Abstract
Monocytes are a subset of circulating blood cells with remarkable plasticity. They can develop into a wide range of terminally differentiated cells and perform versatile functions during infection, tumor formation and in the setting of chronic inflammation. This review focuses on the role of monocytes during microbial infection and summarizes our understanding of the diverse roles that monocytes play in defense against different pathogens.
Monocytes are a subset of circulating blood cells with remarkable plasticity. They can develop into a wide range of terminally differentiated cells and perform versatile functions during infection, tumor formation and in the setting of chronic inflammation. This review focuses on the role of monocytes during microbial infection and summarizes our understanding of the diverse roles that monocytes play in defense against different pathogens.
Overview: subsets of monocytes and their functions
Although circulating monocytes and their potential role as macrophage progenitors have a long history that extends to the early decades of the last century, it was not until much later, with the advent of flow cytometry, monoclonal antibody generation and murine genetic engineering, that the complexity and plasticity of circulating monocytes could be explored and increasingly defined (Geissmann et al., 2003). Since then, comprehensive phenotypic and functional analyses have been carried out, which demonstrate that monocytes play important and at times divergent roles in various pathological conditions.
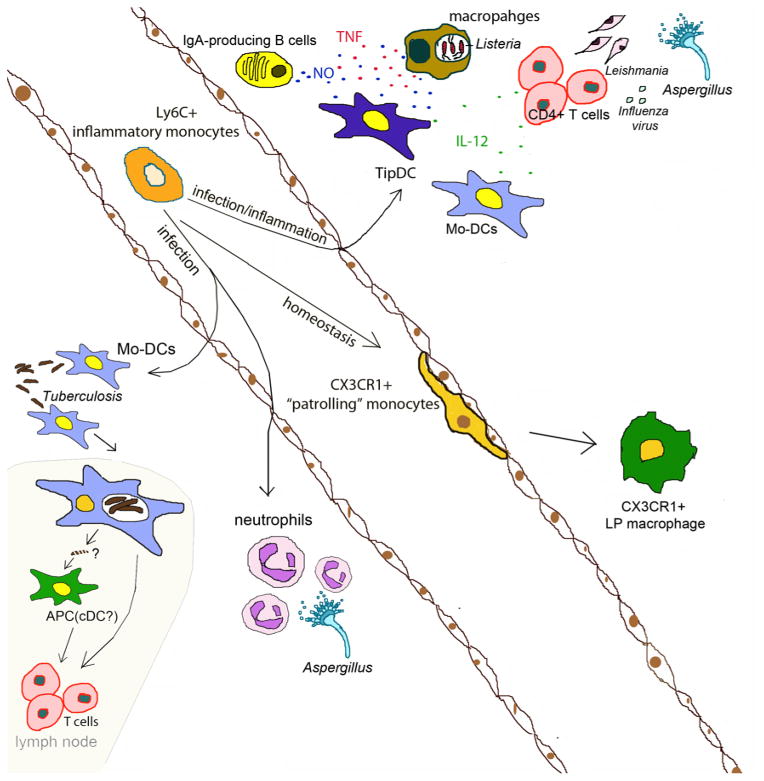
Monocytes are a heterogeneous population, which quickly respond to stimuli including TLR signaling following exposure to pathogens and to inflammatory cytokines (Auffray et al., 2009). Murine monocytes are divided into two categories. Ly6Chi inflammatory monocytes express high levels of CC chemokine receptor 2 (CCR2) but low levels of CX3CR1 (Geissmann et al., 2003), and circulate between blood and bone marrow under homeostatic conditions. They infiltrate tissue upon infection and serve as precursors of different kinds of effector cells, including TNF-and-iNOS Producing Dendritic Cells (TipDCs) during Listeria monocytogenes infections (Serbina et al., 2003b). The diverse role of monocyte-derived cells, which includes secreting cytokines, transporting antigens and priming/polarizing T cells has been demonstrated following infection with different microbial pathogens. (Hohl et al., 2009; Rivera et al., 2011; Samstein et al., 2013) The function of recruited monocytes is highly dependent on the context of infection, the site and the microenvironment. In other settings, such as chronic inflammation or in tumors, monocytes differentiate into so-called myeloid-derived-suppressive cells (MDSC) or macrophages and produce a complex spectrum of cytokines and growth factors, such as IL-10, Arginase, TGF-β and M-CSF, that can suppress immune responses and promote tumor growth (Gabrilovich et al., 2012; Motz and Coukos, 2013).
