Abstract
The objective of this study was to enhance the solubility as well as to mask the intensely bitter taste of the poorly soluble drug, Mefenamic acid (MA). The taste masking and solubility of the drug was improved by using Eudragit® E PO in different ratios via hot melt extrusion (HME), solid dispersion technology. Differential scanning calorimetry (DSC) studies demonstrated that MA and E PO were completely miscible up to 40% drug loads. Powder X-ray diffraction analysis indicated that MA was converted to its amorphous phase in all of the formulations. Additionally, FT-IR analysis indicated hydrogen bonding between the drug and the carrier up to 25% of drug loading. SEM images indicated aggregation of MA at over 30% of drug loading. Based on the FT-IR, SEM and dissolution results for the extrudates, two optimized formulations (20% and 25% drug loads) were selected to formulate the orally disintegrating tablets (ODTs). ODTs were successfully prepared with excellent friability and rapid disintegration time in addition to having the desired taste-masking effect. All of the extruded formulations and the ODTs were found to be physically and chemically stable over a period of 6 months at 40°C/75% RH and 12 months at 25°C/60% RH, respectively.
Keywords: Hot-melt extrusion, Orally disintegrating tablets, Solid dispersion, Solubility enhancement, Taste masking effect, Molecular interaction
1. INTRODUCTION
The oral route of drug administration has many advantages over other routes (i.e. simple self-administration, painlessness compared to the parenteral route, and often excellent patient compliance) and, is generally considered to be the most important route for a drug delivery system [1–4]. However, some patients may find it difficult to swallow intact tablets or capsules. This is particularly true for both pediatric and geriatric populations, who have a tendency to suffer from dysphagia, or among those who suffer from certain mental disorders. These problems could be overcome, in part, by developing orally disintegrating tablets (ODT). These tablets have the capability to dissolve in the mouth rapidly without the need of additional water, or they can dispersed directly in water to make a suspension which is then administered as a liquid [5, 6]. The U.S. FDA has defined ODTs as “A solid dosage form containing medical substance or active ingredient which disintegrates rapidly, usually within a matter of seconds when placed upon the tongue” therefore the disintegration time for ODT’s are limited from seconds up to a minute [7]. In addition to rapid disintegration concerns, ODT formulations must also provide sufficient taste masking as the tablet dissolves in the oral cavity thereby allowing the drug to come into direct contact with the patient’s taste buds. The bitter taste of the drug could be prevented from coming in directly contact with the patient’s tongue, via the formulation of a solid dispersion system [8].
According to the drug discovery field, more than 50% of the active pharmaceutical ingredients (APIs) belongs to class II of Biopharmaceutical classification system (BCS), characterized as poorly soluble compounds resulting in low bioavailability, which is a major disadvantage in oral drug delivery systems [9]. The bioavailability can be enhanced by increasing the apparent solubility of the API [10–12]. Various strategies for improving solubility of poorly water soluble drugs have been developed, such as micronization, chemical modification, pH adjustment, solid dispersion formation, complexation, co-solvency, micellar solubilization, hydrotropy etc [14]. Amorphous solid dispersion formulation is a technique that leads to the conversion of the crystalline lattice to the amorphous phase and disperses the drug molecules in a suitable carrier in order to enhance the solubility of the drug [15]. High drug load in the drug delivery system, especially in solid dispersion systems, is crucial when high dosing is required. Thus, the present study has employed hot-melt extrusion (HME) technology to improve the solubility and to mask the bitter taste of the API in the ODT formulations with maximized drug load.
Over the last decade, HME has been used as a processing technique in the pharmaceutical industry for the formulation of oral solid dosage forms. HME has multiple inherent advantages such as elimination of organic solvents and fewer processing steps compared to the other pharmaceutical technologies [13, 16]. HME has been used to form solid dispersions in order to increase the bioavailability of many drugs by increasing their solubility. This is especially true for BCS class II drugs [17–19]. Also, HME has been utilized for taste masking purposes [20]. Mefenamic acid (MA) [2-(2,3-dimethyl phenyl) aminobenzoic acid], is a non-steroidal anti-inflammatory agent, which acts by inhibiting the activity of cyclo-oxygenase-2 and thereby the production of prostaglandin [21]. It is an odorless white or light gray powder with a melting point of 230°–231°C, with an unpleasant taste, which could lead to patient compliance issues [22, 23] was used as a model drug.
Based on our knowledge, there is no MA ODT available on the market. In addition, there are no taste masking studies reported in the literature regarding the bitter taste of MA. Therefore, the objectives of the present study were to mask the bitter taste and to enhance the solubility of MA using HME solid dispersion techniques, and to optimize the HME processing conditions to manufacture stable orally disintegrating tablets by incorporating milled extrudates. In this study, the authors selected Eudragit® E PO (aminoalkyl methacrylate copolymer) and explored its solubilizing and taste making potential for MA by HME.
2. MATERIALS AND METHODS
2.1. MATERIALS
Mefenamic acid (MA) was purchased from Sigma Aldrich (Bellefonte PA. USA); Eudragit® E PO and Aerosil® was gifted by Evonik (Evonik Industries, Germany); Avicel® 200 was gifted by FMC Biopolymers (Philadelphia, PA. USA); PolyplasdoneTM crospovidone was gifted by ISP Technologies (ISP Technologies, Inc., Wayne, NJ, USA); Magnesium stearate was purchased from Mallinckrodt (St. Louis, MO, USA). All other chemicals used were of analytical grade.
2.2. PREPARATION METHODS
2.2.1. Preparation and Evaluation of Hot-Melt Extrudates
2.2.1.1. Thermal Gravimetric Analysis (TGA)
The thermal stabilities of Eudragit® E PO, Mefenamic acid and the physical mixtures, were determined in the temperature range of 30°C to 250°C, at heating rate of 20°C/min by TGA (Pyris 1 TGA, Perkin Elmer) using Pyris manager software (PerkinElmer Life and Analytical Sciences, 719 Bridgeport Ave., CT, USA). The analysis was performed on samples of approximately 3–5 mg and evaluated as a function of weight loss.
2.2.1.2. Preparation of Hot Melt Extrudates
Mefenamic acid was blended with Eudragit® E PO at drug loadings of 20%, 25%, 30%, and 40% using a V-shell blender (GlobePharma, Maxiblend®, New Brunswick, NJ). The binary mixtures of drug and polymer were extruded using a co-rotating twin-screw extruder (16 mm Prism Euro Lab, ThermoFisher Scientific) at 110°C with a screw speed of 100 rpm. The extrudates were further processed using a comminuting mill (Fitzpatrick, Model L1A), which was sieved by USP mesh (#35).
2.2.1.3. Determination of Drug Loading using Differential Scanning Calorimetry (DSC)
DSC (Diamond DSC, Perkin Elmer) equipped with Pyris manager software, was utilized to determine the physical properties, stability and miscibility of binary mixture of MA with the Eudragit® E PO in each physical mixture and the extrudates. Samples were prepared by weighing 2–4 mg each in hermetically sealed aluminum pans and analyzed at a heating rate of 20°C/minute under an inert nitrogen atmosphere at a flow rate of 20ml/min, over a temperature range of 30°C to 250°C.
