Abstract
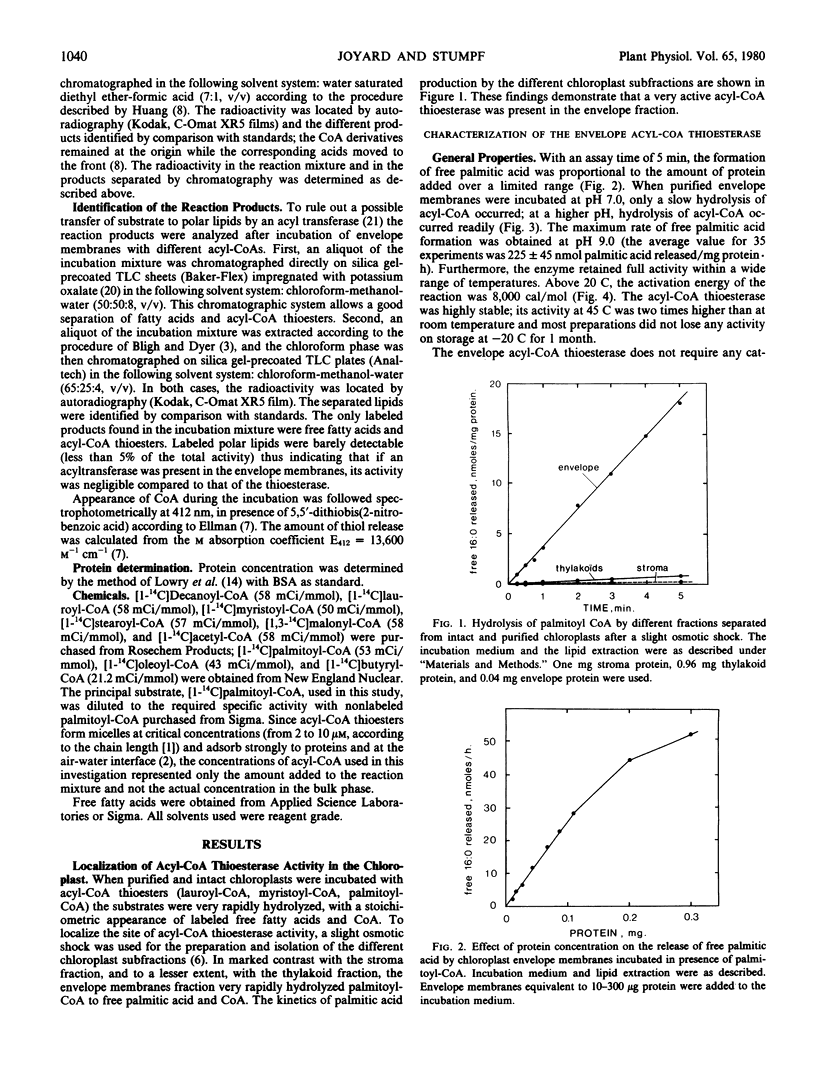
The enzymic hydrolysis of acyl-coenzyme A occurs in intact and purified chloroplasts. The different components of spinach chloroplasts were separated after a slight osmotic shock and the purified envelope membranes were shown to be the site of very active acyl-CoA thioesterase activity (EC 3.1.2.2.). The enzyme, which had a pH optimum of 9.0, was not affected by sulfhydryl reagents or by serine esterase inhibitors. However, the acyl-CoA thioesterase was strongly inhibited by unsaturated fatty acids, especially oleic acid, at concentrations above 100 micromolar. In marked contrast, saturated fatty acids had only a slight effect on the thioesterase activity. Substrate specificities showed that the velocity of the reaction increased with the chain length of the substrate from decanoyl-CoA to myristoyl-CoA and then decreased with the chain length from myristoyl-CoA to stearoyl-CoA. Interestingly, oleoyl-CoA was only slowly hydrolyzed. These results suggest that the envelope acyl-CoA thioesterase coupled with an envelope acyl-CoA synthetase may be involved in a switching system which indirectly allows acyl transfer from acyl carrier protein derivatives to unsaturated acyl-CoA derivatives and ensures the predominance of unsaturated 18 carbon fatty acids in plants. Furthermore, the position of both acyl-CoA thioesterase and synthetase in the envelope membranes suggest that these two enzymes may be involved in the transport of oleic acid from the stroma phase to the cytosol compartment of the leaf cell.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barden R. E., Cleland W. W. 1-Acylglycerol 3-phosphate acyltransferase from rat liver. J Biol Chem. 1969 Jul 10;244(13):3677–3684. [PubMed] [Google Scholar]
- Barden R. E., Cleland W. W. Alteration of the concentrations of dilute palmityl-CoA solutions by surface adsorption. Biochem Biophys Res Commun. 1969 Mar 10;34(5):555–559. doi: 10.1016/0006-291x(69)90773-6. [DOI] [PubMed] [Google Scholar]
- Douce R., Holtz R. B., Benson A. A. Isolation and properties of the envelope of spinach chloroplasts. J Biol Chem. 1973 Oct 25;248(20):7215–7222. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Joyard J., Douce R. Site of synthesis of phosphatidic acid and diacyglycerol in spinach chloroplasts. Biochim Biophys Acta. 1977 Feb 23;486(2):273–285. doi: 10.1016/0005-2760(77)90023-6. [DOI] [PubMed] [Google Scholar]
