Summary
The distribution of atherosclerotic plaque burden in the human coronary arteries is not uniform. Plaques are located mostly in the left anterior descending artery (LAD), then in the right coronary artery (RCA), circumflex branch (LCx) and the left main coronary artery (LM) in a decreasing order of frequency. In the LAD and LCx, plaques tend to cluster within the proximal segment, while in the RCA their distribution is more uniform. Several factors have been involved in this phenomenon, particularly flow patterns in the left and right coronary artery. Nevertheless, it does not explain the difference in lesion frequency between the LAD and the LCx as these are both parts of the left coronary artery. Branching points are considered to be the risk points of atherosclerosis. In the LCx, the number of side branches is lower than in the LAD or RCA and there are no septal perforators with intramuscular courses like in the proximal third of the LAD and the posterior descending artery (PDA). We hypothesized that septal branches generate disturbed flow in the LAD and PDA in a similar fashion to the myocardial bridge (myocardial bridging effect). This coronary architecture determines the non-uniform plaque distribution in coronary arteries and LAD predisposition to plaque formation.
MeSH Keywords: Atherosclerosis, Haemodynamics, Myocardial Bridge, Septal Branch
Background
The distribution of atherosclerotic plaque burden in human coronary arteries is not uniform. For example, the septal branches are protected against atherosclerosis but the proximal segment of the left anterior descending artery (LAD) is predisposed to plaque formation. Several local hemodynamic factors have been involved in this phenomenon. The purpose of this review was to discuss well-known risk factors involved in plaque formation and to introduce the new ones, in relation to the plaque distribution pattern which is not random.
Plaque Distribution
There is not much research relating to coronary artery atherosclerosis distribution. A high-volume study concerning location of coronary atherosclerosis was published by Montenegro et al. in the year 1968 [1]. They examined 2964 hearts at the autopsy. In his study, atherosclerosis was more prevalent in the left coronary artery (LCA), in particular in the LAD, in comparison to the right coronary artery (RCA). Another autopsy study on 600 male and 600 female human hearts [2,3] and clinical studies on a few hundred patients also confirmed LAD predisposition to plaque formation [4–6]. Giannoglou et al. assessed a cohort of 17,323 patients who underwent coronary angiography [7]. In his survey, 6.5% of patients had an RCA-only disease, while 34.7% had an LCA-only disease.
The amount of coronary artery calcium correlates with plaque burden and the calcium score roughly reflects the magnitude of coronary atherosclerosis [8]. According to tomographic studies, higher spatial distribution of calcalcifications and plaques is found in the LAD, then in RCA, circumflex branch (LCx) and the left main stem (LM) in a decreasing order of frequency [9–13].
Moreover, there was not only a difference in the frequency of plaque distribution in the right and left coronary artery but also the RCA plaques had more uniform distribution along the coronary artery. However, in the LAD or the LCx, they clustered within the proximal segments [14–16]. The present study tried to give a comprehensive explanation of uneven plaque distribution and proximal LAD susceptibility to plaque formation.
Endothelial Shear Stress
Traditional risk factors may influence the evolution and development of atherosclerosis. However, systemic in nature, they do not explain uneven plaque distribution in each coronary artery. Some factors may be involved in the local nature of atherosclerosis but endothelial shear stress (ESS) seems to be crucial. ESS is the tangential stress derived from the friction of the flowing blood on the endothelial surface and is expressed in units of force/unit area (N/m2 or Pascal [Pa] or dyne/cm2; 1 N/m2=1 Pa=10 dyne/cm2). In definition, ESS is proportional to product of the blood viscosity (μ) and the spatial gradient of blood velocity (ESS=μ × dv/dy).
Mechanotransduction
Endothelial cells are equipped with numerous mechanoreceptors (membrane integrins, ion channels, platelet-derived growth factor receptors and G proteins) capable of responding to ESS stimuli. The mechanical forces are converted into biological signals. This phenomenon is termed mechanotransduction. Disturbed, low and low-oscillatory ESS results in intracellular pathways, such as the mitogen-associated protein kinases (MAPKs) and the nuclear factor kappa-light-chain-enhancer of activated B cells pathway, which lead to the activation of several transcription factors and subsequent proatherogenic gene expression [17,18].
In arterial segments (usually straight) with laminar flow, where ESS varies within a physiological range, the endothelial cells (ECs) express atheroprotective genes [17,19–22].
Low and low-oscillatory shear stress causes suppression of atheroprotective genes, whereas the pro-atherogenic genes are upregulated, thereby promoting plaque formation [19,20]. In addition to this, disturbed flow enhances DNA synthesis in ECs, which results in intensified ECs proliferation [23].
Although the process of arterial calcification is not completely understood, the mechanism resembles the process of osteogenesis, involving various cells like calcifying vascular cells (CVCs), proteins like osteopontin (OPN), osteonectin (ON), osteoprotegerin (OPG) and inflammatory cytokines that lead to tissue mineralization [24,25]. Mechanotransduction is also observed in bones where extracellular fluid shear stress, generated in lacunar or canalicular spaces, generates a mechanical stimulus which enhances proliferation of osteocytes and their differentiation into osteoblasts [26].