2.2.1.4. Powder X-Ray Diffraction (PXRD)
PXRD measurements were used to study the crystallinity of the MA in melt extrudates. The PXRD studies were performed using a powder X-ray diffraction apparatus (Bruker AXS, Madison, MI) at room temperature using CuK a radiation at 15 mA and 30 kV, 4°/min, and diffraction angles (2θ) of 1–40°.
2.2.1.5. Fourier Transform Infrared Spectroscopy (FT-IR)
FT-IR spectroscopic analysis was performed on the extruded samples to study the drug-polymer interactions and corroborate the miscibility results obtained by DSC. FT-IR was conducted on a Cary 660 bench (Agilent Technologies, Santa Clara, CA.). The bench was equipped with an ATR (Pike Technologies MIRacle ATR, Madison, WI), which was fitted with a single bounce diamond coated ZnSe internal reflection element.
2.2.1.6. Scanning Electron Microscope (SEM)
The surface morphology of the pure drug and milled extrudates were evaluated and studied using SEM. Samples were mounted on adhesive carbon pads placed on aluminum stubs prior to sputter coating. A Hummer® 6.2 sputtering system (Anatech LTD, Springfield, VA) in a high vacuum evaporator were used to sputter-coated the samples with gold. SEM (JEOL JSM-5600) operating at an accelerating voltage of 10 kV was used for imaging. Three magnificent (500X, 1000X, 1500X) were used to give more accurate and clear results comparing each blend with pure MA.
2.2.1.7. Quantitative evaluations on extrudates
All of the prepared extrudates were evaluated for content uniformity and dissolution profiles. The milled extrudates were filled into hard HPMC capsules forin vitro drug release studies, each capsule containing the equivalent amount of 100 mg MA. The capsule dissolution tests were conducted in 500 ml of acetate buffer (pH 5.5) dissolution medium utilizing a USP apparatus II (Hanson SR8) at 37 ± 0.5°C for 120 minutes with a rotation speed of 100 rpm (n=3) [24]. All of the samples from the content uniformity test and dissolution studies were analyzed using a Waters HPLC-UV system.
2.2.1.8. HPLC Method
A Waters HPLC (Waters Corp, Milford, MA, USA) and Empower 2 software were used to analyze the samples and data. The stationary phase consisted of a Symmetry Shield C18, 250Å~4.6 mm, 5μm particle size reverse-phase column. The mobile phase consisted of methanol: water: acetonitrile (80:17.5:2.5 v/v) with the pH adjusted to 3.0 with phosphoric acid (85%). The detection wavelength was set at 225 nm and the mobile phase flow rate was maintained at 1.0 mL/min. The injection volume for all samples was 20 μL [25]. All assays studies were performed in triplicate (n=3).
2.2.2. Preparation and Evaluation for Orally Disintegrating Tablets
2.2.2.1. Tablet Preparations
20% and 25% drug loading extrudates were selected to make ODTs based on the FT-IR, SEM and dissolution results. Selected milled extrudates were mixed with excipients (microcrystalline cellulose, Polyplasdone™ crospovidone, colloidal silicon dioxide and magnesium stearate) and sieved using US # 35 of mesh size, and compressed at 4–4.1 kN on a manual tablet press (MCTMI, Globe Pharma Inc., New Brunswick, NJ) using a 12 mm flat punch to a final tablet weight of 650 mg (Table 1).
Table I.
Compositions of Mefenamic Acid ODTs
| Excipients | 20% MA/EPO EXTRUDATES | 25% MA/EPO EXTRUDATES | ||
|---|---|---|---|---|
| % (W/W) | Weight (mg/tablet) | % (W/W) | Weight (mg/tablet) | |
| Mefenamic acid | 15.38 | 100.00 | 15.38 | 100.00 |
| Eudragit® E PO | 61.53 | 400.00 | 46.15 | 300.00 |
| Avicel®102 | 12.58 | 81.75 | 27.96 | 181.75 |
| AEROSIL® 200 | 5.0 | 32.5 | 5.0 | 32.5 |
| Polyplasdone™ crospovidone | 5.0 | 32.5 | 5.0 | 32.5 |
| Magnisium Stearate | 0.5 | 3.25 | 0.5 | 3.25 |
| Total | 100.00 | 650.00 | 100.00 | 650.00 |
2.2.2.2. Compressibility Test to Make ODTs
Prior to compression, compressibility test was carried out with the final mixtures. The bulk density (ρb) was determined by placing a specific amount of the mixture (M) in 100 ml graduated cylinder to measure the bulk volume (Vb). Bulk density was calculated using the following equation [35]:
| Eq. 1 |
Tap density (ρt) was determined by placing a known amount of the mixture into a 100 mL graduated cylinder and measuring its volume. The mixture was then tapped for 100 times and its volume was measured again. The tap density was calculated using the following equation [35]:
| Eq. 2 |
Where M is the amount of the blend and Vt is the tapped volume.
Compressibility index (Carr’s index, I) was determined by the following equation [35]:
| Eq.3 |
2.2.2.3. Tablet Characterization
The compressed tablets were evaluated for thickness, diameter, hardness, friability and disintegration test. A digital vernier caliper (Fisher Scientific) was used to evaluate the thickness and diameter of the tablets. A hardness tester (VarianVK200, Agilent technologies, 13000 Weston Pkwy, Cary, NC was used to evaluate the hardness of the tablets (n=6). Friability studies were performed by Roche friabilator) for 4 min at 25 rpm (n=10, total weight-6.5 g). Disintegration testing was performed on Dr. Schleuniger Pharmatron USP disintegration apparatus (n=6) at 37±0.5°C [5]. The tablet dissolution test for drug release was carried out using the previously outlined conditions for capsules.
2.2.2.4 In vitro taste masking evaluation of OTDs
In vitro dissolution studies were carried out by two different methods to analyze the taste-masking efficiency. In vitro oral drug release was conducted in 150 ml of simulated saliva fluids adjusted to pH 6.8 (Table 3). A dissolution apparatus II (Hanson SR8), which equipped with UV-Vis probes (Rainbow Dissolution Monitor, pION) set at 225 nm with collecting samples every 5 seconds for 120 seconds, was used and maintained at 37 ± 0.5°C with a shaft rotation speed of 100 rpm (n=3). In addition, a 500 ml of water using USP apparatus II (Hanson SR8) at 37 ± 0.5°C with a rotation speed of 50 rpm (n=3) was conducted and samples were collected at time points of 0.5, 1, 2, 3, and 5 minute.
Table III.
