM based on perinatal risks, the International Association of Diabetes and Pregnancy Study Groups (IADPSG) convened a consensus panel that selected the diagnostic criteria for GDM presented in Table 1.4 The individual glucose cut-off points are only modestly different from cut-off points that were already in use in many countries. However, the new criteria require only one abnormal value on a 75 g OGTT to make the diagnosis of GDM, compared with the two abnormal values commonly required in the past. As a result, many more women than before will meet the criteria for GDM if the IADPSG criteria are used. For example, when the new criteria were applied retrospectively to data from the HAPO cohort, approximately 18% of women met the criteria forGDM.4 This incidence rate is approximately twice that reported with old diagnostic approaches.
Table 1.
Thresholds for classifying hyperglycaemia in pregnancy1
| Diagnosis and test type | Glucose level (mmol/l) |
|---|---|
| Gestational diabetes mellitus2 | – |
| Fasting plasma glucose | ≥5.1 |
| 1 h plasma glucose | ≥10.0 |
| 2 h plasma glucose | ≥8.5 |
| Overt diabetes3 | – |
| Fasting plasma glucose | ≥7.0 |
| Random plasma glucose4 | ≥11.1 |
| HbA1C | ≥6.5% |
As proposed by the International Association for Diabetes and Pregnancy Study Groups.4
One or more values must be met or exceeded for diagnosis of gestational diabetes mellitus.
One value must be met or exceeded for diagnosis of overt diabetes in pregnancy.
Should be confirmed by fasting plasma glucose or HbA1C level.
One advantage of the new approach is that a large majority of patients can be identified from the fasting and 1 h values on the OGTT.4 This finding suggests that a one-step approach employing a 75 g OGTT can be used to simplify the detection of GDM in many settings. However, the 2 h value on the OGTT could be particularly important in some regions or ethnic groups.5 Controversies surrounding the widespread adoption of the IADPSG approach for the diagnosis of GDM have been summarized in several articles.6–8 Some professional organizations have adopted the new criteria for GDM, whilst others are waiting for evidence of the beneficial effects of treatment in this expanded patient population before recommending any change in criteria to clinicians.
One additional recommendation of the IADPSG consensus panel was to create a category of overt diabetes in pregnancy. Women are given this diagnosis if their glucose levels meet criteria for diabetes mellitus outside of pregnancy (Table 1). This distinction is not artificial.Women with this level of hyperglycaemia have an increased risk of birth defects in their infants, something that does not occur in GDM with lower glucose levels.9 Also, women with overt diabetes mellitus could have chronic diabetic complications that alter risks of hypertensive disorders and visual deterioration during pregnancy. Thus, these women should be managed as if they had pre-existing diabetes mellitus, a topic beyond the scope of this Review.
Frequency of GDM
Diagnostic criteria for GDM have varied widely by geography and over time. As a result, it has been difficult to compare the incidence of GDM among ethnic groups or to determine whether GDM rates have changed over time. Two studies from the Kaiser Permanente health systems in the USA10,11 assessed incidence rates in pregnant women of multiple ethnicities after a standardized diagnostic approach for GDM had been applied over 9–10 year periods between 1991 and 2002. In both studies, incidence rates of GDM rose over time, from slightly less than 4% to more than 6%. GDM was most common among people of Asian ancestry or Hispanic ethnicity and least common in people of European ancestry. The GDM rate in African Americans fell in between. GDM rates rose in parallel in all ethnic groups, which indicated a true increase in the incidence of GDM over time. A report from Kaiser Permanente Southern California health system published last year suggests a continued rise in GDM rates. The study spanned a period from 1995–2009. The overall incidence rate of GDM was 10%, with higher rates in Asian (17%) and Hispanic (11%) women and lower rates in non-Hispanic white (7%) and black (7%) women.12 As noted above, adoption of the IADPSG criteria would approximately double these rates of GDM.
Mechanisms Underlying GDM
GDM is a form of hyperglycaemia. Similar to other forms of hyperglycaemia, GDM is a disease of the pancreatic β cells, which do not produce sufficient insulin to meet the increased requirements of late pregnancy. The simplicity of this description belies a more complex set of aetiologies for GDM. Mechanistic studies of GDM reveal at least three separate underlying causes of β-cell dysfunction. First, some women have circulating immune markers (for example, anti-islet cell antibodies or antibodies to glutamate decarboxylase 65) that are diagnostic of evolving type 1 diabetes mellitus (T1DM). The frequency is generally <10% of all women with GDM and it tends to parallel the background prevalence of type 1 diabetes mellitus in the population.13–16
Second, some women have genetic variants that are diagnostic of monogenic forms of diabetes. These include genes for subtypes of maturity onset diabetes of the young (MODY) and maternally inherited diabetes.17–19 Systematic data on the frequency of these monogenic forms of diabetes in GDM are limited, but they appear to be infrequent, accounting for 1–5% of cases. The third general setting in which the β-cell defects that underlie GDM occur is that of obesity and chronic insulin resistance. This group represents a majority of cases of GDM, leading many clinicians to view GDM as a form of evolving type 2 diabetes mellitus (T2DM). This view may be accurate for the majority of cases, but the full spectrum of GDM includes other causes of inadequate β-cell function in relatively young women. Appropriate care for mothers, especially after pregnancy, requires an understanding of this fact, as will be discussed below.
Understanding of the role of the acquired insulin resistance of pregnancy in the pathogenesis of the hyperglycaemia that defines GDM has evolved over time. Traditionally, it has been thought and taught that GDM develops when β cells fail to keep pace with the increasing insulin resistance that occurs during the second half of pregnancy. The resultant increasing imbalance between insulin demand and supply manifests itself as rising glucose levels, especially during the second half of pregnancy when insulin resistance is the greatest. In this scenario, glucose regulation returns to normal postpartum, only to resurface years later as impaired glucose levels and diabetes mellitus, usually T2DM.
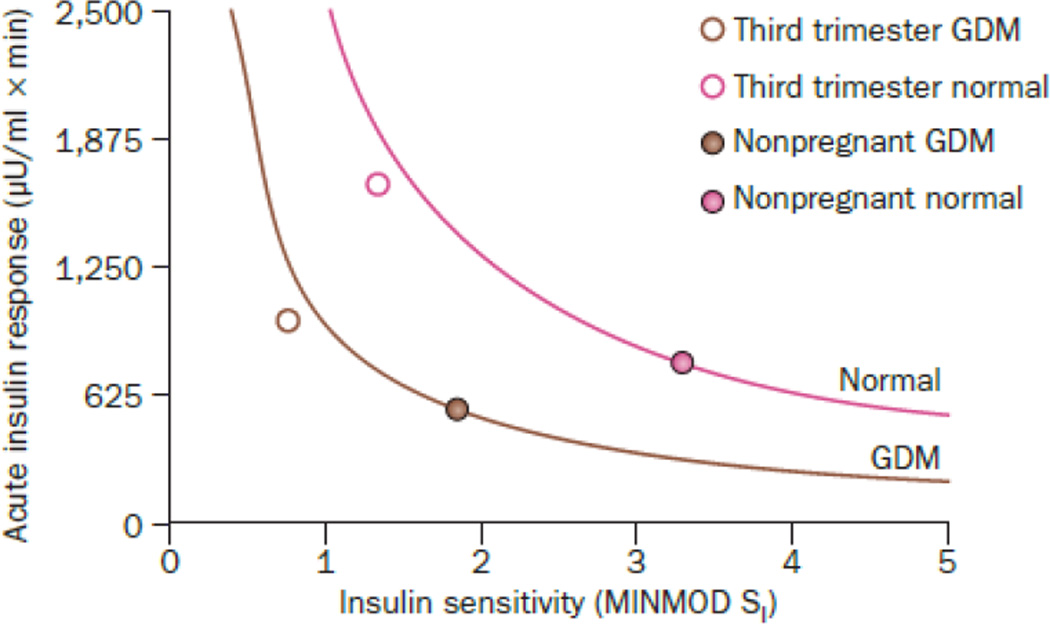
Serial studies of insulin resistance and β-cell function in women who develop GDM provide quite a different picture. A large majority of the insulin secretory defect that is present in the third trimester of pregnancy is present before20,21 and soon after2,22 pregnancy. In fact, insulin secretion during pregnancy increases in parallel in women with and without GDM (Figure 1), but from a lower starting point in women the condition. Clinical characteristics of the women in these studies suggest that they fall into the sub-type of GDM that is related to T2DM. Thus, many and perhaps most women with GDM appear to have a β-cell defect that is chronic rather than acquired during pregnancy. This concept is consistent with the fact that GDM tends to occur in women over 30 years old who have had multiple pregnancies and who are obese. Obesity and pregnancy are conditions that promote chronic β-cell dysfunction that is detected during pregnancy, when glucose tolerance is tested for the first time in many women’s lives.