Moreover, oscillatory ESS induces the formation of bone morphogenic protein 4 (BMP4) in ECs. BMP4 is a critical mechanosensitive, proinflammatory, autocrine cytokine. It increases reactive oxygen species (ROS) production from nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, which then initates the inflammatory cascades, subsequently leading to monocyte adhesion and further atherosclerotic plaque formation. Laminar flow and ESS within physiological range inhibit the expression of BMP4 [27].
ESS-modulating factors
The different factors influencing hemodynamics generate low shear stress (approx. 5 dyn/cm2) and low-oscillatory shear stress (±5 dyn/cm2):
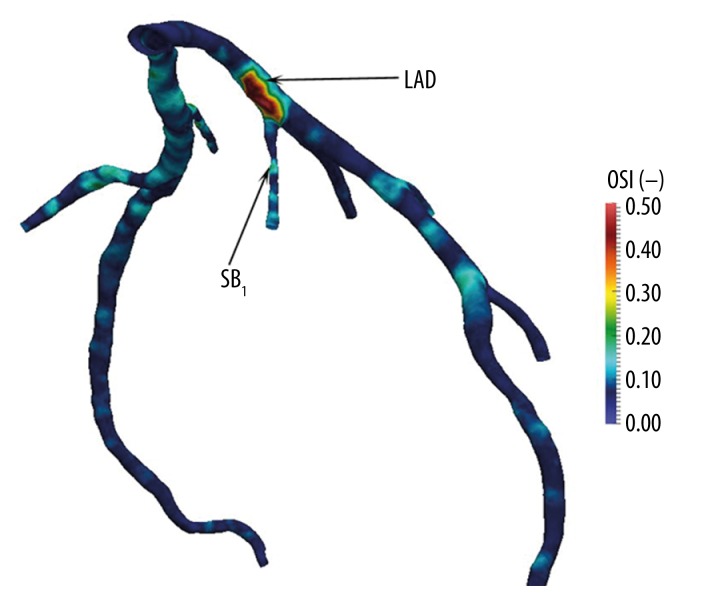
1. In regions such as branching points, the outer wall of bifurcations, and the inner walls of curvatures, where its occurs disturbed flows, generating low and low-oscillatory ESS, which was shown both in coronary and non-coronary arteries in ex vivo and in vivo investigations [20,28] (Figure 1).
Figure 1.
Numerical calculation of the oscillatory shear index (OSI) in the LAD. Increased OSI values are observed at the origin of the septal perforator branch and co-exist with decreasing wall shear stress.
This phenomenon occurs not only in the coronary arteries but also in the entire arterial system, for example in carotid bifurcations, aortic arch and femoral arteries [23].
2. Coronary blood flow pattern varies between the LCA and the RCA. In the LCA it is biphasic, low during systole, showing an abrupt increase of velocity in the early stages of diastole, and gradually declining after that. As much as 85% of the anterograde flow in the LCA occurs during diastole [29]. On the other hand, blood flow in the RCA shows less variation and is almost consistent during the cardiac cycle with a small systolic predominance [29,30].
Differences between the RCA and the LCA flow and shear stress fluctuations follow the same patterns [29,30].
Myocardial Bridge
Myocardial bridge (MB) is a congenital anomaly in which a segment of a coronary artery is surrounded by the myocardium (the artery covered by the myocardium is called a tunneled artery). MB is most commonly found in the middle segment of the LAD [31,32].
Prevalence of MB varies between different analyses and imaging methods used. In autopsy studies it ranges from 5% to 86% of the general population [32]. In conventional contrast-enhanced, coronary angiography it occurs in <5% of patients [32] and in computed tomography angiography more frequently (from 5.7% to 58%) [33,34].
Numerous studies have shown that the myocardial bridge is associated with the development of atherosclerosis proximal to the tunneled artery [20,35,36], wherein the intramyocardial segments is spared which has been shown repeatedly by autopsy studies [37] as well as by several series using cardiac catheterization and intravascular ultrasound (IVUS) [38].
Nakaura et al. recognized MB in the mid-LAD segment as an independent risk factor for coronary atherosclerosis in the proximal LAD [39].
There are two mechanisms related to this phenomenon:
Tensile stress (TS) is a force exerted circumferentially (perpendicular both to the axis and to the radius of the object) in both directions on every particle in the cylinder (artery) wall. It can be approximated by an equation related to Laplace’s law (T=P[r/t]), where P is the blood pressure, r is the lumen radius and t is the wall thickness. As it was described before, coronary flow occurs in the LCA in diastole mainly, whereas systolic blood is stored in dilated arterial walls. However, in the case of myocardial bridging, dilatation of the arterial wall is impossible. In fact, tunneled segments at the systole undergo compression and lumen radius reduction. However, the blood pressure exhibits a significant increase within the bridged segments compared with the adjacent proximal regions [40]. Reduction of the lumen artery radius and subsequent decrease of TS [41] protect coronary arteries from atherosclerosis (high ESS) [42]. At the same time, high TS occurs in the segment proximal to the bridge and a proatherogenic environment is generated [43].
Within the bridged artery, there is no anterograde blood flow in systole followed by accelerated forward flow in diastole. Proximally to the bridge a retrograde flow is generated, similarly followed by accelerated forward flow in diastole [40,42]. Therefore, in the proximal segments, bidirectional flow occurs and generates low and low-oscillatory ESS, whereas in the bridged segments, normal or even high ESS is preserved [44]. In addition to low ESS, increased residence time of proatherogenic blood particles above the tunneled coronary segments promotes their subendothelial accumulation [45].