An interesting subset of circulating monocytes expresses high levels of CX3CR1 and low levels of Ly6C and CCR2 (Geissmann et al., 2003). This subset is characterized by “patrolling” the luminal endothelium. Patrolling by this monocyte subset depends on LFA-1 and ICAM-1 interaction between CX3CR1hi monocytes and the endothelial cells, and experimental studies demonstrate that monocytes contribute to surveillance, wound healing and vascular-endothelial growth (Auffray et al., 2007; Nahrendorf et al., 2007). CX3CR1hi monocytes can differentiate into CX3CR1+ intestinal LP macrophages (Geissmann et al., 2010; Medina-Contreras et al., 2011). Their roles in infections are less well understood, although it has been suggested that they are involved in very early defense against Listeria monocytogenes (Auffray et al., 2009). Recent studies of the two monocyte subsets suggest that CX3CR1intLy6C+ inflammatory monocytes are precursors for CX3CR1+Ly6C monocytes in the intestine under homeostasis (Yona et al., 2013). With inflammation, however, CX3CR1intLy6C+ monocytes develop into pro-inflammatory DCs and prime Th1 responses (Rivollier et al., 2012).
In humans, monocytes can be divided into three subsets based on CD14 and CD16 expression. CD14+CD16+ and CD14+CD16 monocytes resemble mouse inflammatory monocytes and express CCR2 and traffic to the site of infection or inflammation to carry out antimicrobial or pro-inflammatory functions (Geissmann et al., 2003; Serbina et al., 2008). Human CD14loCD16+ cells express CX3CR1 and are involved in endothelial patrolling similar to mouse CX3CR1hi monocytes(Cros et al., 2010).
Monocyte recruitment to the site of infection
Inflammatory monocytes traffic between the bone marrow and bloodstream and constitute a small population of white blood cells under steady state conditions. Upon infection, however, egress of monocytes from the bone marrow to the bloodstream is enhanced and mediated by CCR2-CCL2 interaction. CCR2 is highly expressed on Ly6C+ monocytes and CCL2 (MCP1) expression is induced on PDGFR-b+Nestin+ bone marrow mesenchymal stromal cells (MSCs) and CD31+ bone marrow endothelial cells in a TLR-dependent manner (Serbina and Pamer, 2006; Shi et al., 2011). CCR2-CCL2 interaction is believed to drive inflammatory monocytes migration towards endothelial cells, followed by trafficking into the bloodstream. In the absence of TLR-MyD88 signaling, inflammatory monocyte emigration from the bone marrow can be induced by TNF and type I IFN (Jia et al., 2009), suggesting that inflammatory cytokines may play a compensatory and partially overlapping role with TLR-MyD88 signaling to drive CCR2-CCL2-mediated monocyte trafficking. In addition to CCL2, a second CCR2 ligand, CCL7, is also involved in monocyte trafficking (Jia et al., 2008; Tsou et al., 2007). CCR2KO animals have a profound deficiency in monocyte circulation whereas CCL2KO and CCL7KO mice have a partial reduction under homeostatic conditions and after L.monocytogenes infection (Jia et al., 2008; Shi et al., 2011). Consequently, CCR2KO animals are more susceptible to L.monocytogenes infection than CCL2KO or CCL7KO mice (Jia et al., 2008). These findings suggest that CCL2 and CCL7 make parallel contributions to monocyte trafficking. It is notable that CCL7, but not CCL2, is upregulated in the kidney post infection, suggesting a possible tissue-specific role of CCL7 in antimicrobial defense (Jia et al., 2008). On the other hand, neither CCL2 nor CCL7 seems to be required for circulating monocytes to enter the infected tissue or to differentiate to effector cells. This is illustrated by the finding that CCR2KO monocytes traffic efficiently to sites of infection following adoptive transfer into the bloodstream (Bosschaerts et al., 2010; Serbina and Pamer, 2006; Shi et al., 2010) and differentiate to TipDCs (Jia et al., 2008; Serbina and Pamer, 2006). ICAM-1 has been suggested to mediate monocyte infiltration to target organs. (Shi et al., 2010).