Composition of artificial saliva media adjusted to pH 6.8
| Compounds | Concentration (g/L) | |
|---|---|---|
| 1) | CaCl2.2H2O | 0.228 |
| 2) | MgCl2.6H2O | 0.061 |
| 3) | NaCl | 1.017 |
| 4) | K2CO3.1.5H2O | 0.603 |
| 5) | Na2HPO4.7H2O | 0.204 |
| 6) | NaH2PO4.H2O | 0.273 |
2.2.3. Stability Studies
Stability testing was performed over a period of 6 months at accelerated conditions and a year at normal conditions to evaluate the physical and chemical stability of the extrudates and ODTs. The extrudates and ODTs samples were stored in closed glass vials at 40°C/75%RH and 25°C/60% RH, respectively. DSC, FT-IR, and XRD were utilized to determine the influence of the temperature and humidity conditions on the physical stability of the formulations. The extrudates and ODTs samples were evaluated for chemical stability by measuring drug content and content uniformity, which were compared with fresh ones. Additionally, the in vitro drug release studies in aqueous and 0.1 M acetate buffer media were also performed on the samples and the release profiles were compared with the fresh ones using the similarity factor (f2 value) [26]. All of the samples from the drug content, content uniformity test and dissolution studies were analyzed using a Waters HPLC-UV system.
3. RESULTS AND DISCUSSION
3.1 Thermal Analysis
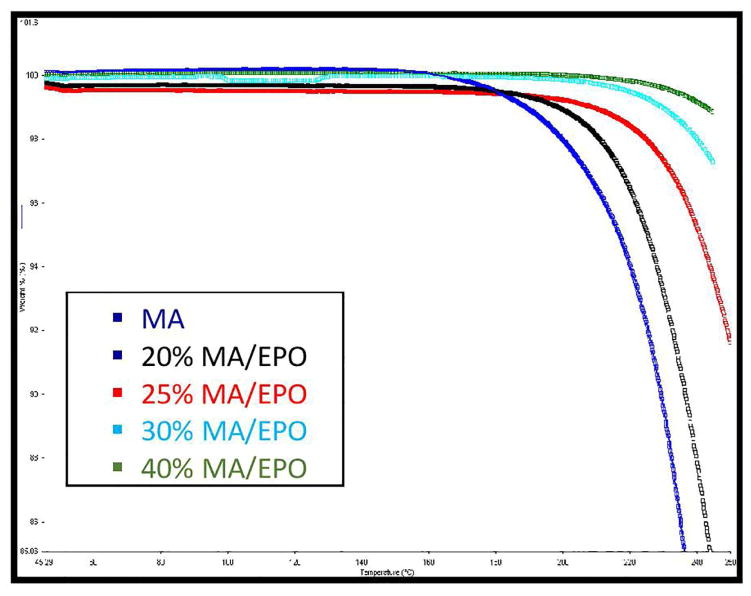
TGA thermogram demonstrated the thermal stability of pure MA and all physical mixtures at the employed processing temperatures (Figure 1). Mefenamic acid and all of the physical mixtures showed no significant degradation up to 200° C. Less than 2% of degradation was considered as residual water of MA and other excipients. Therefore, all of the formulations were assumed to be thermally stable during the hot melt extrusion process.
Fig. 1.
Thermogravimetric analysis of various MA/EPO mixtures
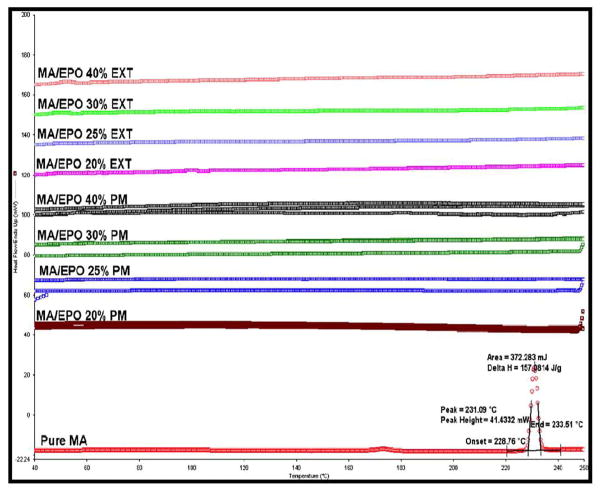
DSC studies of the physical mixtures and the extrudates showed complete miscibility between the MA and Eudragit® E PO at 20%, 25%, 30%, and 40% of drug loadings (Figure 2). Moreover, the DSC studies revealed that MA exhibited an endothermic peak at 230°C. However, the disappearance of the MA melting peak in both of the physical mixtures up to 40% drug load and the melt extrudates confirmed the formation of amorphous solid dispersions of MA in Eudragit® E PO. Additionally, the absence of the melting peak in the thermograms indicates that the drug was completely solubilized in the melt-extruded matrices [27].
Fig. 2.
MA/EPO miscibility studies using DSC at temperatures of 30–250°C and heating rate of 20 °C/min
3.2 Hot Melt Extrusion Process
The hot melt extrusion process is affected by the different physical and chemical properties of the drug and the carrier, which also affects the final product as well [28, 29]. Thus, the process condition should be adjusted and optimized according to the physicochemical properties of both the API and the excipients. Based on the TGA and DSC studies as well as other preliminary studies, the extruder temperature and screw speed seemed to be well adjusted. Therefore, four drug loads (20%, 25%, 30%, and 40%) were extruded at 110° C with a screw speed of 100 rpm to produce uniform rod extrudates. All of the extruded formulations possessed exceptional drug content (>98%) as well as content uniformities (<3% RSD) after hot melt extrusion processing. This is indicative of a robust formulation and process. However, 20% and 25% of drug loaded extrudates were transparent in appearance, whereas 30% and 40% drug loaded extrudates were opaque due to increasing the amount of drug and decreasing the fraction of the Eudragit® E PO.
Eudragit® E PO is a cationic copolymer, consists of dimethyl amino ethyl methacrylate and neutral methacrylic acid ester, which is in the amorphous form. According to the literature on solid dispersion, E PO has multiple uses in the pharmaceutical field such as taste and odor masking [36]. In this work E PO has been selected as a primary carrier due to its cationic nature, which forms counter ionic intermolecular interaction with the weakly acidic drug. The interaction between Eudragit® E PO and MA was later confirmed by FT-IR analysis. This interaction may have aided in improving taste masking efficiency as well as drug release. The advantage of using E PO is that it is very stable thermally, and has a very low glass transition temperature, which is beneficial during HME processing [16, 24]. MA has a high melting point of 230 °C. However, the binary mixtures could be extruded at a low temperature of 110 °C without the addition of a plasticizer, which indicates that MA acted as a plasticizer with E PO.
3.3. In Vitro Dissolution Studies on Extrudates
Mefenamic acid is known to be increasingly soluble when raising the buffer’s pH from pH 1.2 to 9.0. MA is a weak acid (pKa=4.32) and easily ionizes at higher pH [30]. Thus, the US Pharmacopeia recommended pH 9.0 buffer as a dissolution media to produce sink conditions. E PO is basic material (butylated methacrylate copolymer) with high solubility in the gastric media (1.2–5.0 pH), which cannot dissolve or swell above pH 5. This property of E PO makes it an attractive copolymer for taste masking applications. Therefore, choosing acetate buffer of pH 5.5 as a dissolution media is a reasonable choice for this extruded formulation, and has been previously reported in the literature [24]. Drug release studies were performed to understand the dissolution behavior of MA from the binary solid dispersion matrices.