- Kleinig H., Liedvogel B. On the energy requirements of fatty acid synthesis in spinach chloroplasts in the light and in the dark. FEBS Lett. 1979 May 15;101(2):339–342. doi: 10.1016/0014-5793(79)81039-x. [DOI] [PubMed] [Google Scholar]
- Kumar S. Functional deacylases of pigeon liver fatty acid synthetase complex. J Biol Chem. 1975 Jul 10;250(13):5150–5158. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mancha M., Stokes G. B., Stumpf P. K. Fat metabolism in higher plants. The determination of acyl-acyl carrier protein and acyl coenzyme A in a complex lipid mixture 1,2. Anal Biochem. 1975 Oct;68(2):600–608. doi: 10.1016/0003-2697(75)90655-7. [DOI] [PubMed] [Google Scholar]
- Neuburger M., Joyard J., Douce R. Strong binding of cytochrome C on the envelope of spinach chloroplasts. Plant Physiol. 1977 Jun;59(6):1178–1181. doi: 10.1104/pp.59.6.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kuhn D. N., Stumpf P. K. Subcellular localization of acyl carrier protein in leaf protoplasts of Spinacia oleracea. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1194–1198. doi: 10.1073/pnas.76.3.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlrogge J. B., Shine W. E., Stumpf P. K. Fat metabolism in higher plants. Characterization of plant acyl-ACP and acyl-CoA hydrolases. Arch Biochem Biophys. 1978 Aug;189(2):382–391. doi: 10.1016/0003-9861(78)90225-4. [DOI] [PubMed] [Google Scholar]
- Roughan P. G., Slack C. R. Long-chain acyl-coenzyme A synthetase activity of spinach chloroplasts is concentrated in the envelope. Biochem J. 1977 Feb 15;162(2):457–459. doi: 10.1042/bj1620457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman M. D., Radin N. S. Oxalate-silica gel thin-layer system for free 2-hydroxy fatty acids and for fatty acyl coenzyme A. J Lipid Res. 1972 May;13(3):422–423. [PubMed] [Google Scholar]
- Vijay I. K., Stumpf P. K. Fat metabolism in higher plants. XLVI. Nature of the substrate and the product of oleyl coenzyme A desaturase from Carthamus tinctorius. J Biol Chem. 1971 May 10;246(9):2910–2917. [PubMed] [Google Scholar]