The morphology of ECs proves the importance of the local hemodynamic milieu in the processes of atherosclerosis development and progression. In scanning electron microscopy of the human LAD, ECs proximal to the MB are polygonal and flat in shape, whereas those beneath MB become spindle-shaped, engorged and aligned along the blood flow direction. Spindle-shaped ECs are found in the areas of high ESS, and polygonal-shaped cells are characteristic for low ESS regions. Moreover, under the physiological ESS, actin fibers of ECs are elongated, well organized, situated in the central region of the cell and the distribution of intercellular junctional proteins is continuous. In contrast, in points of disturbed flow, actin fibers are short, randomly oriented and located mainly at the periphery of ECs with discontinuity of junctional proteins. As a consequence, lipoprotein accumulation is increased [23].
Vertebral arteries might be another example of the atheroprotective role of surrounding tissue. In this case, the intraosseal portions of the vertebral arteries are free from atherosclerosis, which then localizes in segments between bone canals [46]. The same mechanism can explain why septal perforators with intramyocardial courses are protected against plaque formation.
Septal Perforators
Anatomy of septal perforators
Interventricular septum is the most densely vascularized portion of the heart and receives blood both from the left and the right coronary artery [47].
a. Anterior septal perforator arteries originate from the proximal part of the LAD and irrigate 2/3 of the upper part of the interventricular septum. They vary in number (usually from 4 to 13) with an average of eight branches [48]. The length of these vessels ranges from 40 to 80 mm and tends to become shorter as they reach the apex [49].
The first septal artery is usually the largest and the longest one (4 to 6 cm). Its external diameter at the origin ranges from 1.0 mm to 2.35 mm [50]. Most commonly it originates close to the takeoff of the first diagonal branch. Less frequently the first septal perforator might be a short artery or there are two or three major septal arteries comparable in size [48]. This pattern seems to protect the LAD against atherosclerosis.
Infrequently septal perforators arise from the diagonal branch, the RCA or the LM [51–54].
b. Posterior septal perforator arteries originate from the posterior descending artery (PDA) and irrigate 1/3 of the lower part of the interventricular septum. The PDA usually arises as a branch or continuation of the RCA, at the level of the crux cordis on the posterior surface of the heart. It gives rise to small inferior septal branches (varying in number from 6 to 20, length up to 15 mm, diameter of 1 mm on average) which interconnect with anterior septal branches from the LAD and create a network of potential collateral channels [48].
Pro-atherogenic role of the septal perforators
The prevalence of atherosclerosis in the LCA in comparison to the RCA can be attributed to different flow patterns, which was mentioned previously. However it does not explain the disparity between the LAD and the LCx, as these are both parts of the LCA. In the LAD and the LCx, plaques are located in the proximal segments. Nevertheless, in the LCx they occur significantly less frequently than in the LAD or even in the RCA [1–7,14–16] (Figures 2–7).
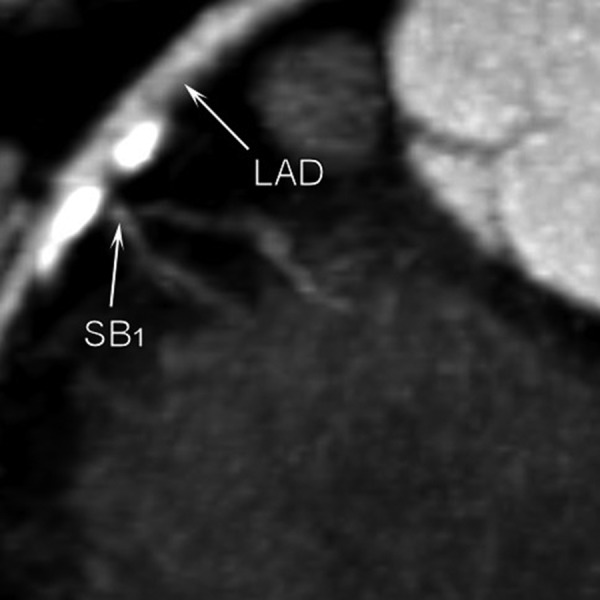
Figure 2.
Multi-slice computed tomography examination. Calcifications are visible proximally to the origin of the first septal branch and on the outer wall of the LM bifurcation.
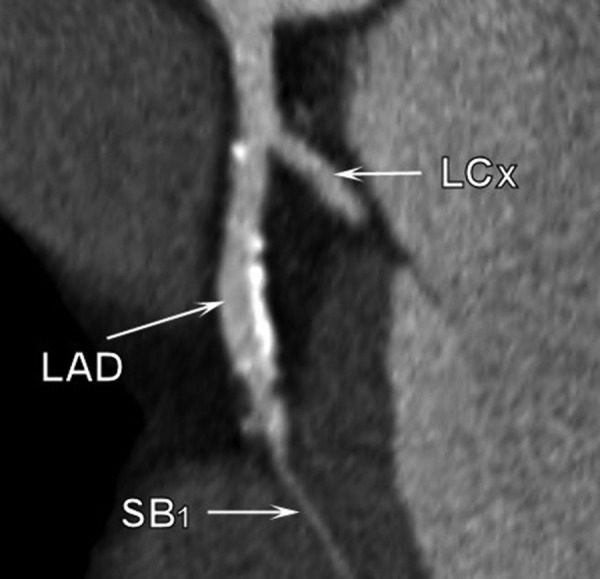
Figure 3.