Monocytes directly exert microbicidal effects through TNF and iNOS
As demonstrated in the L.monocytogenes model, monocytes are swiftly recruited and activated at the site of infection. Importantly, activated monocytes can secrete TNF and produce inducible nitric oxide synthase (iNOS, also termed NOS2)(Serbina et al., 2003b). Production of TNF and iNOS by monocytes requires infection by live, virulent bacteria, is dependent on TLR-MyD88 signaling and IFN-γ, and is negatively regulated by Th2 cytokines such as IL-10, IL-4 or IL-13(Bosschaerts et al., 2010; De Trez et al., 2009; Serbina et al., 2003a). TNF and iNOS play an important role in clearing Listeria infection (MacMicking et al., 1995; Nakane et al., 1988; Pfeffer et al., 1993) through distinct mechanisms. TNF functions mainly through TNFR1 during infections, as TNFR1KO animals are highly susceptible and succumb to L. monocytogenes infection (Rothe et al., 1993). TNF-TNFR1 binding activates NF-κB signaling, leading to transcription of inflammatory genes (Chen and Goeddel, 2002; Micheau and Tschopp, 2003), stimulation of IFN-γ production (Tripp et al., 1993) and enhancement of macrophage listericidal activity (Bancroft et al., 1989; Nakane et al., 1988). Membrane-bound TNF has been shown to partially protect infected mice, indicating the requirement for cell-cell interactions (Torres et al., 2005). iNOS production leads to the production of nitric oxide (NO)in phagocytic cells, including monocytes, which likely kills bacteria by inducing DNA damage and disrupting bacterial metabolism. (Nathan and Shiloh, 2000)
Similar to the L.monocytogenes infection model, TNF and/or iNOS produced by monocyte-derived DCs has been implicated in the control of infections caused by Toxoplasmagondii (Dunay et al., 2008; Mordue and Sibley, 2003; Robben et al., 2005), Salmonella typhimurium (Rydstrom and Wick, 2007) Trypanosomabrucei (Bosschaerts et al., 2010), and Influenza virus infections(Aldridge et al., 2009; Lin et al., 2008). In some of these diseases, monocyte functions in addition to TNF and iNOS have been implicated, such as IL-12 production (Dunay et al., 2008). In some cases, monocyte-produced TNF and iNOS can be deleterious. For example, following influenza virus infections, monocyte responses can exacerbate inflammation, causing tissue damage and suppression of wound healing (Karupiah et al., 1998; Lai et al., 2009; Lin et al., 2008).
Monocytes transport antigens to T cells
In addition to differentiating into TipDCs, inflammatory monocytes play versatile and active roles in modulating the development, recruitment and activation of other immune cells during infection. Monocytes can differentiate into dendritic cells and prime CD4+ T cells in various infection models including fungal (Hohl et al., 2009), bacterial (Samstein et al., 2013) and viral infections (Aldridge et al., 2009). The mechanism by which monocytes prime T cell responses has been intensively investigated. Recently, it has been demonstrated, by using a murine Mycobacterium tuberculosis model, that monocytes transport live bacteria to the draining lymph node. (Samstein et al., 2013) Depletion of Ly6Chi monocytes in CCR2-DTR mice at the early and late stages of infection results in distinct outcomes, and that monocyte depletion at early time points significantly reduces the number of live bacteria in mediastinal lymph nodes, which leads to decreased proliferation of M.tuberculosis -specific T cells and thus hampers bacterial clearance. This indicates that live bacterial trafficking to the LN is mediated by inflammatory monocytes and is required for efficient T cell priming. This process does not require MHCII expression on the monocytes, but rather, requires MHCII expression on classical dendritic cells (cDC) in the LN. Taken together, these observations suggest a model in which monocytes carry antigen to lymph nodes and transfer it to cDCs, which present antigens and activate antigen-specific T cells (Samstein et al., 2013). This was similarly observed in a dermal fungal infection model, where monocyte-derived DCs carry attenuated yeast to skin-draining lymph nodes but fail to present the fungal antigen in the node. Instead, dermal and lymph node-resident DCs present fungal antigen and prime naive antigen-specific T cells (Ersland et al., 2010).
Monocytes orchestrate functions of other cells
In addition to antigen transportation, inflammatory monocytes modulate T cell responses. In a pulmonary infection model using the fungal pathogen Aspergillus fumigatus, monocytes are involved in both T cell priming at early time points and T cell polarization at later time points (Hohl et al., 2009; Rivera et al., 2011). Inflammatory monocytes induce Th1 polarization by inducing T-bet expression in responding CD4 T cells (Rivera et al., 2011). Th1 polarization depends on IL-12 and IFN-γ and monocyte-derived cells can produce IL-12 to facilitate priming and expansion of Th1 CD4 T cells in response to infection (Kim et al., 2011; Leon et al., 2007; Schreiber et al., 2013; Wuthrich et al., 2012).