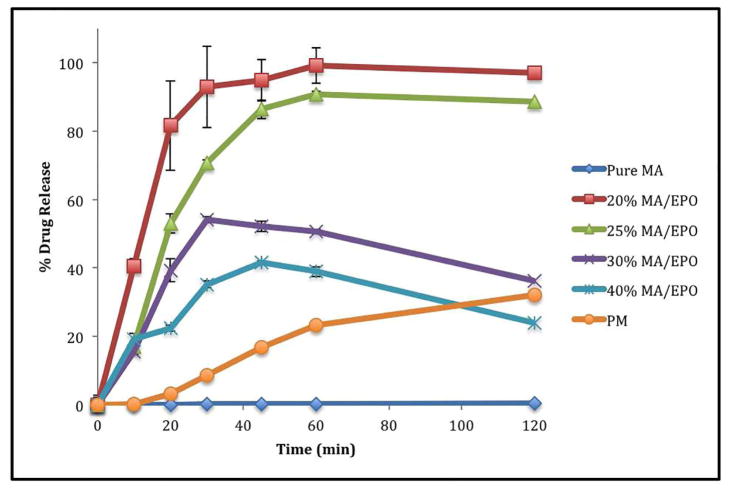
According to thein vitro dissolution studies, drug release from all of the extruded formulations (acetate buffer) demonstrated improved dissolution as compared to the pure drug. The drug release from the 20% and 25% drug loaded extrudates reached more than 85% within 40 minutes, whereas less than 1% drug release within 40 minutes was observed for the pure drug, and less than 15% release for the physical mixture (20% of drug load) under the same conditions (Figure 3). However, the 20% drug loaded formulation demonstrated approximately 99% release within 60 minutes. Thus, the dissolution of MA was greatly enhanced by increasing the portion of Eudragit® E PO, and up to 25% drug loading. This indicates that the melt extruded molecular dispersion with Eudragit® E PO plays an integral role in enhancing the dissolution rate of MA. The mechanism by which the drug release was enhanced in these formulations is discussed in greater detail in the ‘physico-chemical properties of extrudates’ section.
Fig. 3.
In vitro dissolution profiles of MA/EPO extrudates in 0.1 M acetate buffer (pH 5.5)
3.4. Physico-Chemical Properties of Extrudates
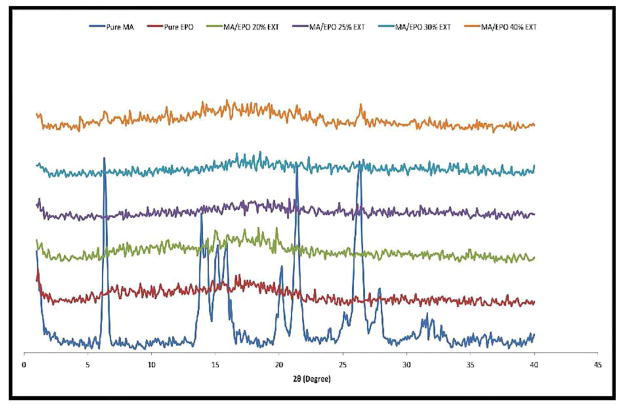
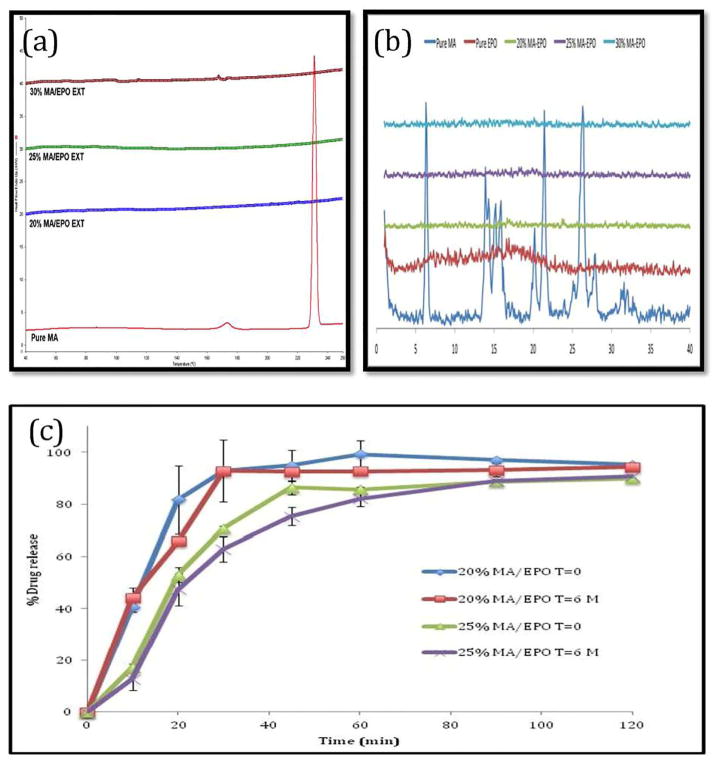
The characteristic PXRD peaks of crystalline MA were observed at 2θ = 6.3, 21.4, and 26.3. These peaks indicated that the pure MA is in a crystalline state. Eudragit® E PO showed no characteristic peaks, indicating the amorphous structure of the polymer. The MA peaks were absent in the hot melt extruded binary formulations with up to 40% drug loading (figure 4). These results confirm that crystalline MA was transformed into the amorphous phase during the HME processing with Eudragit® E PO.
Fig. 4.
PXRD analysis for pure MA, E PO and various MA/EPO extrudates.
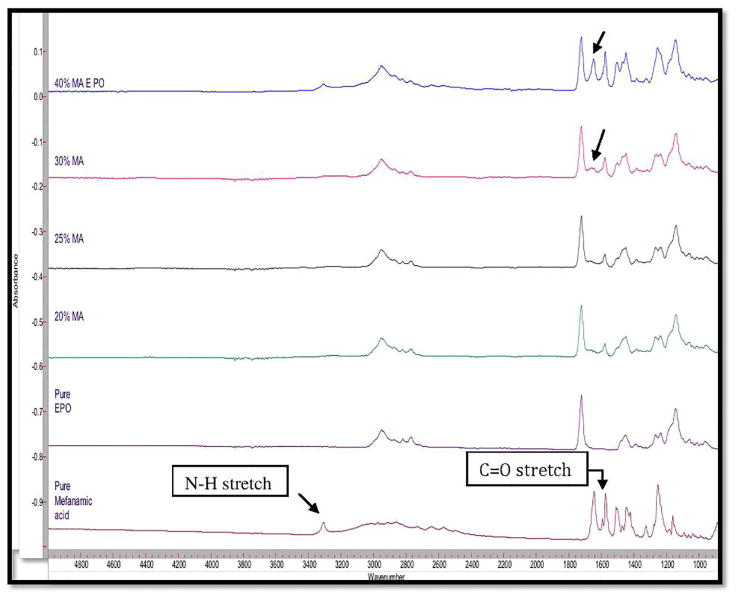
FT-IR analysis was used to observe the drug-polymer interactions usually resulting in peak shifting or the appearance/absence of absorbance peaks. MA showed three significant stretching absorbance peaks at 3,308 cm−1, 1,646 cm−1, and 1,573 cm−1 representative of the functional groups of N-H, C=O, and C=C respectively (figure 5). Additionally, E PO spectra showed one significant peak from the stretching of a carbonyl C=O at 1,730 cm−1. Furthermore, from the chemical structure of MA, it can be seen that available carboxyl group (proton-donating group) and the aminoalkyl group (proton-accepting group) in E PO may have a strong interaction. This interaction was confirmed by the absence of the peaks at 1,646 cm−1 and 1,573 cm−1. Therefore, FTIR analysis clearly indicated strong intermolecular interactions (hydrogen bonding) were induced between MA and the E PO by HME processing. This interaction is presumed to have led to the enhanced MA release profile. However, the MA peak at 1,646 cm−1 reappeared when drug loading increased to 40%. Based on these findings, it is apparent that 30% drug loading saturated the hydrogen bonding capacity of the polymer. Consequently, 30% and 40% of drug loadings showed relatively lower drug release as well as precipitation.