Multi-slice computed tomography examination. Atherosclerotic plaques are located at the origin of the first septal branch.
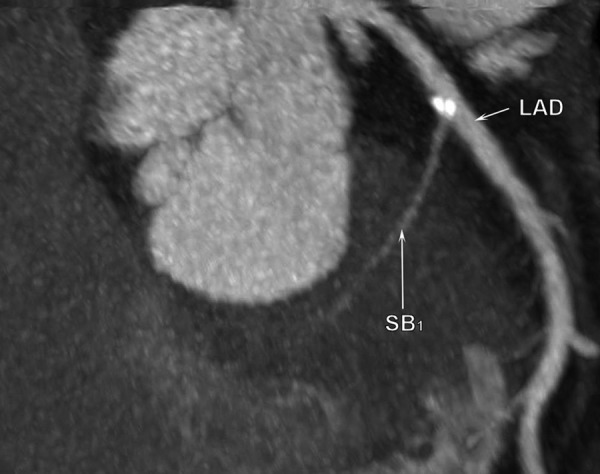
Figure 4.
Multi-slice computed tomography examination. Calcifications are located on the septal side of the LAD, proximally and distally to the first septal branch origin.
Figure 5.
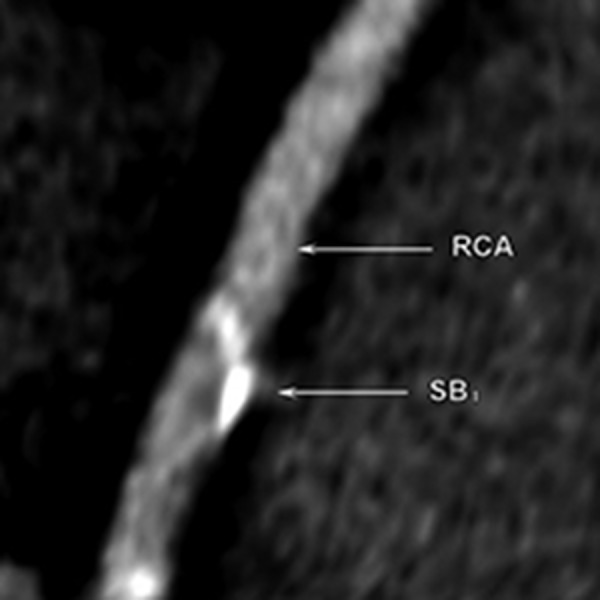
Multi-slice computed tomography examination. The septal branch emerging from the distal portion of the RCA. The calcified plaques are present at the RCA in the proximity to the septal branch take-off.
Figure 6.
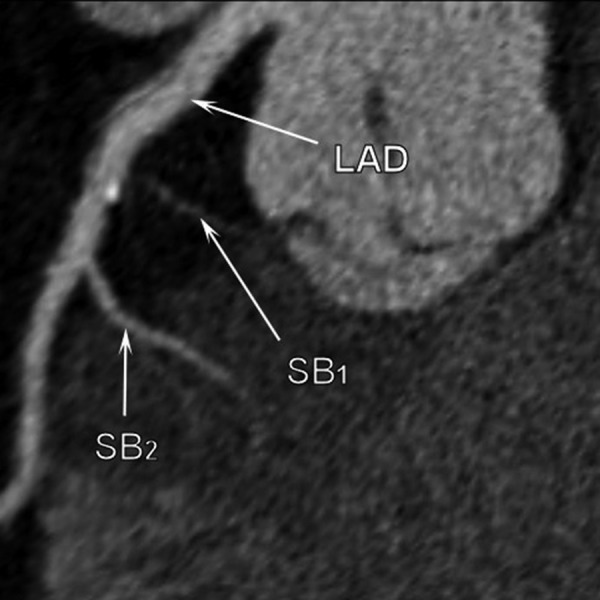
Multi-slice computed tomography examination. Calcifiations are visible on the septal side of the LAD between the origin of first and the second septal branch.
Figure 7.
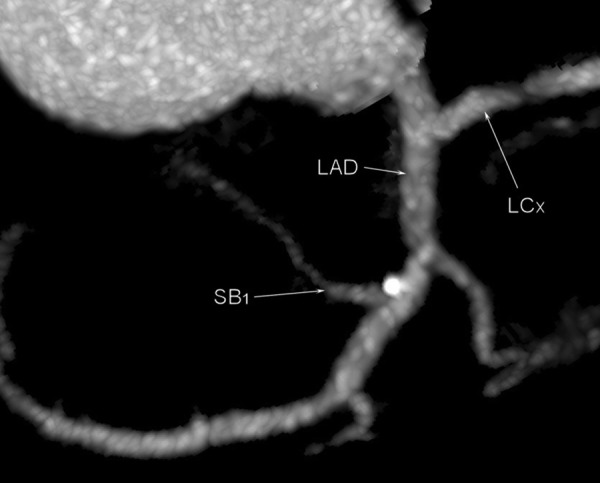
Multi-slice computed tomography examination. Atherosclerotic plaque is located at the origin of the first septal branch and in the close proximity to the origin of the first diagonal branch.
