Abstract
Abstract
Prosthetic joint infection (PJI) still remains a significant problem. In line with the forecasted rise in joint replacement procedures, the number of cases of PJI is also anticipated to rise. The formation of biofilm by causative pathogens is central to the occurrence and the recalcitrance of PJI. The subject of microbial biofilms is receiving increasing attention, probably as a result of the wide acknowledgement of the ubiquity of biofilms in the natural, industrial, and clinical contexts, as well as the notorious difficulty in eradicating them. In this review, we discuss the pertinent issues surrounding PJI and the challenges posed by biofilms regarding diagnosis and treatment. In addition, we discuss novel strategies of prevention and treatment of biofilm-related PJI.
PJI is a devastating complication of joint arthroplasty, with an average 1-year incidence of 0.25–1.0% for primary THR and 0.4–2% for primary TKR (Peersman et al. 2001, Blom et al. 2003, Joseph et al. 2003, Meehan et al. 2009). The incidence rate of infection in revision surgery is even higher, with an estimated rate of 3.2–5.6% for both hips and knees (Montanaro et al. 2011). Moreover, infection accounts for up to 12% of the indications for revision hip arthroplasty, and 22% for revision knee arthoplasty, as recorded in the National Joint Registry (10th Annual Report 2013). The overall infection burden is projected to rise by 4% between 2005 and 2030 for both primary and revision hip and knee arthroplasties (Kurtz et al. 2007, Bozic et al. 2009).
The formation of biofilms is intrinsic to the pathogenesis of PJI, and in this review we consider the impact of biofilms in PJI, explore the diagnostic challenges of biofilm-related prosthetic joint infections (BRPJIs), and evaluate the various measures that are aimed at their eradication.
The significance of biofilms in arthroplasty
Over 65% of all human infections are estimated to be biofilm-related (McLean et al. 2012, Williams and Costerton 2012). In addition, over 12 million people in the USA are reported to be affected by biofilm-related infections (BRIs) every year, with an estimated annual economic burden of $6 billion (O’Toole 2002, Wolcott and Ehrlich 2008). Of these, BRI in orthopedic practice is one of the most significant, due to bone and joint sequelae. The surfaces of commonly used orthopedic components such as titanium (and its alloys), stainless steel, cobalt-chromium, various polymeric biomaterials (e.g. ceramics, hydroxyapatite, and polyethylene), and polymethylmethacrylate (PMMA) cement are all susceptible to colonization by biofilm-forming bacteria (Gristina and Costerton 1985, Gristina 1987, Rochford et al. 2012).
The biofilm life cycle
A biofilm can be described as a structured aggregation of microbial cells of one or several species, encased in a self-produced matrix and adherent to a biotic or an abiotic surface (Cramton et al. 1999, Rice et al. 2007, O’Neill et al. 2008). The biofilm matrix is composed of exopolysaccharides (also called extrapolymeric substances), proteins, teichoic acids, lipids, and extracellular DNA (Arciola et al. 2012). The reason why antibiotics have poor activity against biofilms is not entirely understood. It is thought that the existence of slow or non-growing cells within the biofilm, the presence of bacterial subpopulations with different phenotypic levels of resistance within biofilms, overexpression of genes, and stress responses to hostile environmental conditions all contribute to the resistance of biofilms (Costerton et al. 1999, Lewis 2001, Mah and O’Toole 2001). Although, biofilms are often described as being attached to surfaces, they can also form at interfaces of spatially distinct microenvironments or as aggregated masses of free-floating cells, which can exhibit features similar to those of a typical surface-associated biofilm (Costerton 2007, Hall-Stoodley et al. 2012).
The development of a biofilm on an orthopedic implant can be described as a 4-stage process: (1) cell adhesion, (2) cell aggregation, (3) biofilm maturation, and (4) cellular detachment.
Stage 1: Cell adhesion: This process starts within the first few seconds and extends to approximately 2 h of exposure (O’Neill et al. 2008). It is mediated by factors such as the implant surface charge, hydrophobicity, topography, and exposure time (Rochford et al. 2012). During arthroplasty, host proteins such as fibrinogen, fibronectin, and vitronectin are absorbed onto the surfaces of orthopedic implants shortly after insertion, resulting in the formation of a conditioning film (Watnick and Kolter 2000, Rochford et al. 2012). This state of the biomaterial surface enhances bacterial colonization through interactions between bacterial proteins and host proteins (Heilmann et al. 1997, Legeay et al. 2006).