Fig. 5.
FTIR analysis for pure MA, EPO and various MA/EPO extrudates.
Kojima et al. (2012) used MA and E PO to prepare solid dispersion formulations, which were subsequently stabilized in a supersaturated state utilizing cryogenic milling at −180°C for 90 min. They reported that the strong intermolecular interactions between MA and E PO resulted in enhanced bioavailability as well as long-term storage stability in the solid state [24]. In this current research, solid dispersions composed of MA and E PO prepared by hot melt extrusion technology demonstrated the same strong intermolecular interactions, which were made evident by FT-IR analysis.
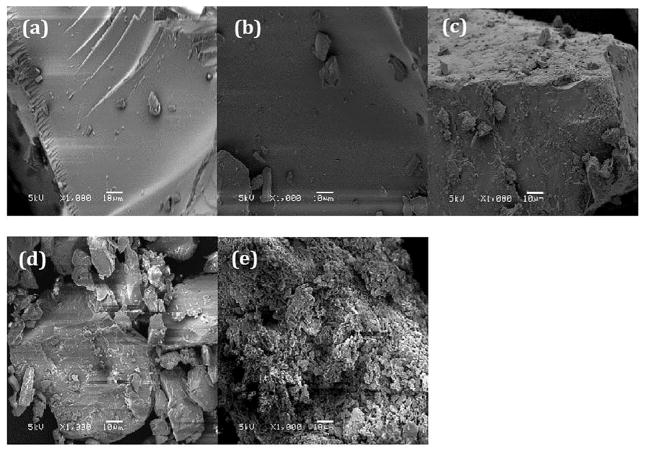
Scanning Electron Microscope (SEM) showed a remarkable difference between pure MA and processed binary mixtures (figure 6). Pure MA (6-e) seemed to consist of microcrystalline aggregates with a coarse surface. On the other hand, 20% (6-a) and 25% (6-b) drug loaded dispersions exhibited smooth surfaces and no aggregation suggesting a completely miscible single-phase binary solid dispersion system. However, increase in drug loading further results in increase in agglomeration as shown in fig.6-c (30%) and 6-d (40%).
Fig. 6.
SEM images: (a) 20% MA/EPO extrudate; (b) 25% MA/EPO exrudate; (c) 30% MA/EPO extrudate; (d) 40% MA/EPO extrudate; (e) Pure MA.
The above analysis confirms that the extrudates up to 25% drug loading produced completely miscible systems. Consequently, 20% and 25% of drug loading hot melt extrudates showed very high dissolution rates as compared to the pure API. Though the drug loadings of 30% and 40% were changed to an amorphous phase, there was an occurrence of precipitation and decrease in the drug release which may be due to the weak or absent intermolecular interaction and agglomeration.. Based on these findings, only 20% and 25% drug loadings were chosen for further studies with orally disintegrating tablets (ODT).
3.5. Preparation and Characterization of Orally Disintegrating Tablets
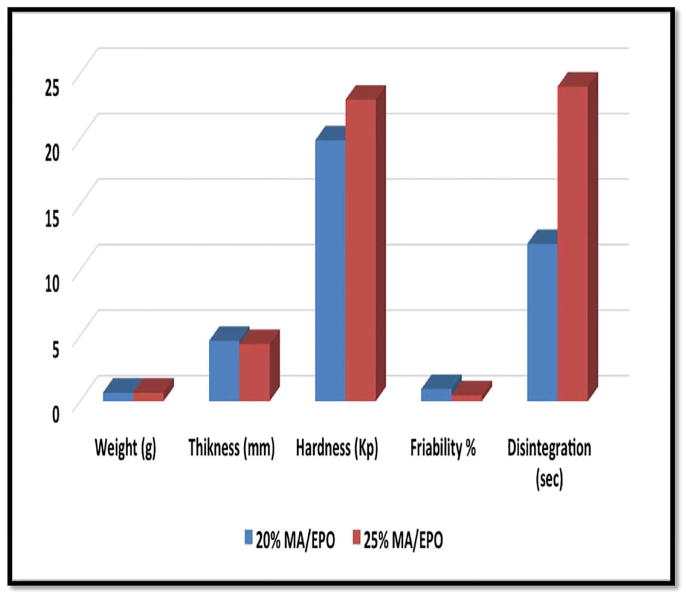
Based on the previous findings, 20% and 25% drug loaded hot-melt extrudates were selected to make orally disintegrating tablets. The selected extrudates and corresponding physical mixtures were mixed with tableting excipients (microcrystalline cellulose, colloidal silicon dioxide, polyplasdoneTM crospovidone) for 10 min using V-shell blender (GlobePharma, Maxiblend® ). Magnesium stearate was added during the final two minutes of mixing. The resulting mixtures were tested for bulk density, tap density and Carr’s index (Table 2). These mixtures were compressed into tablets and evaluated by tablet characterization tests. The results of tablet weight, thickness, hardness, friability and disintegrating time were shown in Figure 7. The ODT with 25% drug loading showed improved hardness and friability values compared to 20% of drug loading, which may be due to decreasing the amount of extrudate and increasing the amount of microcrystalline cellulose. Both ODT formulations exhibited less than 1% friability, which meets the USP specification. The super-disintegrant, cros-povidone, played a key role in disintegration, and consequently, both of the tablets disintegrated in less than 25 seconds. These ODTs showed almost ideal drug content as well as content uniformity for 20% and 25 % drug loaded tablets which were 98.8% ± 2.5 and 102.8% ± 1.0, respectively.
Table II.
Physical Properties of Final Blends (n=3)
| 20% MA/EPO + Excipients | 25% MA/EPO + Excipients | |
|---|---|---|
| Bulk density (g/cm3) | 0.4 | 0.38 |
| Tapped density (g/cm3) | 0.5 | 0.52 |
| Carr’s index (%) | 20.0 | 26.90 |
Fig. 7.