Studies that evaluated plaque distribution in the LAD by ultrasound revealed that in the LAD segments adjacent to the first septal branch, the mean atheroma area and the mean intimal thickness were substantially greater on the septal side than on the antiseptal side [55].
Among patients with myocardial hypertrophy, systolic retrograde flow was observed in septal perforators and adjacent LAD segments. Watanabe et al. with the use of echocardiographic examinations, showed in patients with hypertrophic cardiomyopathy systolic flow reversal in septal perforators as well as in the LAD, caused by systolic compression of the intramyocardial coronary arteries [56], which was previously shown in angiographic studies as well [57].
The compression of SB at systole and reversed or disturbed flow occur as well in patients with isolated aortic stenosis [58,59] and with left ventricular hypertrophy in association with aortic stenosis [60]. It might be an important issue, considering the fact that left ventricular hypertrophy was observed as an important risk factor for sub-clinical atherosclerosis and calcifications [61] and that left ventricular mass, and septal and posterior wall thicknesses of the left ventricle were associated with an increased extent of calcium coronary calcifications [62].
Anatomy of coronary branching. An additional risk factor for non-uniform plaque distribution
In 50–78% of cases the first RCA branch is the conus artery, usually arising from the first 2 cm of that artery [63,64]. The atrial branches of the RCA vary considerably in number, size, and location. Nevertheless, in about 63% of cases the first atrial branch is the sinoatrial node artery. It is also the biggest atrial branch with an external diameter of about 1.7 mm [65]. Additionally, the right superior septal perforator artery might be present, originating from the proximal segment of the RCA. It was detected in 3.2–3.9% of the cases in angiography [52] and computed angiography [54], and in 27% in autopsy studies [53].
The right ventricular branches arise from the middle segment of the RCA. The number of these branches varies greatly and is inversely proportional to the diameter of such vessels [66].
Acute marginal branches are further arteries arising from the RCA [66]. Additionally, in 14–15.1% of cases, usually after the acute marginal branch, there follows the posterior right diagonal artery [67,68].
The atrioventricular node artery originates in 90% of cases from the RCA at the level of the crux and is considered as the first inferior septal perforating branch [65].
At the level of the crux, the RCA divides into two terminal branches: the PDA and the posterolateral artery. The PDA gives rise to posterior septal branches [66].
In the case of a right-dominant pattern of heart circulation which occurs in approximately 85% of patients, the left circumflex artery has two or three obtuse marginal branches [66]. Additionally, in 25% of cases, the sinoatrial node artery derives from the LCx [66]. At the end of the proximal segment, there arises the left atrial branch [66].
From the LAD, except for septal branches described before, usually three diagonal branches arise [66]. Moreover, short right ventricular branches might be present.
This description of the coronary tree shows that the LCx has fewer branches compared with the RCA, and no septal perforators like LAD or PDA. As it was mentioned before, branching points and intramyocardial septal perforators are known to generate a number of risk points with a complex disturbed flow. The frequency of branching and SB distribution determines the plaque pattern and explains why the most prevalent location of atherosclerosis is the proximal LAD and why in the RCA the plaques are distributed more evenly. Finally, it also explains why the LCx is relatively protected against atherosclerosis with a predominance for plaque formation in close proximity to the first side branches (obtuse margin).
Conclusions
Plaque distribution is not uniform. Atherosclerosis occurs more commonly in the LCA than in the RCA but the LCx is less frequently affected. Septal perforators arising from the LAD and RCA cause higher prevalence of atherosclerosis in those two arteries. This effect is intensified by multiple branching points of the RCA and LAD in comparison to the LCx. The architecture of coronary anatomy branching explains the non-uniform distribution of atherosclerotic plaques and prevalence of atherosclerosis in the LAD and RCA in comparison to the LCx.
Footnotes
Disclosures
Authors have no conflicts of interests/financial to disclose.