Stage 2: Cellular aggregation: At this stage, there is a multilayer cellular proliferation, as well as cell-to-cell adhesion, culminating in the formation of microcolonies of one or several species. These organized structures are then surrounded by a self-produced extracellular polysaccharide matrix (slime) with a resultant enclosed volume of high microbial density (Hoiby et al. 2011). Thus, a biofilm is progressively established on the colonized surface. This process is mediated both by microbial surface components recognising adhesive matrix molecules (MSCRAMMs) and the polysaccharide intercellular adhesin (PIA) (Patti et al. 1994, Heilmann et al. 1996, Mack et al. 1996, Rupp et al. 1999a, Rupp et al. 1999b, Speziale et al. 2009). For biofilm- forming staphylococci which do not produce PIA, cell-to-cell adhesion is mediated by biofilm-adhesive proteins such as the accumulation-associated protein (Aap), extracelluar matrix protein (Emp), protein A, and Staphylococcus aureus surface protein G (SasG) (Hussain et al. 1997, Rohde et al. 2005, Conrady et al. 2008, Merino et al. 2009, Christner et al. 2010, Geoghegan et al. 2010). At this stage, the biofilm is still relatively unstable and susceptible to eradication.
Stage 3: Biofilm maturation: To achieve maturation, physiological changes such as regulation of pili, flagellae, and exopolysaccharides occur within the biofilm (Costerton et al. 1995, Lee et al. 2011). This stage is mainly regulated by the accessory gene regulator (Agr) quorum-sensing system (Vuong et al. 2000, Vuong et al. 2003, Vuong et al. 2004b, Periasamy et al. 2012). When mature, the biofilms assume sessile forms, which are more resistant to eradication (Hoiby et al. 2011).
Stage 4: Cellular detachment: On maturation, large biofilms may release planktonic (free-floating) forms from their surfaces, which then disperse to cause further local invasion or seeding of distant sites, thus initiating an entirely new cycle. Proteases and the Agr system regulate this phase.
Classification of PJIs
A number of classifications are offered in the literature, one of the most popular being by Trampuz and Zimmerli (Zimmerli et al. 2004, Trampuz and Zimmerli 2008). These authors classified PJIs—according to the onset of symptoms after implantation—into: (1) early infection (< 3 months postoperatively), typically caused by highly virulent microorganisms such as Staphylococcus aureus or Gram-negative bacilli (such as E. coli); (2) delayed infection (3–24 months postoperatively), typically caused by less virulent bacteria such as coagulase-negative staphylococci or Priopionibacterium acnes; and (3) late infection ( > 24 months), typically caused by virulent bacteria such as Staphylococcus aureus, streptococci, and Gram-negative bacilli.
Both early and delayed infections usually occur as a result of perioperative contamination and are considered to be the most common cause of biomaterial-related infections (Ahlberg et al. 1978, Glynn and Sheehan 1983, Lidwell et al. 1983). These infections are generally associated with both local and systemic symptoms, and in addition induce inflammatory responses that are accompanied by raised laboratory inflammatory markers such as C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and white cell count levels. Blood cultures and tissue cultures can also be used to detect infection during the early stages.
Late infections generally occur after a relatively asymptomatic postoperative period and are usually consequent to hematogenous seeding—most commonly from skin and soft tissue infections (Ainscow and Denham 1984, Maderazo et al. 1988). Seeding may also occur from urinary, respiratory, or gastrointestinal tract infections.
Definition and diagnosis of PJI
There is as yet no universally accepted definition of PJI, but 2 large international infection workgroups—the international consensus group on periprosthetic joint infection (Concensus-Report 2013, Parvizi et al. 2013) and the Infectious Diseases Society of America (Osmon et al. 2013)—recently published consensus documents aimed at standardizing the definition of PJI, which readers may consult.
Based on an extensive review of the literature published between 1966 and 2011, a randomized clinical trial from a single-center, non-randomized retrospective case series, and case reports, the Infectious Diseases Society of America (IDSA) recommended that from the history and physical examination, PJI should be suspected in patients with any of the following: (1) a sinus tract or persistent wound drainage over a joint, (2) acute onset of a painful prosthesis, or (3) a chronically painful prosthesis at any time postoperatively, particularly in the absence of a pain-free period in the first few years after implantation or if there is a history of wound-healing problems or superficial wound infection (Osmon et al. 2013).