Tablet Properties (weight, thickness, hardness, friability and disintegration time) for 20% and 25% of drug loaded ODTs.
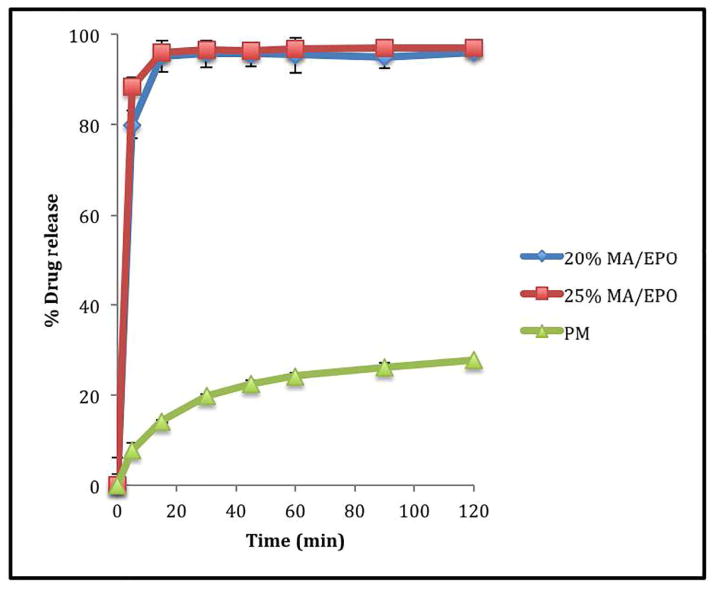
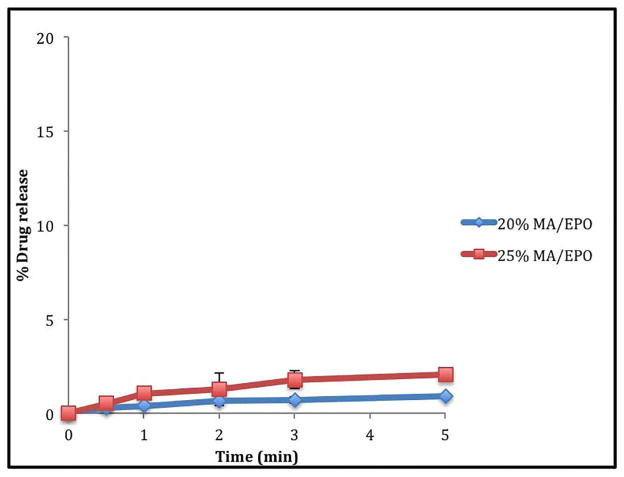
All tablets containing extruded material consistently demonstrated enhanced drug release profiles when compared to tablets containing their corresponding physical mixtures (20% drug load). The drug release from the 20% and 25% drug loaded tablets containing extrudates in pH 5.5 acetate buffer was more than 80% within 5 minutes compared to that less than 10% of drug release was seen with tablets containing physical mixture under the same conditions (Figure 8). However, the drug release from the tablets in the water media for taste masking assessment, which can be placed in a glass of water and administered as a suspension, were less than 2% during the first five minutes (Figure 9) as well as the drug release from the simulated salivary fluid, which can be administered directly into the mouth, were less than 4% during the first 2 minutes (Figure 10) indicating that the bitter taste of the drug was suppressed when processed by hot melt extrusion [37–40].
Fig. 8.
In vitro dissolution profiles of ODTs in 0.1 M acetate buffer (pH 5.5).
Fig. 9.
In vitro taste masking dissolution testing of ODTs in aqueous media.
Fig. 10.
In vitro taste masking dissolution testing of ODTs in artificial saliva media (pH 6.8).
3.6. Stability Tests
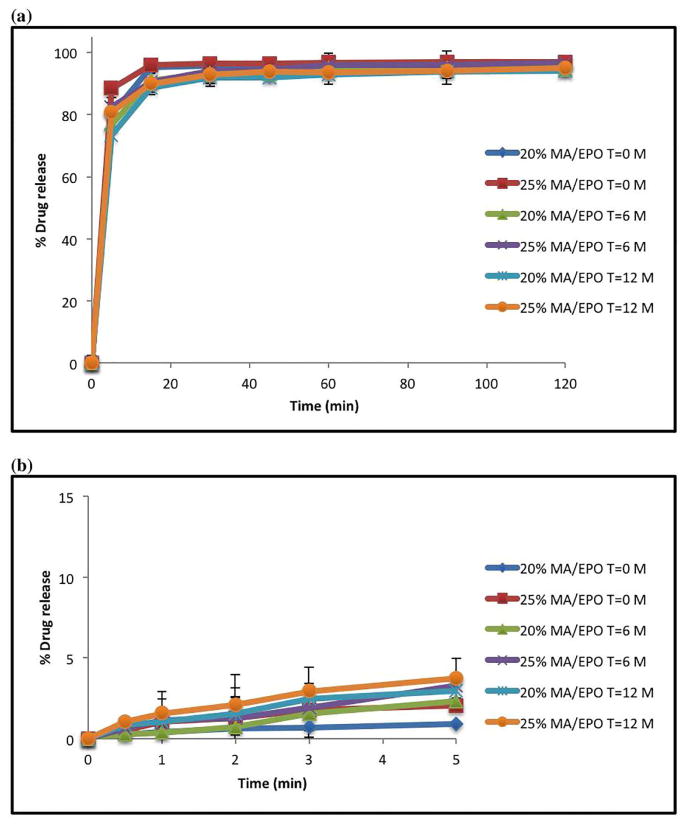
The rate of recrystallization for amorphous drugs during storage is of considerable concern as they are often physically unstable due to their high-energy states [31, 32]. Therefore, there are many polymers commercially available that assist in maintaining drugs in the amorphous form for extended periods of time. Drug-polymer interactions may increase the physical stability by increasing their energy barrier for nucleation [33, 34]. In this work, all of the extruded formulations and ODTs were found to be physically stable when stored under an accelerated stability conditions (40°C/75% RH) and (25° C/60% RH) for a period of 6 months and 12 months, respectively. DSC analysis data showed absence of MA melting endothermic peaks, which confirms the miscibility status of drug, indicating that the MA is still in the amorphous form even after 6 months (Figure 11-a). Additionally, the MA crystalline peaks in XRD for the extrudates were absent as well (Figure 11-b). The FT-IR results also indicated no differences between, before and after stability testing (data not shown). The contents for two drug loaded extrudates (20% and 25%) after 6 months were identical with the results obtained before the stability study. Dissolution studies were also utilized to verify any post-storage changes. The drug release profiles were assessed by similarity factor (f2 value), which was developed to evaluate the degree of similarity between two in vitro dissolution profiles. If the similarity factor is between 50 and 100, that would suggest that two release profiles are similar [26]. The f2 value of 20% and 25% drug loaded extrudates were 59.3 and 62.3 respectively under accelerated condition (40°C/75% RH). The ODTs in 0.1 M acetate buffer media showed more than 85% drug release in 15 minutes, which is considered similar and also it was further supported by f2 value measurements which were 67 and 64. Also the f2 value were 88 (20% drug loading) and 82 (25% drug loading) in aqueous media. It can be considered that the drug release profiles after long-term storage under normal and accelerated conditions were similar to the initial ones (Figure 11-c and Figure 12).