References
- 1.Montenegro MR, Eggen DA. Topography of atherosclerosis in the coronary arteries. Lab Invest. 1968;18(5):586–93. [PubMed] [Google Scholar]
- 2.Ackerman RF, Dry TJ, Edwards JE. Relationship of Various Factors to the Degree of Coronary Atherosclerosis in Women. Circulation. 1950;1(6):1345–54. doi: 10.1161/01.cir.1.6.1345. [DOI] [PubMed] [Google Scholar]
- 3.White NK, Edwards JE, Dry TJ. The Relationship of the Degree of Coronary Atherosclerosis with Age, in Men. Circulation. 1950;1(4):645–54. [Google Scholar]
- 4.Schmermund A, Baumgart D, Möhlenkamp S, et al. Natural history and topographic pattern of progression of coronary calcification in symptomatic patients: An electron-beam CT study. Arterioscler Thromb Vasc Biol. 2001;21(3):421–26. doi: 10.1161/01.atv.21.3.421. [DOI] [PubMed] [Google Scholar]
- 5.Tuzcu EM, Kapadia SR, Tutar E, et al. High prevalence of coronary atherosclerosis in asymptomatic teenagers and young adults: evidence from intravascular ultrasound. Circulation. 2001;103(22):2705–10. doi: 10.1161/01.cir.103.22.2705. [DOI] [PubMed] [Google Scholar]
- 6.Halon DA, Sapoznikov D, Lewis BS, Gotsman MS. Localization of lesions in the coronary circulation. Am J Cardiol. 1983;52(8):921–26. doi: 10.1016/0002-9149(83)90506-4. [DOI] [PubMed] [Google Scholar]
- 7.Giannoglou GD, Antoniadis AP, Chatzizisis YS, Louridas GE. Difference in the topography of atherosclerosis in the left versus right coronary artery in patients referred for coronary angiography. BMC Cardiovasc Disord. 2010;10:26. doi: 10.1186/1471-2261-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Rourke RA, Brundage BH, Froelicher VF, et al. American College of Cardiology/American Heart Association Expert Consensus Document on Electron-Beam Computed Tomography for the Diagnosis and Prognosis of Coronary Artery Disease: Committee Members. Circulation. 2000;102(1):126–40. doi: 10.1161/01.cir.102.1.126. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Choi E-K, Chang H-J, et al. Subclinical coronary artery disease as detected by coronary computed tomography angiography in an asymptomatic population. Korean Circ J. 2010;40(9):434–41. doi: 10.4070/kcj.2010.40.9.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lai HM, Holtzman D, Aronow WS, et al. Association of coronary artery calcium with severity of myocardial ischemia in left anterior descending, left circumflex, and right coronary artery territories. Clin Cardiol. 2012;35(1):61–63. doi: 10.1002/clc.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enrico B, Suranyi P, Thilo C, et al. Coronary artery plaque formation at coronary CT angiography: morphological analysis and relationship to hemodynamics. Eur Radiol. 2009;19(4):837–44. doi: 10.1007/s00330-008-1223-3. [DOI] [PubMed] [Google Scholar]
- 12.Erciyes D, Şener M, Duran C, et al. Segmental distribution of calcium scores in the coronary arteries. Arch Turk Soc Cardiol. 2012;40:671–80. [Google Scholar]
- 13.Wasilewski J, Niedziela J, Nowakowski A, et al. The Distribution of Coronary Artery Atherosclerosis Burden. The Impact of Septal Perforators on Plaque Localization at the Left Anterior Descending Artery; The Effect of Myocardial Bridging. Am J Cardiol; [OP-064] 10th International Congress of Update in Cardiology and Cardiovascular Surgery; Antalaya [Turkey]. 13–16.03.2014; 2014. p. S19. abstracts. [Google Scholar]
- 14.Golinvaux N, Maehara A, Mintz GS, et al. An intravascular ultrasound appraisal of atherosclerotic plaque distribution in diseased coronary arteries. Am Heart J. 2012;163(4):624–31. doi: 10.1016/j.ahj.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Hong MK, Mintz GS, Cheol WL, et al. The site of plaque rupture in native coronary arteries: A three-vessel intravascular ultrasound analysis. J Am Coll Cardiol. 2005;46(2):261–65. doi: 10.1016/j.jacc.2005.03.067. [DOI] [PubMed] [Google Scholar]
- 16.Cheruvu PK, Finn AV, Gardner C, et al. Frequency and Distribution of Thin-Cap Fibroatheroma and Ruptured Plaques in Human Coronary Arteries. A Pathologic Study. J Am Coll Cardiol. 2007;50(10):940–49. doi: 10.1016/j.jacc.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 17.Li Y-SJ, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38(10):1949–71. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 18.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259(4):381–92. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 19.Gimbrone MA, Topper JN, Nagel T, et al. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–39. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–40. [DOI] [PubMed] [Google Scholar]
- 20.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA. 1999;282(21):2035–42. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85(1):9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 22.Resnick N, Yahav H, Shay-Salit A, et al. Fluid shear stress and the vascular endothelium: For better and for worse. Prog Biophys Mol Biol. 2003;81:177–99. doi: 10.1016/s0079-6107(02)00052-4. [DOI] [PubMed] [Google Scholar]