Identification of biofilm-related PJI can be more challenging, as these infections can develop over a period of a few months to years, exist innocuously, and give few clinical signs (Khoury et al. 1992). Conventional antimicrobial therapy is able to resolve systemic symptoms from pathogens in their planktonic form while the sessile forms remain unaffected (Nickel et al. 1985, Hall-Stoodley et al. 2012, Percival et al. 2012). Failure of conventional culture methods to isolate a causative pathogen in these cases can often result in a diagnostic conclusion of “aseptic failure”, even in the presence of compelling clinical signs (Arciola et al. 2011, Costerton et al. 2011). In an attempt to address this dilemma, Hall-Stoodley et al. (Hall-Stoodley et al. 2012) recently described a number of indicators of a possible BRI. These are not intended for use as diagnostic criteria (and thus are not accompanied by a scoring system) but are meant for use as an adjunct for diagnosis. They include: (1) history of persistent or recurrent joint infection, (2) infection localized to a particular implant site (evidenced by features such as swelling, pain, redness, warmth, tenderness, and functional limitation), (3) recalcitrance of infection despite adequate use of appropriate antibiotic therapy (based on antibiotic sensitivity testing for cultured pathogens), (4) ineffective treatment as evidenced by the persistent presence of cell clusters (identified microscopically), together with host inflammatory cells at the same site of infection, (5) culture-negative results despite a high degree of clinical suspicion of infection, and (6) direct visualization, by microscopy, of cellular aggregation of matrix-encased bacteria, associated with a surface.
Analytical challenges of biofilm-related PJI
Conventional culture was originally developed by Robert Koch more than 150 years ago and it is still the approved method for detecting and identifying bacteria in medical microbiology (Arciola et al. 2012, Ehrlich and Arciola 2012). The sensitivity rate of culture can be as low as 19% (Neut et al. 2003, Hall-Stoodley et al. 2006, Trampuz et al. 2007b, Piper et al. 2009) due to the inability to detect bacteria growing in biofilms. To improve detection of infection, other investigative methods are being explored—2 examples of which are molecular techniques and ultrasound.
Molecular methods
Polymerase chain reaction (PCR) and fluorescence in situ hybridization (FISH) are 2 popular types of molecular methods that are capable of identifying pathogens up to 80–100% of the time in cases of chronic/persistent infection (Post et al. 1995, Hall-Stoodley et al. 2006, Stoodley et al. 2011, Esteban et al. 2012, Portillo et al. 2012). By combining 16S rRNA gene PCR analysis with direct confocal microscopic examination (CLSM) of effusions from the affected ear, Hall-Stoodley et al. were able to diagnose a biofilm-based infection in an otherwise culture-negative otitis media (Hall-Stoodley et al. 2006).
The high cost, heavy reliance on expertise, susceptibility to sample contamination, and the lack primers relevant to diagnosis of PJI currently limit the routine use of molecular techniques in medical microbiology. At present, they are probably best reserved for culture-negative cases (van Belkum et al. 2007, Levy and Fenollar 2012, Zimmerli 2012).
Ultrasound
This is a cheaper and more readily available tool, which has been shown to improve infection detection rates (Trampuz et al. 2007b, Kobayashi et al. 2009, Monsen et al. 2009, Sorli et al. 2012). Upon application of ultrasound to liquid medium containing explanted orthopedic prostheses, ultrasonic waves are propagated through the liquid, which creates millions of microscopic air bubbles (a process called cavitation). These bubbles then implode, generating energy high enough to disrupt adherent biofilms and to release the bacteria within them into the liquid (Trampuz et al. 2003). These disaggregated bacterial cells can then be cultured. Trampuz et al. (2007b) prospectively compared cultures of samples obtained by sonication of explanted hip and knee prostheses from 331 patients (252 with aseptic failure and 79 with PJI) with conventional culture of periprosthetic tissue. The sensitivities of periprosthetic tissue cultures and sonicated fluid cultures regarding infection were 61% and 79%, respectively. Sonication is being increasingly used in many orthopedic centers, with reported benefits (Tunney et al. 1998, Esteban et al. 2008, Holinka et al. 2012, Evangelopoulos et al. 2013, Janz et al. 2013a, Janz et al. 2013b). Combination of sonication with PCR further enhances the sensitivity to infection (Achermann et al. 2010, Esteban et al. 2012, Gomez et al. 2012, Bereza et al. 2013).