Figure 1.
β-cell compensation for insulin resistance in GDM and control pregnancies. Data are from 99 Hispanic women who had GDM and 7 Hispanic women who maintained normal glucose tolerance during and after pregnancy. Both groups were studied with frequently-sampled intravenous glucose tolerance tests to measure acute insulin response and insulin sensitivity in the third trimester and then again remote from pregnancy. Curved lines represent insulin sensitivity-secretion relationships defined by the product of insulin sensitivity and acute insulin response in non-pregnant women for each group. Adapted from2
In summary, GDM is a form of generally mild hyperglycaemia that reflects inadequate β-cell compensation for the body’s insulin needs. In some cases, the acquired insulin resistance of pregnancy could create insulin demands that exceed β-cell capacity to supply insulin for the limited time frame of pregnancy. However, most cases appear to represent chronic β-cell dysfunction that is detected during pregnancy, when glucose tolerance is measured as part of routine care and often for the first time in a young woman’s life. As discussed below, β-cell function is not just deficient during pregnancy in women with GDM; it deteriorates over time, which results in women who have had GDM being at a high risk of developing diabetes mellitus in the years following the index pregnancy.
WHY IS GESTATIONAL DIABETES IMPORTANT?
Antepartum and Perinatal Considerations
Overt maternal diabetes mellitus can adversely influence intrauterine development. Spontaneous abortions and major congenital anomalies may be induced in the first trimester. Excessive foetal growth, neonatal hypoglycaemia, jaundice, polycythaemia and stillbirth may be induced during the second and third trimesters. As noted above, excess birth defects are generally limited to cases of gestationally diagnosed hyperglycaemia that meets criteria for overt diabetes mellitus. The frequency of the other adverse outcomes across the full range of maternal glycaemia that defines true GDM is difficult to determine. The reason is simple—women in the upper part of that range almost always receive some form of treatment.
Results from the HAPO study provide useful insight into the frequency of perinatal complications in women with relatively mild GDM in the absence of treatment. In the HAPO study, women found to have fasting plasma glucose levels of >5.8 mmol/l or a glucose level of >11.1 mmol/l on the 75 g, 2 h OGTT were referred for treatment of GDM. Women with lower glucose levels than these thresholds received no diagnosis or care related to their glycaemic control. Retrospective application of the new IADPSG criteria for GDM to these untreated women revealed statistically significant increases in ten different adverse perinatal outcomes in women who ‘had’ GDM compared with those who did not (Table 2). Although the relative increase in risk ranges from 17—202% across the ten complications, the absolute risk (the difference in complication rates between the GDM and control groups) was not more than 11% for any complication. Similar patterns have been observed in many smaller studies; for example, 10% absolute risk of caesarean delivery and 14% absolute risk of macrosomia in women with untreated, borderline GDM in the Toronto Tri-Hospital GDM project.23
Table 2.
Perinatal outcomes in the HAPO study when IADPSG criteria for GDM are applied1
| Outcome | Frequency in GDM (%) |
Frequency in non-GDM (%) |
Frequency difference (%) |
|---|---|---|---|
| Pre-eclampsia | 9.1 | 4.5 | 4.6 |
| Delivery at <37 weeks | 9.4 | 6.4 | 3.0 |
| Primary caesarean delivery | 24.4 | 16.8 | 7.6 |
| Shoulder dystocia or birth injury | 1.8 | 1.3 | 0.5 |
| Intensive neonatal care | 9.1 | 7.8 | 1.3 |
| Clinical neonatal hypoglycaemia | 2.7 | 1.9 | 0.8 |
| Neonatal hyperbilirubinaemia | 10.0 | 8.0 | 2.0 |
| Birthweight >90th percentile | 16.2 | 8.3 | 7.9 |
| Cord C-peptide >90th percentile | 17.5 | 6.7 | 10.8 |
| Percent body adipose tissue content >90th percentile | 16.6 | 8.5 | 8.1 |
IADPSG criteria appear in Table 1.
Abbreviations: GDM, gestational diabetes mellitus; HAPO, Hyperglycemia and Adverse Pregnancy Outcomes; IADPSG, International Association for Diabetes and Pregnancy Study Groups. Data are from the on-line supplement to reference (4)7
The main message is that the diagnosis of GDM imparts some excess risk of perinatal complications. However, only a minority of pregnancies have an adverse outcome that could be attributed to GDM. This fact will become important when approaches to antepartum management are discussed below.
Long-Term Health of the Mother
Women who are diagnosed with GDM are at high risk of developing diabetes mellitus later in life. An estimated ~10% of women with GDM have diabetes mellitus soon after delivery. The rest appear to develop diabetes mellitus at rates of 20–60% within 5–10 years after the index pregnancy in the absence of specific interventions to reduce their risk of diabetes mellitus. Limited long-term data from O’Sullivan24 suggest that not all women with GDM will get diabetes mellitus, but certainly the majority will. Thus, as is true for perinatal complications, GDM is a risk factor for diabetes mellitus after pregnancy. However, the risk of diabetes mellitus in the mother after GDM is much higher than the risk of perinatal complications associated with GDM. Thus, GDM can be reasonably considered to be a form of prediabetes similar to impaired glucose tolerance in nonpregnant individuals.
As discussed above, diabetes mellitus after GDM can take several forms, but the majority of patients fit the phenotype of pre-T2DM. Longitudinal studies of glucose regulation after GDM reveal falling β-cell compensation for chronic insulin resistance that may also worsen over time.25 Risk factors for relatively early development of diabetes mellitus after pregnancy include markers of relatively severe decompensation, which include high glucose levels, marked insulin resistance and poor β-cell function. Women with these characteristics do not have to deteriorate much to cross the line to glucose levels that define diabetes mellitus. Risk factors for relatively high rates of the deterioration in β-cell function that causes diabetes mellitus include weight gain, insulin resistance, rising levels of C-reactive protein and falling levels of adiponectin.26 These findings suggest that metabolic effects of obesity are important determinants of the β-cell deterioration that leads to diabetes mellitus. Indeed, as discussed below, amelioration of adverse metabolic effects of obesity through weight loss or the use of medications that improve adipose tissue biology provide the strongest protection against the development of T2DM following GDM.