- 23.Chiu J-J, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–87. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demer LL, Watson KE, Bostrom K. Mechanism of calcification in atherosclerosis. Trends Cardiovasc Med. 1994;4:45–49. doi: 10.1016/1050-1738(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 25.Doherty TM, Asotra K, Fitzpatrick LA, et al. Calcification in atherosclerosis: bone biology and chronic inflammation at the arterial crossroads. Proc Natl Acad Sci USA. 2003;100(20):11201–206. doi: 10.1073/pnas.1932554100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L, Yuan W, Wang J. Mechanisms for osteogenic differentiation of human mesenchymal stem cells induced by fluid shear stress. Biomech Model Mechanobiol. 2010;9:659–70. doi: 10.1007/s10237-010-0206-x. [DOI] [PubMed] [Google Scholar]
- 27.Sorescu GP, Song H, Tressel SL, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–79. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 28.Stone PH, Coskun AU, Yeghiazarians Y, et al. Prediction of sites of coronary atherosclerosis progression: In vivo profiling of endothelial shear stress, lumen, and outer vessel wall characteristics to predict vascular behavior. Curr Opin Cardiol. 2003;18(6):458–70. doi: 10.1097/00001573-200311000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Chatzizisis YS, Giannoglou GD, Parcharidis GE, Louridas GE. Is left coronary system more susceptible to atherosclerosis than right? A pathophysiological insight. Int J Cardiol. 2007;116(1):7–13. doi: 10.1016/j.ijcard.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Chatzizisis YS, Giannoglou GD. Pulsatile flow: A critical modulator of the natural history of atherosclerosis. Med Hypotheses. 2006;67(2):338–40. doi: 10.1016/j.mehy.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Alegria JR, Herrmann J, Holmes DR, et al. Myocardial bridging. Eur Heart J. 2005;26(12):1159–68. doi: 10.1093/eurheartj/ehi203. [DOI] [PubMed] [Google Scholar]
- 32.Mohlenkamp S. Update on Myocardial Bridging. Circulation. 2002;106(20):2616–22. doi: 10.1161/01.cir.0000038420.14867.7a. [DOI] [PubMed] [Google Scholar]
- 33.Nakanishi R, Rajani R, Ishikawa Y, et al. Myocardial bridging on coronary cta: An innocent bystander or a culprit in myocardial infarction? J Cardiovasc Comput Tomogr. 2012;6(1):3–13. doi: 10.1016/j.jcct.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Czekajska-Chehab E, Staśkiewicz G, Orzechowski P, Drop A. Multiple myocardial bridges of coronary arteries in ECG-gated MSCT. Pol J Radiol. 2010;75(1):154–55. [Google Scholar]
- 35.Ishikawa Y, Akasaka Y, Ito K, et al. Significance of anatomical properties of myocardial bridge on atherosclerosis evolution in the left anterior descending coronary artery. Atherosclerosis. 2006;186(2):380–89. doi: 10.1016/j.atherosclerosis.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Ishii T, Asuwa N, Masuda S, Ishikawa Y. The effects of a myocardial bridge on coronary atherosclerosis and ischaemia. J Pathol. 1998;185:4–9. doi: 10.1002/(SICI)1096-9896(199805)185:1<4::AID-PATH50>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 37.Lee SS, Wu TL. The role of the mural coronary artery in prevention of coronary atherosclerosis. Arch Pathol. 1972;93(1):32–35. [PubMed] [Google Scholar]
- 38.Ge J, Erbel R, Rupprecht HJ, et al. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation. 1994;89:1725–32. doi: 10.1161/01.cir.89.4.1725. [DOI] [PubMed] [Google Scholar]
- 39.Nakaura T, Nagayoshi Y, Awai K, et al. Myocardial bridging is associated with coronary atherosclerosis in the segment proximal to the site of bridging. J Cardiol. 2013;63(2):134–39. doi: 10.1016/j.jjcc.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Klues HG, Schwarz ER, vom Dahl J, et al. Disturbed intracoronary hemodynamics in myocardial bridging: early normalization by intracoronary stent placement. Circulation. 1997;96:2905–13. doi: 10.1161/01.cir.96.9.2905. [DOI] [PubMed] [Google Scholar]
- 41.Robicsek F, Thubrikar MJ. The freedom from atherosclerosis of intramyocardial coronary arteries: reduction of mural stress – a key factor. Eur J Cardiothorac Surg. 1994;8:228–35. doi: 10.1016/1010-7940(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 42.Ge J, Jeremias A, Rupp A, et al. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur Heart J. 1999;20:1707–16. doi: 10.1053/euhj.1999.1661. [DOI] [PubMed] [Google Scholar]
- 43.Haga JH, Li Y-SJ, Chien S. Molecular basis of the effects of mechanical stretch on vascular smooth muscle cells. J Biomech. 2007;40:947–60. doi: 10.1016/j.jbiomech.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Chatzizisis YS, Giannoglou GD. Myocardial bridges spared from atherosclerosis: overview of the underlying mechanisms. Can J Cardiol. 2009;25(4):219–22. doi: 10.1016/s0828-282x(09)70065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soulis JV, Giannoglou GD, Chatzizisis YS, et al. Spatial and phasic oscillation of non-Newtonian wall shear stress in human left coronary artery bifurcation: an insight to atherogenesis. Coron Artery Dis. 2006;17:351–58. doi: 10.1097/00019501-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Thubrikar MJ, Robicsek F. Pressure-induced arterial wall stress and atherosclerosis. Ann Thorac Surg. 1995;59(6):1594–603. doi: 10.1016/0003-4975(94)01037-d. [DOI] [PubMed] [Google Scholar]