Fig. 11.
Accelerated stability test (40°C/75% RH) for 6 months of MA/EPO extrudates: (a) DSC results; (b) PXRD data; (c) Drug release of extrudates in 0.1 M acetate buffer (pH 5.5).
Fig. 12.
In vitro drug release after 6 & 12 months stability at (25 °C/60% RH) of MA/EPO ODTs: (a) 0.1 M acetate buffer (pH 5.5); (b) aqueous media.
Based on these results, the solid dispersions produced via hot-melt extrusion processing were very stable during 6 months at accelerated conditions (40°C/75% RH). Therefore, the molecular interaction between MA and E PO is playing a key role in API phase stability when processed by hot melt extrusion.
4. Conclusion
Mefenamic acid was successfully extruded with the various concentrations of Eudragit® E PO using HME technology. The optimized formulations produced very promising solid dispersions for both taste masking and solubility enhancement. The dissolution rate of MA was improved with the increase in the concentrations of Eudragit® E PO, indicating that Eudragit® E PO is playing an important role in the solubilization of this high melting point, poorly soluble drug. The mechanism of solubility enhancement was investigated using multiple methodologies, including TGA, DSC, PXRD, SEM and FT-IR. Finally, Mefenamic acid ODTs were successfully produced using HME solid dispersion techniques demonstrating short disintegration times, sufficient taste masking and high drug release profiles.
Acknowledgments
The authors wish to thank Evonik Industries as well as Grant Number P20GM104932 from the National Institute of General Medical Sciences (NIGMS), a component of NIH for contributions to this project. We also thank Dr. Vijayasankar Raman, School of Pharmacy, University of Mississippi for his valued assistance with the SEM imaging studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chaudhary H, Gauri S, Rathee P, Kumar V. Development and optimization of fast dissolving oro-dispersible films of granisetron HCl using Box–Behnken statistical design. Bulletin of Faculty of Pharmacy, Cairo University. 2013;51(2):193–201. [Google Scholar]
- 2.Hearnden V, Sankar V, Hull K, Juras DV, Greenberg M, Kerr AR, Lockhart PB, Patton LL, Porter S, Thornhill MH. New developments and opportunities in oral mucosal drug delivery for local and systemic disease. Adv Drug Deliver Rev. 2012;64(1):16–28. doi: 10.1016/j.addr.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Park JB, Lim J, Kang CY, Lee BJ. Drug Release-Modulating Mechanism of Hydrophilic Hydroxypropylmethylcellulose Matrix Tablets: Distribution of Atoms and Carrier and Texture Analysis. Curr drug delivery. 2013;10(6):732–741. doi: 10.2174/156720181006131125155652. [DOI] [PubMed] [Google Scholar]
- 4.Sattar M, Lane ME. Oral transmucosal drug delivery–Current status and future prospects. Int J Pharm. 2014;471(1–2):498–506. doi: 10.1016/j.ijpharm.2014.05.043. [DOI] [PubMed] [Google Scholar]
- 5.Abdelbary G, Eouani C, Prinderre P, Joachim J, Reynier J, Piccerelle P. Determination of the in vitro disintegration profile of rapidly disintegrating tablets and correlation with oral disintegration. Int J Pharm. 2005;292(1):29–41. doi: 10.1016/j.ijpharm.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Huang M, Shen-Tu J, Hu X, Chen J, Liu J, Wu L. Comparative Fasting Bioavailability of Dispersible and Conventional Tablets of Risperidone: A Single-Dose, Randomized-Sequence, Open-Label, Two-Period Crossover Study in Healthy Male Chinese Volunteers. Clin Ther. 2012;34(6):1432–1439. doi: 10.1016/j.clinthera.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Narmada G, Mohini K, Prakash Rao B, Gowrinath D, Kumar K. Formulation, evaluation and optimization of fast dissolving tablets containing amlodipine besylate by sublimation method. ARS Pharmaceutica. 2009;50(3):129–144. [Google Scholar]
- 8.Kim JI, Cho SM, Cui JH, Cao QR, Oh E, Lee BJ. In vitro and in vivo correlation of disintegration and bitter taste masking using orally disintegrating tablet containing ion exchange resin-drug complex. Int J Pharm. 2013;455(1):31–39. doi: 10.1016/j.ijpharm.2013.07.072. [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Cao F, Zhang C, Ping Q. Use of polymer combinations in the preparation of solid dispersions of a thermally unstable drug by hot-melt extrusion. Acta Pharmaceutica Sinica B. 2013;3(4):263–272. [Google Scholar]
- 10.Shah S, Maddineni S, Lu J, Repka MA. Melt extrusion with poorly soluble drugs. Int J Pharm. 2013;453(1):233–252. doi: 10.1016/j.ijpharm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Tran PHL, Tran TTD, Piao ZZ, Van Vo T, Park JB, Lim J, Oh KT, Rhee YS, Lee BJ. Physical properties and in vivo bioavailability in human volunteers of isradipine using controlled release matrix tablet containing self-emulsifying solid dispersion. Int J Pharm. 2013;450(1):79–86. doi: 10.1016/j.ijpharm.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Vo CLN, Park C, Lee BJ. Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm. 2013;85(3):799–813. doi: 10.1016/j.ejpb.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Crowley MM, Zhang F, Repka MA, Thumma S, Upadhye SB, Kumar Battu S, McGinity JW, Martin C. Pharmaceutical applications of hot-melt extrusion: part I. Drug Dev Ind Pharm. 2007;33(9):909–926. doi: 10.1080/03639040701498759. [DOI] [PubMed] [Google Scholar]
- 14.Vemula VR, Lagishetty V, Lingala S. Solubility enhancement techniques. Int J Pharm Sci Rev Res. 2010;5(1):41–51. [Google Scholar]
- 15.Khan AW, Kotta S, Ansari SH, Sharma RK, Ali J. Enhanced dissolution and bioavailability of grapefruit flavonoid Naringenin by solid dispersion utilizing fourth generation carrier. Drug Dev Ind Pharm. 2014:1–8. doi: 10.3109/03639045.2014.902466. [DOI] [PubMed] [Google Scholar]
- 16.Madana S, Madanb S. Hot melt extrusion and its pharmaceutical applications. Asian J Pharmacol. 2012;7(2):123–133. [Google Scholar]