The background upon which T2DM develops is one of obesity and related conditions that are often referred to as the metabolic syndrome. As might be expected, women who have had GDM manifest components of the metabolic syndrome more often than do women without GDM.27 A history of GDM is also associated with an increased frequency of cardiovascular risk factors28 and cardiovascular events.29
Long-Term Health of the Offspring
Several30–33 but not all34,35 studies of growth and development of offspring of mothers with diabetes mellitus indicate an increased risk of obesity during childhood and adolescence. Some of this effect could represent simple heredity or shared environment between mothers and children; however, several observations suggest an independent effect of exposure to diabetes mellitus in utero. First, offspring of mothers with diabetes mellitus have a higher risk of developing obesity than the offspring of fathers with diabetes mellitus.36 Second, offspring of mothers with type 1 diabetes mellitus, who are generally not obese, have higher BMI by age 14–17 years and more often have impaired glucose tolerance than offspring of nondiabetic mothers.37 Third and most convincingly, in sibling pairs discordant for exposure to maternal diabetes mellitus, offspring born after the mother developed diabetes mellitus had a higher BMI and a higher risk of developing diabetes mellitus than offspring born before their mother developed diabetes mellitus.36
In Pima Indians, glucose levels in the range diagnostic for GDM were associated with an increased risk of obesity in offspring. These findings suggest that foetal exposure to maternal diabetes mellitus, including GDM, influences important aspects of the regulation of appetite and/or energy expenditure in favour of positive caloric balance. Interestingly, the effect of maternal diabetes mellitus on offspring does not become manifest as increased BMI until after ~2 years of age,37,38 and effects on other components of the metabolic syndrome, including hyperglycaemia, have been observed.37 All of these findings suggest that exposure to maternal diabetes mellitus in utero could be an important contributor to the rising rates of obesity and diabetes mellitus that are occurring in developed countries throughout the world.31
CAN THE RISKS OF GDM BE REDUCED?
Antenatal and Perinatal Complications
Traditionally, most of the evidence about the antenatal and perinatal benefits of diagnosing and treating GDM have come from a mix of clinical observations and non-randomized treatment trials. As a result, expert bodies have made a wide range of recommendations about the importance of diagnosing and treating GDM. At one end of the spectrum are organizations that recommend widespread or universal screening for GDM and stepped care treatment for women with the disease.1,39 At the other end of the spectrum are groups that have questioned the cost-effectiveness of detecting GDM at all.40–42
Two randomized clinical trials have provided evidence that diagnosing and treating GDM can have statistically significant beneficial effects, albeit in a relatively small fraction of patients. In the Australian Carbohydrate Intolerance Study (ACHOIS),43 a 75 g OGTT was performed between 16 and 30 weeks of gestation, and fasting plasma glucose levels of <7.8 mmol/l or 2 h glucose levels 7.8–11.0 mmol/l were used as the criteria for the diagnosis of GDM. Women with GDM were randomly allocated to usual care (patients and providers blinded to the OGTT results) or intervention. In the intervention group, patients and providers were aware of the OGTT results. Treatment included individualized nutritional advice and glucose self-monitoring. Exogenous insulin was given when glucose levels exceeded pre-specified targets. The intervention group had a lower rate of serious perinatal complications (1% versus 4%; mostly shoulder dystocia) but a higher rate of admissions to the neonatal intensive care unit (71% versus 61%).
In a study from the Maternal-Fetal Medical Units Network in the USA, a 100 g OGTT was performed in women between 24 and 30 weeks of gestation; the criteria for the diagnosis of GDM were a fasting plasma glucose level <5.3 mmol/l and at least two timed glucose values that met or exceeded set thresholds (1 h: 10.0 mmol/l; 2 h: 8.6 mmol/l; 3 h: 7.8 mmol/l).44 Patients were randomly allocated to usual care (blinded to the diagnosis) or intervention, as in the ACHOIS study. A composite of clinically significant perinatal outcomes (death, trauma, jaundice, hypoglycaemia or elevated C-peptide levels in the offspring) occurred at similar frequencies in the two groups. Rates of shoulder dystocia were reduced in the intervention group (1.5% versus 4%), similar to the results of the ACHOIS study. In both randomized clinical trials, intervention was associated with significant reductions in mean birth weights (~100–150 g), in rates of infants that were born large for gestational age or that weighed >4,000 g at birth, and in rates of maternal hypertensive disorders. For these last three adverse outcomes, absolute differences in rates between groups were ~6–10%.
Taken together, these studies and a meta-analysis that included them and three smaller studies45 reveal a consistent pattern. Diagnosing GDM and treating it using nutritional advice, glucose self-monitoring and, if required, exogenous insulin lowers the relative risk of foetal overgrowth, shoulder dystocia and maternal hypertensive disorders. However, only a small fraction of pregnancies benefit, since most pregnancies do not incur any adverse perinatal outcomes in the absence of treatment and some pregnancies incur them despite treatment.
A much larger body of evidence exists regarding the effect of intensifying treatment beyond nutritional therapy once GDM has been diagnosed, as reviewed in the meta-analysis by Hovarth et al.45 The methods of intensification varied considerably across the thirteen analyzed studies and included insulin treatment versus diet alone, different intensities of insulin, insulin versus aerobic exercise, glucose monitoring at visits versus glucose self-monitoring by patients, more versus less frequent glucose self-monitoring, glucose self-monitoring versus continuous glucose monitoring, therapy adjustments at visits versus adjustments using telemedicine, caloric restriction versus unrestricted diets with insulin treatment, and the use of ultrasonography to guide decisions on insulin treatment.
The studies were generally small (41–342 individuals each). As might be expected, it was difficult to identify uniform or consistent findings across this heterogeneous group of studies in the meta-analysis. Rates of shoulder dystocia, which was reported in five of the thirteen studies, were reduced significantly in the intervention group (~3% absolute risk reduction). There was a tendency for reduction in the rate of large for gestational age babies in the intervention group that did not reach statistical significance and represented only a 4% absolute risk reduction overall. No important differences in rates of caesarean delivery, birth trauma, macrosomia or perinatal mortality were found. Thus, as is true for diagnosing and treating GDM, intensifying treatment can benefit a relatively small but potentially important subset of patients.
The information presented above highlights a major weakness in the field of GDM. Intensive monitoring and, to a lesser extent, pharmacological treatments are applied to a large number of patients to improve outcomes in a relative few. Even if this approach could be shown to be cost effective,46 it is almost certainly inefficient. In truth, when it comes to perinatal complications, GDM is more of a risk factor than a disease. A very great need exists for more precise methods to identify the subset of pregnant women with GDM who are at the highest risk for perinatal complications so that the most intensive monitoring and treatment efforts can be directed at them. Maternal glucose measurements are a very crude tool in this regard. This issue will be discussed below in relation to approaches to treatment during pregnancy.
Protecting Long-Term Maternal Health
A reasonably robust and growing body of clinical trial evidence exists concerning reducing the risk of diabetes mellitus in high risk individuals. For individuals at high risk of developing T1DM, the evidence is that we do not have any interventions that work well in humans. For individuals at high risk of developing T2DM, the story is very different. Lifestyle interventions, metformin, acarbose and thiazolidinediones have been used to reduce the risk of T2DM by 25–72% in adults with impaired glucose levels.47–52 The best evidence for risk reduction and disease mitigation comes from approaches that either change body adipose tissue content (lifestyle change can produce ~58% risk reductions) or adipose tissue biology (thiazolidinediones can produce 55–72% risk reductions).53 Evidence is less strong for approaches that primarily reduce rates of glucose appearance in the circulation (acarbose and metformin can produce 25–31% risk reductions).53
Two trials provide information for the specific case of women with a high risk of T2DM on the basis of a history of GDM. The Troglitazone in the Prevention of Diabetes (TRIPOD) study was conducted solely in Hispanic women with prior GDM.48 The study demonstrated a 55% reduction in the incidence of T2DM over a 30-month period. Protection from T2DM was associated with reduced secretory demands on β-cells and with significant slowing of loss of β-cell function. Preservation of β-cell function persisted when patients were switched to pioglitazone after troglitazone was withdrawn from clinical use.54 The U.S. Diabetes Prevention Program included women with a history of GDM as one risk factor for diabetes mellitus. A post-hoc analysis of the impact of lifestyle or metformin compared with placebo in women with a self-reported history of GDM revealed a ~50% reduction in the risk of diabetes mellitus with either intervention.55
Interestingly, lifestyle was equally effective in reducing the risk of diabetes mellitus in women with and without a history of GDM (50% versus 49% risk reductions, respectively). By contrast, metformin was more effective in women with than without a history of GDM (50% versus 14%, respectively). Thus, metformin may be particularly effective in reducing the risk of diabetes mellitus in women with a history of GDM, for reasons that are not clear at present. The limited available evidence suggests that assessment and reduction of cardiovascular risk should be an additional component of care for women with prior GDM.28,29
Improving Long-Term Health of the Offspring
Little high-quality evidence exists to help guide clinicians to reduce the risks of obesity and diabetes mellitus in the offspring of mothers with diabetes mellitus. Breastfeeding has been associated with a reduced long-term risk of obesity and diabetes mellitus compared with bottle feeding in several observational studies, some of which included mothers with diabetes mellitus.56,57 The hope that aggressive control of maternal glucose levels in pregnancy might also mitigate the development of obesity in their children remains to be realized. Follow-up of the ACHOIS study, in which diagnosis and treatment of GDM led to a reduction in birth weights and macrosomia rates in offspring, did not reveal any intergroup difference in BMI z-scores of offspring by ages 4–5 years.58
The Metformin in Gestational Diabetes (MiG) trial59 compared metformin with insulin in women with GDM who required intensification of treatment beyond dietary therapy. The two treatments provided similar perinatal outcomes, including birth weights and skin-fold measurements in newborns. At age 2 years, offspring of the two treatment groups had similar body adipose tissue content, but the offspring in the metformin group had small (3–16%) but statistically significant increases in skin-fold thicknesses.60 The authors suggested that this might reflect increased subcutaneous and reduced visceral adipose tissue in the metformin group, but that suggestion remains to be proven. Thus, other than breastfeeding, currently no interventions have been proven to reduce obesity and its complications in offspring exposed in utero to GDM or any other form of diabetes mellitus. However, excess rates of obesity in offspring from mothers with GDM may take years to develop; therefore, long-term studies will be required to provide definitive information on this issue.