In addition to T cells, monocytes also regulate the function of other cell types. In the case of A. fumigatus infection, inflammatory monocytes optimize the function of neutrophils in clearing the infection in the lung by augmenting neutrophil conidiacidal activity (Espinosa et al., 2014). At the mucosal level, TipDCs regulate B cell class switching and promote mucosal IgA production under steady state conditions. (Tezuka et al., 2007)
Conclusion Remarks
Since their discovery, our understanding of the development and function of monocytes in infections has greatly advanced. Monocytes are highly sensitive and reactive to pathogen-derived molecules, and can quickly respond to microbial-stimuli to inhibit pathogens at early stages of infection. Monocyte differentiation and functionality are highly dependent on the context of the infection. They can enhance microbicidal activities by producing TNF and iNOS, in some settings they transport live microbes to the draining LN for presentation to T cells by DCs and, in other infections, they participate T cell priming and Th1 polarization in draining lymph nodes and at the site of infection. The influence of monocytes on neutrophils and B cells are also being increasingly appreciated. The pathways of monocyte development and differentiation into a wide range of distinct effector cells are being defined. It is likely that this new knowledge will eventually be exploited to enhance defense against infectious pathogens and to limit deleterious inflammatory conditions.
Fig. 1.
Differentiation and effector mechanism of monocytes
Footnotes
Conflict of Interest
The authors claim no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aldridge JR, Jr, Moseley CE, Boltz DA, Negovetich NJ, Reynolds C, Franks J, Brown SA, Doherty PC, Webster RG, Thomas PG. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317:666–670. doi: 10.1126/science.1142883. [DOI] [PubMed] [Google Scholar]
- Auffray C, Sieweke MH, Geissmann F. Blood monocytes: development, heterogeneity, and relationship with dendritic cells. Annual review of immunology. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- Bancroft GJ, Sheehan KC, Schreiber RD, Unanue ER. Tumor necrosis factor is involved in the T cell-independent pathway of macrophage activation in scid mice. J Immunol. 1989;143:127–130. [PubMed] [Google Scholar]
- Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, Herin M, De Baetselier P, Beschin A. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-gamma and MyD88 signaling. PLoS pathogens. 2010;6:e1001045. doi: 10.1371/journal.ppat.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Goeddel DV. TNF-R1 signaling: a beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- Cros J, Cagnard N, Woollard K, Patey N, Zhang SY, Senechal B, Puel A, Biswas SK, Moshous D, Picard C, et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33:375–386. doi: 10.1016/j.immuni.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Trez C, Magez S, Akira S, Ryffel B, Carlier Y, Muraille E. iNOS-producing inflammatory dendritic cells constitute the major infected cell type during the chronic Leishmania major infection phase of C57BL/6 resistant mice. PLoS pathogens. 2009;5:e1000494. doi: 10.1371/journal.ppat.1000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD. Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity. 2008;29:306–317. doi: 10.1016/j.immuni.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersland K, Wuthrich M, Klein BS. Dynamic interplay among monocyte-derived, dermal, and resident lymph node dendritic cells during the generation of vaccine immunity to fungi. Cell host & microbe. 2010;7:474–487. doi: 10.1016/j.chom.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa V, Jhingran A, Dutta O, Kasahara S, Donnelly R, Du P, Rosenfeld J, Leiner I, Chen CC, Ron Y, et al. Inflammatory monocytes orchestrate innate antifungal immunity in the lung. PLoS pathogens. 2014;10:e1003940. doi: 10.1371/journal.ppat.1003940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature reviews Immunology. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, Pamer EG. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell host & microbe. 2009;6:470–481. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T, Leiner I, Dorothee G, Brandl K, Pamer EG. MyD88 and Type I interferon receptor-mediated chemokine induction and monocyte recruitment during Listeria monocytogenes infection. J Immunol. 2009;183:1271–1278. doi: 10.4049/jimmunol.0900460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia T, Serbina NV, Brandl K, Zhong MX, Leiner IM, Charo IF, Pamer EG. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karupiah G, Chen JH, Mahalingam S, Nathan CF, MacMicking JD. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. The Journal of experimental medicine. 1998;188:1541–1546. doi: 10.1084/jem.188.8.