- 47.Levin DC. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 1988. pp. 268–310. [Google Scholar]
- 48.Topaz O, DiSciascio G, Vetrovec GW. Septal perforator arteries: from angiographic-morphologic characteristics to related revascularization options. Am Heart J. 1992;124(3):810–15. doi: 10.1016/0002-8703(92)90304-e. [DOI] [PubMed] [Google Scholar]
- 49.James TN, Burch GE. Blood Supply of the Human Interventricular Septum. Circulation. 1958;17(3):391–96. doi: 10.1161/01.cir.17.3.391. [DOI] [PubMed] [Google Scholar]
- 50.Possatti LL, Ramos HF, Rodrigues H, Musso F. Anatomical study of the anterior intraventricular septal branches and their relationschip with the blood supply of the septomarginal trabercula. Braz J Morphol Sci. 2005;22:169–74. [Google Scholar]
- 51.Bream PR, Jones JM, Elliott LP. The anomalous septal perforating artery. Its origin from the first diagonal, first marginal, or circumflex artery. Radiology. 1981;138:301–7. doi: 10.1148/radiology.138.2.7455107. [DOI] [PubMed] [Google Scholar]
- 52.Bream PR, Souza AS, Elliott LP, et al. Right superior septal perforator artery: its angiographic description and clinical significance. Am J Roentgenol. 1979;133(1):67–73. doi: 10.2214/ajr.133.1.67. [DOI] [PubMed] [Google Scholar]
- 53.Von Lüdinghausen M, Ohmachi N. Right superior septal artery with “normal” right coronary and ectopic “early” aortic origin: a contribution to the vascular supply of the interventricular septum of the human heart. Clin Anat. 2001;14(5):312–19. doi: 10.1002/ca.1057. [DOI] [PubMed] [Google Scholar]
- 54.Takeguchi T, Ibukuro K, Fukuda H, et al. Anatomy of right superior septal artery demonstrated on the coronary CT scan. Acta Radiol. 2012;53:23–27. doi: 10.1258/ar.2011.110277. [DOI] [PubMed] [Google Scholar]
- 55.Balghith MA, Schoenhagen P, Foody JM, et al. Atherosclerotic plaque distribution in the left anterior descending coronary artery as assessed by intravascular ultrasound. Am J Cardiol. 2003;91(4):443–45. doi: 10.1016/s0002-9149(02)03242-3. [DOI] [PubMed] [Google Scholar]
- 56.Watanabe N, Akasaka T, Yamaura Y, et al. Intramyocardial coronary flow characteristics in patients with hypertrophic cardiomyopathy: non-invasive assessment by transthoracic Doppler echocardiography. Heart. 2003;89(6):657–58. doi: 10.1136/heart.89.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Navarro-Lopez F, Soler J, Magriña J, et al. Systolic compression of coronary artery in hypertrophic cardiomyopathy. Int J Cardiol. 1986;12:309–20. doi: 10.1016/0167-5273(86)90267-6. [DOI] [PubMed] [Google Scholar]
- 58.Kostis JB, Moreyra AE, Natarajan N, et al. The pathophysiology and diverse etiology of septal perforator compression. Circulation. 1979;59:913–19. doi: 10.1161/01.cir.59.5.913. [DOI] [PubMed] [Google Scholar]
- 59.Kenny A, Wisbey CR, Shapiro LM. Profiles of coronary blood flow velocity in patients with aortic stenosis and the effect of valve replacement: a transthoracic echocardiographic study. Br Heart J. 1994;71(1):57–62. doi: 10.1136/hrt.71.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshikawa J, Akasaka T, Yoshida K, Takagi T. Systolic coronary flow reversal and abnormal diastolic flow patterns in patients with aortic stenosis: assessment with an intracoronary Doppler catheter. J Am Soc Echocardiogr. 1993;6(5):516–24. doi: 10.1016/s0894-7317(14)80471-9. [DOI] [PubMed] [Google Scholar]
- 61.Altunkan S, Erdogan N, Altin L, Budoff MJ. Relation of coronary artery calcium to left ventricular mass and geometry in patients with essential hypertension. Blood Press Monit. 2003;8:9–15. doi: 10.1097/00126097-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Gardin JM, Iribarren C, Detrano RC, et al. Relation of echocardiographic left ventricular mass, geometry and wall stress, and left atrial dimension to coronary calcium in young adults (The CARDIA study) Am J Cardiol. 2005;95:626–29. doi: 10.1016/j.amjcard.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 63.Levin DC, Beckmann CF, Garnic JD, et al. Frequency and clinical significance of failure to visualize the conus artery during coronary arteriography. Circulation. 1981;63:833–37. doi: 10.1161/01.cir.63.4.833. [DOI] [PubMed] [Google Scholar]
- 64.Koşar P, Ergun E, Oztürk C, Koşar U. Anatomic variations and anomalies of the coronary arteries: 64-slice CT angiographic appearance. Diagn Interv Radiol. 2009;15:275–83. doi: 10.4261/1305-3825.DIR.2550-09.1. [DOI] [PubMed] [Google Scholar]
- 65.Pejković B, Krajnc I, Anderhuber F, Kosutić D. Anatomical aspects of the arterial blood supply to the sinoatrial and atrioventricular nodes of the human heart. J Int Med Res. 2008;36(4):691–98. doi: 10.1177/147323000803600410. [DOI] [PubMed] [Google Scholar]
- 66.Fioranelli M, Gonnella C. Clinical Anatomy of the Coronary Circulation. Milano: Springer Milan; 2009. pp. 1–11. [Google Scholar]
- 67.Nerantzis CE, Gribizi JE, Margaris NG, et al. Posterior right diagonal artery. Anat Rec. 1994;238:528–32. doi: 10.1002/ar.1092380412. [DOI] [PubMed] [Google Scholar]
- 68.Margaris NG, Kostopoulos KG, Nerantzis CE, et al. Posterior right diagonal artery. An angiographic study. Angiology. 1997;48:673–77. doi: 10.1177/000331979704800802. [DOI] [PubMed] [Google Scholar]