RECOMMENDATIONS FOR CLINICAL CARE
Antepartum Care
Nutritional therapy is widely recommended as an integral part of the treatment of GDM. Unfortunately, relatively little information from controlled trials exists to guide nutritional recommendations for this condition. In general, nutritional requirements are the same for pregnant women with and without GDM. However, several dietary modifications can lower glucose levels more effectively than a standard diet for pregnant women.These include reducing caloric intake for overweight and obese women (for example, to ~25 kcal/kg of body weight),61 limiting carbohydrate content to 35–40% of total calories62,63 and focusing on complex rather than simple carbohydrates. The second of these modifications has been shown to improve perinatal outcomes compared with higher carbohydrate diets.64 These principles can be applied in practice by individualizing dietary advice under the guidance of a nutritionist who is expert in dietary management of women with diabetes mellitus in pregnancy.
The next step after initiating nutritional therapy is the identification of women who need additional treatment to minimize the risk of perinatal complications. Two general approaches have been applied in this regard. The most common approach employs regular glucose self-monitoring by patients. The optimal timing and frequency of monitoring has not been determined. One study that is often cited as proof that post-meal glucose targets are more important than pre-meal targets in the management GDM65 actually compared relatively low post-meal targets to relatively high pre-meal targets. Thus, the design was biased in favour of post-meal monitoring. Other studies have found fasting glucose levels to be more closely related to perinatal complications that post-challenge glucose levels on the diagnostic OGTT.66,67 In the absence of definitive evidence, it has become common practice to ask patients to measure capillary glucose levels before breakfast and 1–2 h after breakfast, lunch and dinner. Treatment targets have varied among studies that have demonstrated improved perinatal outcomes. Some commonly recommended targets are fasting ≤5.3 mmol/l, 1-hour post-meal ≤7.8 mmol/l and 2-hour post-meal 6.7 mmol/l.
The other general approach to identifying pregnancies that can benefit from intensified metabolic management employs a combination of maternal glucose measurements and foetal morphological measurements. This approach is based on two principles. The first is that certain fasting plasma glucose concentrations, measureable at routine clinic visits, are high enough to warrant intensified treatment because they impart a high risk of preventable perinatal complications. Studies by the authors’ group suggest that a fasting plasma glucose level ≥5.8 mmol/l is useful in this regard.68,69 The second principle is that, among women with lower fasting glucose levels than this, foetal measurements can identify a relatively large fraction of pregnancies that will not incur a perinatal complication in the absence of intensified treatment.
The authors’ group has used foetal abdominal circumference measurements obtained by ultrasonography to identify such pregnancies. In the presence of a fasting plasma glucose level <5.8 mmol/l, a foetal abdominal circumference below the 70th percentile for gestational age between 29–33 weeks of gestation was associated with no excess risk of large for gestational age infants or caesarean deliveries compared with non-diabetic pregnancies.69 Moreover, the findings showed that intensified insulin treatment in pregnancies with foetal abdominal circumference above the 70th percentile could eliminate the excess of large for gestational age infants.69 The capillary glucose targets employed in this last subgroup were fasting <4.4 mmol/l and 2-hr postprandial <6.1 mmol/l). The lower targets can be used because there is virtually no risk of creating small for gestational age infants70 when treatment is directed at foetuses with evidence of increased growth. Note that only women who were placed on insulin require glucose self-monitoring with this approach, providing considerable savings to offset the cost of the foetal ultrasound.
Several options are available for intensifying therapy beyond nutritional management once the decision is made to do so. Traditionally, exogenous insulin was the primary mode of pharmacological treatment. Insulin remains an important option and regimens should be tailored to meet glycaemic targets. No convincing evidence exists to exclude any available insulin from use during pregnancy. Small studies have suggested that regular aerobic exercise can reduce glucose levels as effectively as insulin can.71,72 However, the intensity of exercise employed in these studies was high (for example, 60% of maximal oxygen uptake for 45 min three days a week) and not easy for the women to achieve. Regular exercise of lower intensity, such as walking after meals, is often recommended for women with GDM, although data are lacking on the effect of this level of exercise on pregnancy outcomes.
Two randomized trials have expanded the pharmacological options for GDM to include oral antidiabetic agents. Langer et al.73 compared glyburide with insulin in women who were deemed in need of intensified treatment on the basis of maternal self-monitored glucose level results. They reported equivalent perinatal outcomes in the two groups. Only 4% of women assigned to glyburide received insulin to meet pre-specified glycaemic targets. Rowan et al.59 conducted a similar study, but compared metformin with insulin. Perinatal outcomes were similar in the two treatment groups; 46% of women assigned to metformin received supplemental insulin to achieve glycaemic targets. Patients reported preference for metformin over insulin.
At least two oral agents can, therefore, be used to intensify treatment and achieve good perinatal outcomes. Longer-term information on offspring outcome is available from Rowan et al.,60 who found similar body fat content at age 2 years in offspring of the two treatment groups. As noted above, offspring of mothers assigned to receive metformin had skin-fold evidence of increased subcutaneous fat, which raises the untested possibility that adipose tissue in other locations could be reduced. Information regarding the effects of these agents, which may cross the human placenta, on truly long-term health of offspring are lacking at the present time.
In summary, medical nutritional therapy is recommended for all patients based on principles—reduced caloric intake for overweight and obese women, limited carbohydrate intake and a focus on complex carbohydrates—that are supported by a small amount of good quality evidence. Maternal glucose self-monitoring or a combination of foetal abdominal circumference measurements with fasting plasma glucose measurements in clinic can be used to identify women who may benefit from intensified treatment (or conversely, those who do not need intensification). Insulin, glyburide and metformin are viable options for intensification of treatment in the high-risk groups. Regular exercise is often recommended, but the benefits for pregnancy outcome are largely unknown.
Postpartum Care for Mothers
The main emphasis of postpartum care should be the assessment of the future risk of diabetes mellitus and mitigating that risk. In regard to monitoring, no systematic studies of optimal methods or timing are available. In general, women should have a fasting plasma glucose measurement before hospital discharge to identify the rare patients who have glucose levels in the diabetic range at that time. These women should be treated for diabetes mellitus, whilst other women can be discharged with plans to re-assess glucose levels in the outpatient setting. Glucose tolerance testing 1–4 months postpartum is useful for the identification of additional women with diabetes mellitus and in stratifying the 1–5 year risk of diabetes mellitus.74 The utility of HbA1C testing at this time point is uncertain due to potential influences of blood loss during pregnancy.