Although routine sonication of explanted prostheses may not be necessary, it can be helpful for selection of antimicrobial agents by improving bacterial detection, especially in cases where preoperative joint aspiration has given culture-negative results. For diagnosis, low-intensity ultrasound (US) should be used, as high-intensity US can result in bacterial death.
US of high intensity has been shown to be useful for the eradication of biofilms. Ensing et al. (2005) compared bacteria survival on bone cement implanted into New Zealand rabbits, in the presence or absence of ultrasound. They found that in combination with gentamicin, pulsed US applied continuously for up to 72 h at a frequency of 28.48 Hz and a maximum intensity of 0.5 W/cm2 resulted in a 58–69% reduction in viable E. coli biofilm on bone cement compared to the negative controls.
Previously, Carmen et al. (2004) had performed an in vivo experiment similar to that of Ensing et al., in which they also investigated the effect of ultrasound and antibiotics, but in their study they used vancomycin rather than gentamicin. They infected rabbits with biofilm-producing Staphylococcus epidermidis and, like Ensing et al., applied ultrasound. Statistically significant reductions in viable bacteria were seen with the combination of US and vancomycin after 48h, however, at times shorter than this, there were no significant reductions in viable bacteria counts.
The mechanism by which the combination of US and antibiotic exerts its effect is not clearly understood, but it is postulated that ultrasound probably induces an increase in cement porosity, thereby enhancing elution of the antibiotic. Furthermore, high-intensity US possibly also has a disruptive effect on the bacterial cell wall itself, leading to cell death.
There does not appear to be a clear consensus in the literature regarding the ideal frequency and intensity to use for either diagnostic or therapeutic purposes in PJI management. Despite the fact that pulsed US appears to be safe for use in treating experimentally induced infections in rabbits, the same cannot be said for spontaneous infections in humans—if US is to be used at infection sites with implants in situ.
Advanced imaging of biofilms
Confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM) are advanced imaging techniques that can be used to visualize biofilms.
CLSM allows non-destructive examination of the layers of a biofilm at different depths, and in addition generates high-resolution three-dimensional (3-D) images (Lawrence and Neu 1999, Jones et al. 2005, Psaltis et al. 2007). Using CLSM, Stoodley et al. (2008) were able to demonstrate viable bacteria in biofilm in joint fluid, wound tissue, and bone cement retrieved from an infected total elbow arthroplasty, which had consistently yielded negative cultures for over 5 years.
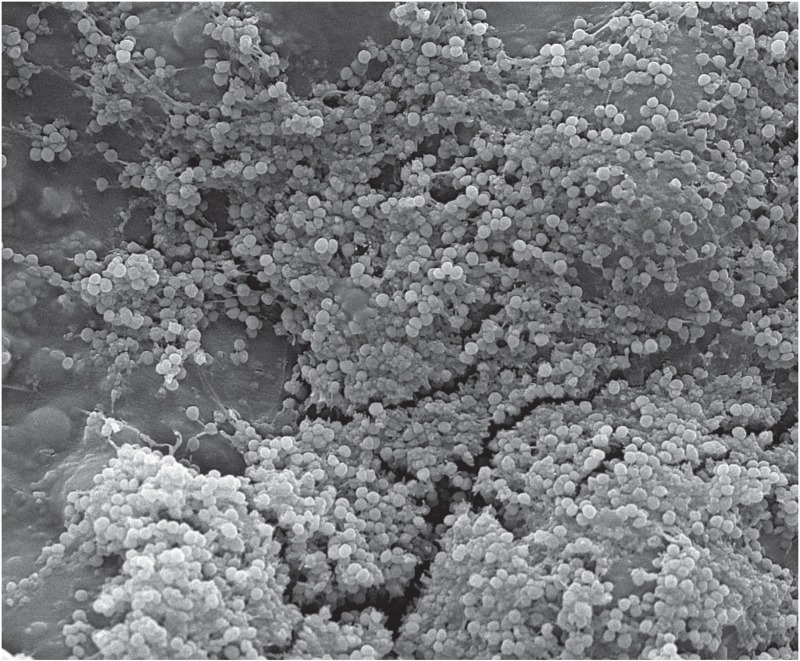
SEM does not generate 3-D images but provides more easily discernable images depicting the co-aggregation of microbial cells of a biofilm. The Figure is an SEM image of a Staphylococcus epidermidis biofilm on bone cement. Unfortunately, SEM images often do not reveal the ECM structure of the biofilm, as the preparation process often results in the loss or distortion of the ECM (Fassel and Edmiston 1999, Kachlany et al. 2001, Walker et al. 2001).