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YG, Kamada N, Shaw MH, Warner N, Chen GY, Franchi L, Nunez G. The Nod2 sensor promotes intestinal pathogen eradication via the chemokine CCL2-dependent recruitment of inflammatory monocytes. Immunity. 2011;34:769–780. doi: 10.1016/j.immuni.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC, Lin WJ, Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. The Journal of clinical investigation. 2009;119:3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- MacMicking JD, Nathan C, Hom G, Chartrain N, Fletcher DS, Trumbauer M, Stevens K, Xie QW, Sokol K, Hutchinson N, et al. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell. 1995;81:641–650. doi: 10.1016/0092-8674(95)90085-3. [DOI] [PubMed] [Google Scholar]
- Medina-Contreras O, Geem D, Laur O, Williams IR, Lira SA, Nusrat A, Parkos CA, Denning TL. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. The Journal of clinical investigation. 2011;121:4787–4795. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Mordue DG, Sibley LD. A novel population of Gr-1+-activated macrophages induced during acute toxoplasmosis. Journal of leukocyte biology. 2003;74:1015–1025. doi: 10.1189/jlb.0403164. [DOI] [PubMed] [Google Scholar]
- Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. The Journal of experimental medicine. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A, Minagawa T, Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infection and immunity. 1988;56:2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer K, Matsuyama T, Kundig TM, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi PS, Kronke M, Mak TW. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- Rivera A, Hohl TM, Collins N, Leiner I, Gallegos A, Saijo S, Coward JW, Iwakura Y, Pamer EG. Dectin-1 diversifies Aspergillus fumigatus-specific T cell responses by inhibiting T helper type 1 CD4 T cell differentiation. The Journal of experimental medicine. 2011;208:369–381. doi: 10.1084/jem.20100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. The Journal of experimental medicine. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robben PM, LaRegina M, Kuziel WA, Sibley LD. Recruitment of Gr-1+ monocytes is essential for control of acute toxoplasmosis. The Journal of experimental medicine. 2005;201:1761–1769. doi: 10.1084/jem.20050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- Rydstrom A, Wick MJ. Monocyte recruitment, activation, and function in the gut-associated lymphoid tissue during oral Salmonella infection. J Immunol. 2007;178:5789–5801. doi: 10.4049/jimmunol.178.9.5789. [DOI] [PubMed] [Google Scholar]
- Samstein M, Schreiber HA, Leiner IM, Susac B, Glickman MS, Pamer EG. Essential yet limited role for CCR2+ inflammatory monocytes during Mycobacterium tuberculosis-specific T cell priming. eLife. 2013;2:e01086. doi: 10.7554/eLife.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber HA, Loschko J, Karssemeijer RA, Escolano A, Meredith MM, Mucida D, Guermonprez P, Nussenzweig MC. Intestinal monocytes and macrophages are required for T cell polarization in response to Citrobacter rodentium. The Journal of experimental medicine. 2013;210:2025–2039. doi: 10.1084/jem.20130903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annual review of immunology. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003a;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nature immunology. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003b;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590–601. doi: 10.1016/j.immuni.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Velazquez P, Hohl TM, Leiner I, Dustin ML, Pamer EG. Monocyte trafficking to hepatic sites of bacterial infection is chemokine independent and directed by focal intercellular adhesion molecule-1 expression. J Immunol. 2010;184:6266–6274. doi: 10.4049/jimmunol.0904160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka H, Abe Y, Iwata M, Takeuchi H, Ishikawa H, Matsushita M, Shiohara T, Akira S, Ohteki T. Regulation of IgA production by naturally occurring TNF/iNOS-producing dendritic cells. Nature. 2007;448:929–933. doi: 10.1038/nature06033. [DOI] [PubMed] [Google Scholar]
- Torres D, Janot L, Quesniaux VF, Grivennikov SI, Maillet I, Sedgwick JD, Ryffel B, Erard F. Membrane tumor necrosis factor confers partial protection to Listeria infection. The American journal of pathology. 2005;167:1677–1687. doi: 10.1016/S0002-9440(10)61250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. The Journal of clinical investigation. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich M, Ersland K, Sullivan T, Galles K, Klein BS. Fungi subvert vaccine T cell priming at the respiratory mucosa by preventing chemokine-induced influx of inflammatory monocytes. Immunity. 2012;36:680–692. doi: 10.1016/j.immuni.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]