In women who do not have diabetes mellitus at postpartum testing, the risk increases linearly for at least the first 5–10 years, during which 30–50% of women develop diabetes mellitus.75 This risk is high enough to warrant testing for diabetes mellitus at least annually. The recommendation for using HbA1C levels to diagnose diabetes mellitus1 made by the American Diabetes Association in 2011 make this annual monitoring relatively simple. Levels of 6.5% or greater indicate diabetes mellitus. Levels of 5.8–6.4% indicate impaired glucose levels and a high risk of diabetes mellitus. Serial HbA1C measurements can also be useful to identify women who are progressing most rapidly toward diabetes mellitus and to assess responses to interventions designed to slow or stop that progression. In this context, changes in HbA1C are more important than an individual value.
In regard to diabetes mellitus risk mitigation, the first step is to decide what type of GDM the patient had. Recall that a small fraction of patients have β-cell dysfunction related to islet autoimmunity or monogenic diabetes. No well-validated approach is available to identify these patients. If a patient does not appear to be insulin resistant (for example, if she is lean) one of these conditions should be considered. Measurement of antibodies to glutamate decarboxylase 65 can identify women who may have evolving T1DM. Although no specific interventions can reduce risk of T1DM, patients may deteriorate rapidly to diabetes mellitus.16 They warrant particularly close monitoring of glucose and/or HbA1C levels. Women with monogenic forms of diabetes who present as GDM generally have a strong family history of diabetes mellitus, consistent with autosomal dominant or maternal inheritance patterns. The diagnosis is complex and consultation with an expert in the genetics of these forms of diabetes is advisable. Some forms respond well to specific therapies, as reviewed by Hattersley and Pearson.76 Genetic counselling is also important for these women.
The large majority of patients with GDM have other risk factors for the development of T2DM, such as obesity or non-European ancestry. Results from the diabetes prevention trials cited above (see Protecting Long-Term Maternal Health) and results of observational studies of the development of T2DM after GDM26 suggest that mitigating the metabolic effects of excess body adipose tissue, especially insulin resistance, is an important approach to reducing the risk of diabetes mellitus. The most logical first step is to reduce body adipose tissue through lifestyle modification. The main principles are to reduce caloric intake through dietary modifications and increase caloric output through exercise. This approach has been proven to reduce the risk of T2DM by ~50–60% in people with impaired glucose tolerance,47,49 including women with a history of GDM.55
Given the high rate of progression to diabetes mellitus after GDM, it is advisable to implement some degree of lifestyle modification in all patients whose diabetes risk appears to be for T2DM. The intensity of the approach may be modified according to the perceived risk. For example, women with prediabetic HbA1C levels (5.8–6.4%) are at sufficiently high risk for diabetes mellitus that they are candidates for the type of intensive lifestyle program utilized in the U.S. Diabetes Prevention Program49 and the Finnish Diabetes Prevention Study.47 Monitoring HbA1C levels for change over time can identify women whose response is appropriate (stable or falling HbA1C levels) and women whose response is inadequate (rising HbA1C levels). However, women who are found to have normal HbA1C levels after pregnancy are still likely to be at an increased risk of diabetes mellitus compared with women who have never had GDM. Controlled trials of diabetes prevention have not been conducted in this group of women, but it makes sense to advise lifestyle changes to reduce body adipose tissue and to intensify lifestyle changes if HbA1C levels rise over time.
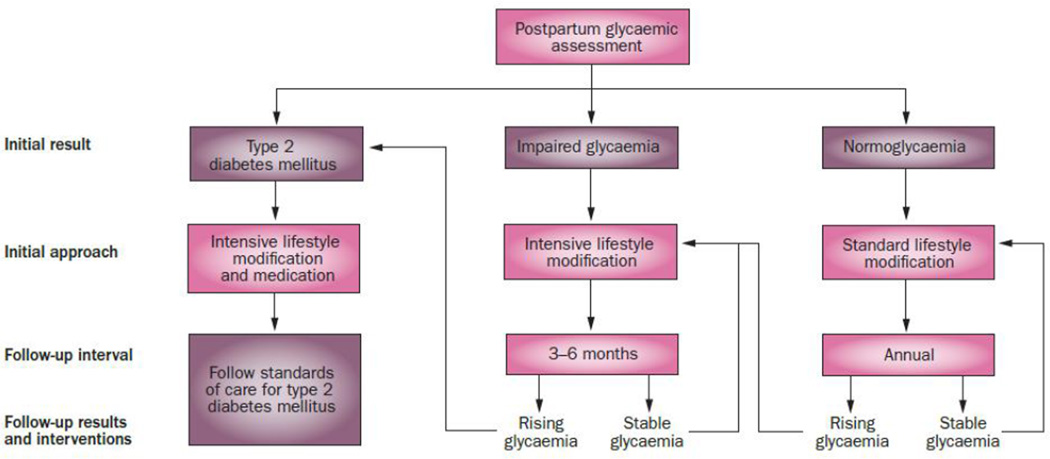
At present, no compelling evidence exists that using medications to prevent diabetes mellitus after GDM provides better long-term outcomes than using medications once diabetes mellitus develops. In addition, medications do not have regulatory approval for treating prediabetes. Only one medication, metformin, was suggested for ‘consideration’ by an expert panel of the American Diabetes Association.77 Our suggestion is to focus on lifestyle changes and monitoring of HbA1C levels in patients who do not have diabetes mellitus. Rising HbA1C levels are indicative of inadequate response to treatment and suggest a need for intensification of lifestyle changes. An HbA1C ≥6.5% indicates development of diabetes mellitus and a need for pharmacological treatment, a topic that is beyond the scope of this Review. See Figure 2 for a summary of our recommendations for management after pregnancy to reduce diabetes risk.
Figure 2.
Suggested management of women with prior gestational diabetes mellitus when risk appears to be for type 2 diabetes mellitus (T2DM). Assessment with oral glucose tolerance test and HBA1C is recommended at 1–4 months postpartum to stratify risk. Women whose initial result is T2DM should begin treatment for that disease. Women whose initial result is impaired glycaemia are at high risk for T2DM. They should participate in intensive lifestyle modification47,49 to reduce weight and they should have HbA1C levels checked every 3–6 months to assess response to treatment. Rising HbA1C levels indicate an inadequate response. Women whose initial postpartum result is normal are at a lower, but still increased risk of diabetes mellitus. They should receive dietary and exercise advice to promote weight loss and be monitored at least annually by measurement of fasting plasma glucose and HbA1C levels. Rising glycaemia, whether glucose or HbA1C, is an indication of deterioration and a need for intensification of treatment. Adapted from reference.82
Several aspects of post-pregnancy care are particularly important to women with prior GDM. One is breastfeeding, which can help women reduce weight after pregnancy, although the effects on the risk of diabetes mellitus are not proven. Breastfeeding has also been associated with reduced obesity of offspring as they grow up (see Improving Long-Term Health of the Offspring), so it is recommended for women with GDM. Another important issue is family planning. The need is great because additional pregnancies can further increase the risk of diabetes mellitus,25,78 and conception after development of diabetes mellitus can lead to major birth defects,9 which could be prevented by appropriate preconceptional glycemic control. Family planning provides an opportunity for prevention of GDM, in particular through weight reduction.79
Although data from randomized controlled trials of contraception after GDM are lacking, observational studies suggest that nearly all forms of contraception are acceptable with one exception. At least two studies have demonstrated an association between an increased risk of diabetes mellitus and use of unopposed systemic progestin contraception. Such an effect was demonstrated for norethisterone in breastfeeding women80 and for depot medroxyprogesterone acetate (DMPA) in women not breastfeeding.81 At least for DMPA, the detrimental effects were associated with weight gain. Thus, we do not recommend using unopposed progestin contraception in women with prior GDM. If this type of contraception is otherwise the best choice, it should be used with careful monitoring for deterioration of glucose or HbA1C levels.