Scanning electron micrograph of biofilms on PMMA cement.
Management of PJI
Antibiotics
Rifampicin has been commended in the literature for its efficiency in treating PJIs, especially those associated with biofilms. Rifampicin, daptomycin, ciprofloxacin, vancomycin, and amikacin have all been reported to penetrate biofilms (Dunne et al. 1993, Darouiche et al. 1994, Zheng and Stewart 2002, Stewart et al. 2009, Singh et al. 2010). It is, however, unclear from these studies whether the antimicrobials had any detrimental effects on the bacteria in the biofilms.
Raad et al. (2007) compared the activities of daptomycin, linezolid, and tigecycline with those of vancomycin, minocycline, and rifampin against catheter-related methicillin-resistant Staphylococcus aureus (MRSA) embedded in biofilm using an in vitro silicon disk biofilm colonization model. They were able to demonstrate that daptomycin, minocycline, and tigecycline were significantly more effective than vancomycin or linezolid in inhibiting MRSA bacteria embedded in biofilm. In addition, they found rifampicin to be the most effective in reducing the bacterial load of MRSA biofilms; however, this effect was short-lived due to the rapid emergence of resistance within a few days of using rifampicin as a sole agent. Rifampicin was then used in combination with other antibiotics, and this was found to expedite the elimination of MRSA colonization in biofilm. John et al. (2009) compared the activity of daptomycin (alone and with rifampicin) with the activities of vancomcyin, linezolid, and levofloxacin against MRSA strain ATCC 43300 in a guinea pig foreign-body infection model. They found that daptomycin at a high once-daily dose (corresponding to the 6 mg/kg safe dose in humans), in combination with rifampicin, showed the highest activity against planktonic and biofilm MRSA. This combination was found to achieve a cure rate higher than that achieved with vancomycin plus rifampicin, and also to prevent the emergence of rifampicin resistance. Moreover, the vancomycin-rifampicin combination did not prevent the emergence of rifampicin resistance. In the same study, the combination of levofloxacin with rifamipcin was also found to be efficacious against planktonic and adherent MRSA with cure rates similar to that of rifampicin in combination with daptomycin (Trampuz et al. 2007a). Rifampicin should ideally be used in combination with other antibiotics to avoid rapid emergence of resistance, which tends to occur when rifampicin is used as monotherapy.
The literature strongly suggests that eradication of biofilms, as well as adequate protection against infections, is better achieved by using combinations of antibiotics rather than single therapy (Valerius et al. 1991, Doring and Hoiby 2004, Hoiby et al. 2005, Pamp et al. 2008). Moreover, combining 2 or more antibiotics can minimize the emergence of resistance, provide synergy, broaden the antimicrobial spectrum, and prolong drug elution (Neut et al. 2006, Hagihara et al. 2012, Worthington and Melander 2013). The Table provides details of some putative biofilm-active antibiotics.
Table 1.