- 17.Mohammed NN, Majumdar S, Singh A, Deng W, Murthy NS, Pinto E, Tewari D, Durig T, Repka MA. Klucel™ EF and ELF polymers for immediate-release oral dosage forms prepared by melt extrusion technology. AAPS PharmSciTech. 2012;13(4):1158–1169. doi: 10.1208/s12249-012-9834-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JB, Kang CY, Kang WS, Choi HG, Han HK, Lee BJ. New investigation of distribution imaging and content uniformity of very low dose drugs using hot-melt extrusion method. Int J Pharm. 2013;458(2):245–253. doi: 10.1016/j.ijpharm.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Reintjes T. Solubility Enhancement With BASF Pharma Polymers: Solubilizer Compendium. BASF SE Pharma Ingredients & Services; Germany: Oct, 2011. [Google Scholar]
- 20.Maniruzzaman M, Boateng JS, Bonnefille M, Aranyos A, Mitchell JC, Douroumis D. Taste masking of paracetamol by hot-melt extrusion: An in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2012;80(2):433–442. doi: 10.1016/j.ejpb.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 21.Zeinolabedini Hezave A, Khademi MH, Esmaeilzadeh F. Measurement and modeling of mefenamic acid solubility in supercritical carbon dioxide. Fluid Phase Equilibria. 2012;313:140–147. [Google Scholar]
- 22.Adam A, Schrimpl L, Schmidt PC. Some physicochemical properties of mefenamic acid. Drug Dev Ind Pharm. 2000;26(5):477–487. doi: 10.1081/ddc-100101258. [DOI] [PubMed] [Google Scholar]
- 23.Dixit M, Kulkarni PK, KRMP, PRASAD MS. Enhancing solubility and dissolution of Mefenamic acid by freeze drying. Bio Physics. 2011;39:5026–5029. [Google Scholar]
- 24.Kojima T, Higashi K, Suzuki T, Tomono K, Moribe K, Yamamoto K. Stabilization of a supersaturated solution of mefenamic acid from a solid dispersion with EUDRAGIT® EPO. Pharm Res. 2012;29(10):2777–2791. doi: 10.1007/s11095-011-0655-7. [DOI] [PubMed] [Google Scholar]
- 25.Sultana N, Arayne MS, Siddiqui R, Naveed S. RP-HPLC method for the simultaneous determination of lisinopril and NSAIDs in API, pharmaceutical formulations and human serum. American Journal of Analytical Chemistry. 2012;3:147–152. [Google Scholar]
- 26.Kim JY, Kim DW, Kuk YM, Park CW, Rhee YS, Oh TO, Weon KY, Park ES. Investigation of an active film coating to prepare new fixed-dose combination tablets for treatment of diabetes. Int J Pharm. 2012;427(2):201–208. doi: 10.1016/j.ijpharm.2012.01.057. [DOI] [PubMed] [Google Scholar]
- 27.Deng W, Majumdar S, Singh A, Shah S, Mohammed NN, Jo S, Pinto E, Tewari D, Durig T, Repka MA. Stabilization of fenofibrate in low molecular weight hydroxypropylcellulose matrices produced by hot-melt extrusion. Drug Dev Ind Pharm. 2013;39(2):290–298. doi: 10.3109/03639045.2012.679280. [DOI] [PubMed] [Google Scholar]
- 28.Repka MA, Shah S, Lu J, Maddineni S, Morott J, Patwardhan K, Mohammed NN. Melt extrusion: process to product. Expert Opin Drug Deliv. 2012;9(1):105–125. doi: 10.1517/17425247.2012.642365. [DOI] [PubMed] [Google Scholar]
- 29.Sarode AL, Sandhu H, Shah N, Malick W, Zia H. Hot melt extrusion (HME) for amorphous solid dispersions: predictive tools for processing and impact of drug–polymer interactions on supersaturation. Eur J Phar Sci. 2013;48(3):371–384. doi: 10.1016/j.ejps.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Klose C, Straub I, Riehle M, Ranta F, Krautwurst D, Ullrich S, Meyerhof W, Harteneck C. Fenamates as TRP channel blockers: mefenamic acid selectively blocks TRPM3. Br J Pharmacol. 2011;162(8):1757–1769. doi: 10.1111/j.1476-5381.2010.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ewing AV, Clarke GS, Kazarian SG. Stability of indomethacin with relevance to the release from amorphous solid dispersions studied with ATR-FTIR spectroscopic imaging. Eur J Phar Sci. 2014;60:64–71. doi: 10.1016/j.ejps.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Keen JM, Martin C, Machado A, Sandhu H, McGinity JW, DiNunzio JC. Investigation of process temperature and screw speed on properties of a pharmaceutical solid dispersion using corotating and counter-rotating twin-screw extruders. J Pharm Pharmacol. 2014;66(2):204–217. doi: 10.1111/jphp.12106. [DOI] [PubMed] [Google Scholar]
- 33.Konno H, Taylor LS. Influence of different polymers on the crystallization tendency of molecularly dispersed amorphous felodipine. J Pharm Sci. 2006;95(12):2692–2705. doi: 10.1002/jps.20697. [DOI] [PubMed] [Google Scholar]
- 34.Sathigari SK, Radhakrishnan VK, Davis VA, Parsons DL, Babu RJ. Amorphous-state characterization of efavirenz—polymer hot-melt extrusion systems for dissolution enhancement. J Pharm Sci. 2012;101(9):3456–3464. doi: 10.1002/jps.23125. [DOI] [PubMed] [Google Scholar]
- 35.Sree Giri Prasad B, Gupta VRM, Devanna N, Siva Subramanian N, Naveen Kumar CH, Maheswar Reddy CH. Formulation and evaluation of taste masked orodispersible tablets of amlodipine besylate by direct compression. Int Res J Pharm. 2013:2230–8407. [Google Scholar]
- 36.Singh A, Majumdar S, Deng W, Mohammed NN, Chittiboyina AG, Raman V, Shah S, Repka MA. Development and characterization of taste masked Efavirenz pellets utilizing hot melt extrusion. J Drug Del Sci Tech. 2013;23(2):157–163. [Google Scholar]
- 37.Chiappetta DA, Carcaboso ÁM, Bregni C, Rubio M, Bramuglia G, Sosnik A. Indinavir-loaded pH-sensitive microparticles for taste masking: toward extemporaneous pediatric anti-HIV/AIDS liquid formulations with improved patient compliance. AAPS PharmSciTech. 2009;10(1):1–6. doi: 10.1208/s12249-008-9168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh J, Cram A, Woertz K, Breitkreutz J, Winzenburg G, Turner R European Formulation Initiative. Playing hide and seek with poorly tasting paediatric medicines: Do not forget the excipients. Adv Drug Deliv Rev. 2014;73:14–33. doi: 10.1016/j.addr.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 39.Wang L, Sun Y, Kuang C, Zhang X. Preparation and evaluation of taste masked oral suspension of arbidol hydrochloride. AJPS. 2014 [Google Scholar]
- 40.Chiappetta DA, Hocht C, Sosnik A. A highly concentrated and taste-improved aqueous formulation of efavirenz for a more appropriate pediatric management of the anti-HIV therapy. Curr HIV Res. 2010;8(3):223–231. doi: 10.2174/157016210791111142. [DOI] [PubMed] [Google Scholar]