Postpartum Care for Offspring
The potential importance of breastfeeding has been mentioned above. On the basis of the increased risk of obesity and diabetes mellitus during childhood and adolescence, it seems prudent to promote healthy eating and regular exercise and to monitor offspring for development of obesity and related complications.
CONCLUSIONS
The Review highlights many of the controversies and knowledge gaps in the clinical care of GDM. A great need exists for high-quality clinical evidence to support many aspects of care. Two areas deserve special attention. First, adoption of the recommendations of the IADPSG for diagnosis of GDM will result in a large increase, perhaps a doubling, of the incidence of the condition.4 Justification exists for this approach based on perinatal outcomes from the HAPO study. Indeed, because the relationship between maternal glucose levels and perinatal complications is not steep, the additional women identified by the new criteria could have perinatal complication rates that are only slightly lower than the rates of the women diagnosed as having GDM in the past. The real problem is that, even in the past, only ~1/3 of women with GDM incurred a perinatal complication of any sort that could be attributed to the effects of the condition. A much smaller fraction (<10%) incurred clinically important complications such as birth trauma or neonatal jaundice. Doubling the number of women diagnosed with GDM will at least double the number of women who have the diagnosis without real perinatal risk. Approaches based on foetal measurements hold great promise to help clinicians sub-stratify antenatal and perinatal risks in GDM so that intensive treatment can be directed at the women with the truly high-risk pregnancies. Such approaches are deserving of expanded evaluation in clinical trials.
The second area deserving special attention is the heterogeneity of GDM. The Review has already alluded to subtypes of GDM based on known causes of β-cell dysfunction. Even among the large majority of women who do not have autoimmunity or monogenic diabetes, considerable heterogeneity exists. Asian women tend to be leaner than many other ethnic groups, and yet they have high rates of GDM. People of African ancestry have lower GDM rates than other ethnic groups, but higher rates of diabetes mellitus after GDM.12 Understanding the genetic and pathophysiological underpinnings of these differences may be useful in developing more targeted approaches to preventing GDM and preventing diabetes mellitus after GDM in mothers and their offspring.
Key Points.
Gestational diabetes mellitus (GDM) is caused by reduced pancreatic β-cell function, the causes of which include the full spectrum of causes of β-cell dysfunction in young women
GDM is associated with a modest increase in adverse perinatal outcomes, an increased risk of obesity in offspring, and a high risk of post-pregnancy diabetes mellitus in mothers
GDM is treated nutritionally; intensification with insulin or oral antidiabetic agents is added if maternal glucose levels and/or foetal growth parameters indicate a sufficiently high risk of perinatal complications
Long term management of mothers includes assessment of the level and type of diabetes risk and, for women at risk for type 2 diabetes mellitus, lifestyle and/or pharmacological approaches
Long-term management of offspring should focus on detection and mitigation of the development of obesity and its complications
An large need exists for high-quality clinical evidence to determine optimal approaches for management of GDM during and after pregnancy
Review criteria.
The manuscript was built on principles of biology and clinical management that our group have developed in >20 years of research in gestational diabetes mellitus. This knowledge was complemented by examination of recent literature in the areas of antepartum management, diabetes prevention and effects of maternal diabetes on offspring. To this effect, PubMed was searched for full text articles in English covering the time period up to September, 2011for the search term gestational diabetes mellitus.
Footnotes
Competing interests
T. A Buchanan declares associations with the following companies: Allergan, Bristol-Myers Squibb, Novo Nordisk, Takeda, Tethys Bioscience. See the article online for full details of the relationships.
Reference List
- 1.American Diabetes Association. Clinical Practice Recommendations. Diabetes Care. 2011;34(Suppl 1) [PubMed] [Google Scholar]
- 2.Buchanan TA, Xiang AH, Kjos SL, Watanabe RM. What is gestational diabetes? Diabetes Care. 2007;30(suppl 2):S105–S111. doi: 10.2337/dc07-s201. [DOI] [PubMed] [Google Scholar]
- 3.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. HAPO Study Cooperative Research Group: Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 4.International Association of Diabetes and Pregnancy Study Group Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sacks DA, Hadden DR, Maresh M, Deerochanawong C, Dyer AR, Metzger BE, Lowe LP, Coustan DR, Hod M, Oats JN, Persson B, Trimble ER. HAPO Study Cooperative Research Group: The Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria. Diabetes Care. 2012;35:526–528. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan EA. Diagnosing gestational diabetes. Diabetologia. 2011;54:480–486. doi: 10.1007/s00125-010-2005-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long H. Diagnosing gestational diabetes: can expert opinions replace scientific evidence. Diabetologia. 2011;54:2211–2213. doi: 10.1007/s00125-011-2228-z. [DOI] [PubMed] [Google Scholar]
- 8.Paglia MJ, Coustan DR. Gestational diabetes: evolving diagnostic criteria. Curr Opin Obestet Gynecol. 2011;23:72–75. doi: 10.1097/GCO.0b013e328342d21e. [DOI] [PubMed] [Google Scholar]
- 9.Schafer UM, Songster G, Xiang A, Berkowitz K, Buchanan TA, Kjos SL. Congenital malformations in offspring of women with hyperglycemia first detected during pregnancy. Am J Obstet Gynecol. 1997;177:1165–1171. doi: 10.1016/s0002-9378(97)70035-8. [DOI] [PubMed] [Google Scholar]
- 10.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Kaiser Permanente of Colorado GDM Screening Program: Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28:579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol. 2004;103:526–533. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- 12.Xiang AH, Li B, Black M, Sacks DA, Buchanan TA, Jacobsen SJ, Lawrence JM. Racial and ethnic dispairities in diabetes risk after gestational diabetes. Diabetologia. 2011;54:3016–3021. doi: 10.1007/s00125-011-2330-2. [DOI] [PubMed] [Google Scholar]
- 13.Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA. Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes mellitus. Diabetes. 1999;48:848–854. doi: 10.2337/diabetes.48.4.848. [DOI] [PubMed] [Google Scholar]
- 14.Petersen JS, Dyrberg T, Damm P, Kuhl C, Molsted-Pedersen L, Buschard K. GAD65 autoantibodies in women with gestational or insulin dependent diabetes mellitus diagnosed during pregnancy. Diabetologia. 1996;39:1329–1333. doi: 10.1007/s001250050578. [DOI] [PubMed] [Google Scholar]
- 15.Catalano PM, Tyzbir ED, Sims EAH. Incidence and significance of islet cell antibodies in women with previous gestational diabetes. Diabetes Care. 1990;13:478–482. doi: 10.2337/diacare.13.5.478. [DOI] [PubMed] [Google Scholar]
- 16.Mauricio D, Corcoy RM, Codina M, Balsells M, Puig-Domingo M, Pou JM, de Levia A. Islet cell antibodies identify a subset of gestational diabetic women with higher risk of developing diabetes shortly after pregnancy. Diab Nutr Metab. 1992;5:237–241. [Google Scholar]
- 17.Kousta E, Ellard S, Allen LI, Saker PJ, Huxtable SJ, Hattersley AT, McCarthy MI. Glucokinase mutations in a phenotypically selected multiethnic group of women with a history of gestational diabetes. Diabetic Med. 2001;18:683–684. doi: 10.1046/j.1464-5491.2001.00530.x. [DOI] [PubMed] [Google Scholar]
- 18.Weng J, Ekelund M, Lehto M, Li H, Ekberg G, Frid A, Aberg A, Groop LC, Berntorp K. Screening for MODY mutations, GAD antibodies, and type 1 diabetes--associated HLA genotypes in women with gestational diabetes mellitus. Diabetes Care. 2002;25:68–71. doi: 10.2337/diacare.25.1.68. [DOI] [PubMed] [Google Scholar]
- 19.Chen Y, Liao WX, Roy AC, Loganath A, Ng SC. Mitochondrial gene mutations in gestational diabetes mellitus. Diabetes Res Clin Pract. 2000;48:29–35. doi: 10.1016/s0168-8227(99)00138-2. [DOI] [PubMed] [Google Scholar]
- 20.Catalano PM, Tzybir ED, Wolfe RR, Calles J, Roman NM, Amini SB, Sims EAH. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264:E60–E67. doi: 10.1152/ajpendo.1993.264.1.E60. [DOI] [PubMed] [Google Scholar]
- 21.Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes. Am J Obstet Gynecol. 1999;180:903–916. doi: 10.1016/s0002-9378(99)70662-9. [DOI] [PubMed] [Google Scholar]
- 22.Homko C, Sivan E, Chen X, Reece EA, Boden G. Insulin secretion during and after pregnancy in patients with gestational diabetes mellitus. J Clin Endocrinol Metab. 2001;86:568–573. doi: 10.1210/jcem.86.2.7137. [DOI] [PubMed] [Google Scholar]
- 23.Naylor CD, Sermer M, Chen E, Sykora K. Cesarean delivery in relation to birth weight and gestational glucose tolerance: pathophysiology or practice style? JAMA. 1996;275:1165–1170. [PubMed] [Google Scholar]
- 24.O'Sullivan JB. Diabetes after GDM. Diabetes. 1991;40(Suppl 2):131–135. doi: 10.2337/diab.40.2.s131. [DOI] [PubMed] [Google Scholar]
- 25.Xiang AH, Kjos SL, Takayanagi M, Trigo E, Buchanan TA. Detailed physiological characterization of the development of type 2 diabetes in Hispanic women with prior gestational diabetes mellitus. Diabetes. 2010;59:2625–2630. doi: 10.2337/db10-0521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiang AH, Kawakubo M, Trigo E, Kjos SL, Buchanan TA. Declining beta-cell compensation for insulin resistance in Hispanic women with recent gestational diabetes mellitus: association with changes in weight, adiponectin, and C-reactive protein. Diabetes Care. 2010;33:396–401. doi: 10.2337/dc09-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Retnakaran R, Qi Y, Connelly PW, Sermer M, Zinnman B, Hanely AJ. Glucose intolerance in pregnancy and risk of metabolic syndrome in young women. J Clin Endocrinol Metab. 2010;95:670–677. doi: 10.1210/jc.2009-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan SD, Umans JG, Ratner R. Gestational diabetes; implications for cardiovascular health. Curr Diab Rep. 2011 doi: 10.1007/s11892-011-0238-3. e-pub (10-27-2011) [DOI] [PubMed] [Google Scholar]
- 29.Retnakaran R, Shah BR. Mild glucose intolerance in pregnancy and risk of cardiovascular disease: a population-based cohort study. CMAJ. 2009;181:371–376. doi: 10.1503/cmaj.090569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverman BL, Green OC, Cho NH, Winter RJ, Ogata ES, Richards GE, Metzger BE. Long-term prospective evaluation of offspring of diabetic mothers. Diabetes. 1991;40(Suppl 2):S121–S125. doi: 10.2337/diab.40.2.s121. [DOI] [PubMed] [Google Scholar]
- 31.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 32.Dabelea D, Mayer-Davis EJ, Lamichhane AP, D'Agostino RB, Liese AD, Vehik KS, Narayan KM, Zeitler P, Hamman RF. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH case-control study. Diabetes Care. 2008;31:1422–1426. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnaveni JV, Veena SR, Hill JC, Kehoe S, Karat SC, Fall CH. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care. 2010;33:404. doi: 10.2337/dc09-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whitaker RC, Pepe MS, Seidel KD, Wright JA, Knopp RH. Gestational diabetes and the risk of offspring obesity. Pediatrics. 1998;101:e9:1–e9:7. doi: 10.1542/peds.101.2.e9. [DOI] [PubMed] [Google Scholar]
- 35.Catalano PM, Farrell K, Thomas A, Houston-Presley L, Mencin P, de Mouzon SH, Amini SB. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr. 2009;90:1303–1313. doi: 10.3945/ajcn.2008.27416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, Roumain J, Bennett PH, Knowler WC. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- 37.Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. Diabetes Care. 1998;21(Suppl 2):B142–B149. [PubMed] [Google Scholar]
- 38.Crume TL, Ogden L, Daniels S, Hamman RF, Norris JM, Dabelea D. The impact of in utero exposure to diabetes on childhood body mass index growth trajectories: the EPOCH study. J Pediatr. 2011;158:941–946. doi: 10.1016/j.jpeds.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. American Diabetes Association, European Associatoin for Study of Diabetes: Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabete. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.U.S.Preventive Services Task Force. Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;148:759–765. doi: 10.7326/0003-4819-148-10-200805200-00008. [DOI] [PubMed] [Google Scholar]
- 41.Scott DA, Loveman E, McIntyre I, Waugh N. Screening for gestational diabetes: a systematic review and economic evaluation. Health Technol Assess. 2002;6:1–161. doi: 10.3310/hta6110. [DOI] [PubMed] [Google Scholar]
- 42.Canadian Task Force on the Periodic Health Examination. The Canadian Guide to clinical preventive health care. Health Canada. 1994:15–23. [Google Scholar]
- 43.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Australian Carbohydrate Intolerance Study in Pregnat Women Trial Group: Effect of treating gestatoinal diabetes mellitus on pregnancy outcomes. N Engl J Med. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 44.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, Wapner RJ, Varner MW, Rouse DJ, Thorp JM, Sciscione A, Catalano P, Harper M, Saade G, Lain KY, Sorokin Y, Peaceman AM, Tolosa JE, Anderson GB. Eunice Kennedy Shriver National Institute for Child Health and Human Development Maternal-Fetal Medicine Units Network: A multicenter randomized trial of treatemnt for mild gestational diabetes. N Engl J Med. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horvath K, Koch K, Jeitler K, Matyas E, Bender R, Bastian H, Lange S, Siebenhofer A. Effects of treatment in women with gestational diabets mellitus: systematic review and meta-analysis. BMJ. 2010;340 doi: 10.1136/bmj.c1395. c1395:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohno MS, Sparks TN, Cheng YW, Caughey AB. Treating mild gestatoinal diabetes: a cost-effectiveness analysis. Am J Obstet Gynecol. 2011;205 doi: 10.1016/j.ajog.2011.06.051. 282.e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Saliminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 48.Buchanan TA, Xiang AH, Peters RK, Kjos SL, Marroquin A, Goico J, Ochoa C, Tan S, Berkowitz K, Hodis HN, Azen SP. Preservation of pancreatic B-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance in high-risk Hispanic women. Diabetes. 2002;51:2796–2803. doi: 10.2337/diabetes.51.9.2796. [DOI] [PubMed] [Google Scholar]
- 49.Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaisson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M STOP-NIDDM Research Group. Acarbose for prevention of type 2 diabetes mellitus: The STOP-NIDDM randomized trial. The Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 51.DREAM Trial Investigators. Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: randomised controlled trial. Lancet. 2006;368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 52.DeFronzo RA, Tripathy D, Schwenke DC, Banerji MA, Bray G, Buchanan TA, Clement SC, Henry RR, Hodis HN, Kitabchi AE, Mack WJ, Mudaliar S, Ratner RE, Williams K, Stentz FB, Musi N, Reaven PD. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011;364:1104–1115. doi: 10.1056/NEJMoa1010949. [DOI] [PubMed] [Google Scholar]
- 53.Buchanan TA. (How) Can we prevent type 2 diabetes? Diabetes. 2007;56:1502–1507. doi: 10.2337/db07-0140. [DOI] [PubMed] [Google Scholar]
- 54.Xiang A, Peters RK, Kjos SL, Marroqiun A, Goico J, Ochoa C, Kawakubo M, Buchanan TA. Effect of pioglitazone on pancreatic beta cell function and diabetes risk in Hispanic women with prior gestational diabetes. Diabetes. 2006;55:517–522. doi: 10.2337/diabetes.55.02.06.db05-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ratner RE, Christophi CA, Metzger BE, Dabelea D, Bennett PH, Pi-Sunyer X, Fowler S, Kahn SE. Diabetes Prevention Program Research Group: Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle intervention. J Clin Endocrinol Metab. 2008;93:4774–4779. doi: 10.1210/jc.2008-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pettitt DJ, Knowler WC. Long-term effects of the intrauterine environment, birth weight, and breast-feeding on Pima Indians. Diabetes Care. 1998;21(Suppl 2):B138–B141. [PubMed] [Google Scholar]
- 57.Mayer-Davis EJ, Dabelea D, Lamichhane AP, D'Agostino RB, Jr., Liese D, Thomas J, McKeown RE, Hamman RF. Breast-feeding and type 2 diabetes in the youth of three ethnic groups: the SEARCh for diabetes in youth case-control study. Diabetes Care. 2008;31:470–475. doi: 10.2337/dc07-1321. [DOI] [PubMed] [Google Scholar]
- 58.Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes on obesity in the next generation. Diabetes Care. 2010;33:964–968. doi: 10.2337/dc09-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rowan JA, Hague WM, Gao W, Battin MR, Moore PM MiG Trial Investigators. Metformin versus insulin for treatment of gestational diabetes. N Engl J Med. 2008;358:2003–2015. doi: 10.1056/NEJMoa0707193. [DOI] [PubMed] [Google Scholar]
- 60.Rowan JA, Rush EC, Obolonkin V, Battin M, Wouldes T, Hague WM. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU) Diabetes Care. 2011;34:2279–2284. doi: 10.2337/dc11-0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Knopp RH, Magee MS, Raisys V, Benedetti T. Metabolic effects of hypocaloric diets in management of gestational diabetes. Diabetes. 1991;40(Suppl 2):165–171. doi: 10.2337/diab.40.2.s165. [DOI] [PubMed] [Google Scholar]
- 62.Peterson CM, Jovanovic-Peterson L. Percentage of carbohydrate and glycemic response to breakfast, lunch and dinner in women with gestational diabetes. Diabetes. 1991;40(Suppl 1):172–174. doi: 10.2337/diab.40.2.s172. [DOI] [PubMed] [Google Scholar]
- 63.Clapp JEI. Effect of dietary carbohydrate on the glucose and insulin response to mixed caloric intake and exercise in both non-pregnant and pregnant women. Diabetes Care. 1998;21(Suppl 2):B107–B112. [PubMed] [Google Scholar]
- 64.Major CA, Henry MJ, de Veciana M, Morgat MA. The effects of carbohydrate restriction in patients with diet-controlled gestational diabetes. Obstet Gynecol. 1998;91:600–604. doi: 10.1016/s0029-7844(98)00003-9. [DOI] [PubMed] [Google Scholar]
- 65.de Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, Evans AT. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333:1237–1241. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 66.Naylor CD, Sermer M, Chen E, Sykora K. Cesarean delivery in relation to birthweight and gestational glucose tolerance: pathophysiology or practice style? JAMA. 1996;265:1165–1170. [PubMed] [Google Scholar]
- 67.HAPO Study Cooperative Research Group. Hyperglycemia and Adverse Perinatal Outcomes Study: associations with neonatal anthropometrics. Diabetes. 2009;58:453–459. doi: 10.2337/db08-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kjos SL, Schaefer-Graf U, Sardesi S, Peters RK, Buley A, Xiang AH, Byrne JD, Sutherland C, Montoro MN, Buchanan TA. A randomized controlled trial utilizing glycemic plus fetal ultrasound parameters vs glycemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycemia. Diabetes Care. 2001;24:1904–1910. doi: 10.2337/diacare.24.11.1904. [DOI] [PubMed] [Google Scholar]
- 69.Buchanan TA, Kjos SL, Montoro MN, Wu PYK, Madrilejo NG, Gonzalez M, Nunez V, Pantoja PM, Xiang A. Use of fetal ultrasound to select metabolic therapy for pregnancies complicated by mild gestational diabetes. Diabetes Care. 1994;17:275–283. doi: 10.2337/diacare.17.4.275. [DOI] [PubMed] [Google Scholar]
- 70.Langer O, Levy J, Brustman L, Anyaegbunam A, Merkatz R, Divon M. Glycemic control in gestational diabetes mellitus: how tight is tight enough: small for gestational age versus large for gestational age? Am J Obstet Gynecol. 1989;161:646–653. doi: 10.1016/0002-9378(89)90371-2. [DOI] [PubMed] [Google Scholar]
- 71.Bung P, Artal R, Khodiguiab N, Kjos S. Exercise in gestational diabetes. An optional therapeutic approach? Diabetes. 1991;40(Suppl 2):182–185. doi: 10.2337/diab.40.2.s182. [DOI] [PubMed] [Google Scholar]
- 72.Jovanovic-Peterson L, Peterson CM. Is exercise safe or useful for gestational diabetic women? Diabetes. 1991;40(Suppl 2):179–181. doi: 10.2337/diab.40.2.s179. [DOI] [PubMed] [Google Scholar]
- 73.Langer O, Conway DL, Berkus MD, Xenakis EM, Gonzales O. A comparison of glyburide and insulin in women with gestational diabetes mellitus. N Engl J Med. 2000;343:1134–1138. doi: 10.1056/NEJM200010193431601. [DOI] [PubMed] [Google Scholar]
- 74.Kjos SL, Peters RK, Xiang A, Henry OA, Montoro MN, Buchanan TA. Predicting future diabetes in Latino women with gestational diabetes: utility of early postpartum glucose tolerance testing. Diabetes. 1995;44:586–591. doi: 10.2337/diab.44.5.586. [DOI] [PubMed] [Google Scholar]
- 75.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 76.Hattersley AT, Pearson ER. Minireview: pharmacogenetics and beyond: the interaction of therapeutic response, beta-cell physiology, and genetics in diabetes. Endocrinology. 2006;147:2657–2663. doi: 10.1210/en.2006-0152. [DOI] [PubMed] [Google Scholar]
- 77.Nathan D, Davidson M, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. Impaired fasting glucose and impaired glucose tolerance. Diabetes Care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 78.Peters RK, Kjos SL, Xiang A, Buchanan TA. Long-term diabetogenic effect of a single pregnancy in women with prior gestational diabetes mellitus. Lancet. 1996;347:227–230. doi: 10.1016/s0140-6736(96)90405-5. [DOI] [PubMed] [Google Scholar]
- 79.Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol. 2011;117:1323–1330. doi: 10.1097/AOG.0b013e31821aa358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kjos SL, Peters RK, Xiang A, Thomas D, Schafer U, Buchanan TA. Oral contraception and the risk of type 2 diabetes in Latino women with prior gestational diabetes. JAMA. 1998;280:533–538. doi: 10.1001/jama.280.6.533. [DOI] [PubMed] [Google Scholar]
- 81.Xiang AH, Kawakubo M, Kjos SL, Buchanan TA. Long-acting injectable progestin contraception and risk of type 2 diabetes in Latino women with prior gestational diabetes mellitus. Diabetes Care. 2006;29:613–617. doi: 10.2337/diacare.29.03.06.dc05-1940. [DOI] [PubMed] [Google Scholar]
- 82.Buchana TA, Page KL. Approach to the patient with gestational diabetes after delivery. J Clin Endocrinol Metab. 2011;96:3592–3598. doi: 10.1210/jc.2011-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Metzger BE, Buchanan TA, Coustan DR, de Levia A, Dunger DB, Hadden DR, Hod M, Kitzmiller JL, Kjos SL, Oats JN, Pettitt DJ, Sacks DA, Zoupas C. Summary and recommendations of the 5th International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]