Putative biofilm-active antibiotics
| Antibiotic | Class | MOA | Spectrum of activity | Important side effects b |
| Rifampicin at1/2: 4 h | Rifamycin | BactericidalInhibition of bacterial RNA synthesis | Gram-positive and -negative bacteria | Nausea, gastrointestinal disturbances, hepatotoxicity, thrombocytopenia, rash, red discoloration of urine, flu-like symptoms |
| Daptomycint1/2: 9 h | Lipopetide | BactericidalInsertion of hydrophobic tail into cell membrane, resulting in membrane depolarization and cell death | Gram-positive bacteria including MRSA, VRSA, VRE, and PRSP. Log- and stationary-phase of bacteria | Nausea, vomiting, diarrhea, hypertension and hypotension, myopathy, neuropathy, urethritis, anemia, hypokalemia, arthralgia |
| Linezolidt1/2: 6 h | Oxazolidinones | BacteriostaticBinds to the bacterial 23S ribosomal RNA of the 50S subunit, thus preventing the formation of a functional 70S complex. Production by MSSA and MRSA | Gram-positive bacteria including MRSA, MSSA, CoNS, and enterococci including VRE.Good tissue distribution and bioavailability | Nausea, vomiting, diarrhea, thrombocytopenia, myelosuppression, reversible optic neuritis, irreversible peripheral neuropathy, serotonin syndrome |
| Tigecyclinet1/2: 42 h | Glycylcylines (synthetic derivative of tetracyclines) | BacteriostaticBinds 30S bacterial ribosomal subunit and prevents binding of tRNA to the mRNA ribosome complex | Active against Gram-positive bacteria (including VRE and MRSA), Gram-negative bacilli, and anaerobes | Nausea, vomiting, diarrhea, sore mouth and throat, dysphagia, vitamin B complex deficiency, dental abnormalities, hepatotoxicity |
| Minocyclinet1/2: 15 h | Tetracyclines | Same as tigecycline | Similar to tigecycline and also active against Neisseria meningitidis | Similar to tigecycline and in addition, vestibular disturbances with dizziness, tinnitus, and impaired balance—especially in women |
| Vancomycint1/2: 8 h | Glycopeptides | BactericidalInhibits bacterial cell wall formationInterferes with peptidoglycan synthesis | Gram-positive bacteria. MRSA | Tinnitus, deafness (reversible on cessation of drug), nephrotoxicity, maculopapular rash (with rapid i.v. infusion) |
Should ideally be used as combination therapy to avoid rapid emergence of resistance.
MOA: mechanism of action; MRSA: methicillin-resistent Staphylococcus aureus; MSSA: methicillin-sensitive Staphylococcus aureus;
VRE: vancomycin-resistent enterococcus; PRSP: penicillin-resistant Streptococcus pneumoniae; CoNS: coagulase-negative staphylococci; t1/2: serum half-life of drug.
These side effects are generally attributed to systemic administration.
The ideal course of treatment of PJI using systemically administered antibiotics is still under debate. Trampuz and Zimmerli (2006) suggested a total period of between 3 and 6 months, with intravenous administration being continued for 2–6 weeks prior to a switch to oral alternatives.
Since its conceptualization by Buchholz and Engelbrecht in 1970 (Buchholz and Engelbrecht 1970, Buchholz et al. 1981), the use of antibiotic-loaded acrylic cement (ALAC) for the management of PJI has been common practice among many orthopedic surgeons. Furthermore, ALAC is an independent factor proven to reduce the incidence of PJI (Dale et al. 2009, Jamsen et al. 2009, Nowinski et al. 2012). Unfortunately, only about 10% of the antibiotic incorporated is ever released from the cement (Webb and Spencer 2007). Moreover, the potential of ALAC to induce antibiotic resistance due to late release of antibiotic at sub-inhibitory concentrations, is of significant concern, although the use of combination antimicrobial therapy could alleviate this problem (Hagihara et al. 2012).
Bacteriophages
Bacteriophages are viruses that act as obligate parasites capable of invading bacterial cells, injecting their genomic material, and taking over the host metabolic system. These viruses can then go on to replicate inside the bacteria and produce specific proteins that can induce lysis of the bacterial cell wall (endolysin) and degradation of the polysaccharide matrix of biofilms (Yilmaz et al. 2013). Bacteriophages were first discovered about 100 years ago, but their development as therapeutic agents was dampened by the advent and success of antibiotics in the 1930s and 1940s (Wittebole et al. 2014). Research into bacteriophages has been rejuvenated in recent years, in the search for alternative antimicrobials. Yilmaz et al. (2013) undertook an in vivo study to evaluate the antimicrobial activities of bacteriophages against 2 different types of bacterial infection (MRSA and Pseudomonas aeruginosa) in rats. They found that when used as monotherapy, both antibiotics and bacteriophages were able to reduce the viable bacterial count. Furthermore, the combination of antibiotics and bacteriophages was able to reduce the viable bacterial count to a significantly lower level than when either agent was used as monotherapy. In addition, only the combination of antibiotic and bacteriophage resulted in significant reduction in biofilm.
Bacteriophages are inherently non-toxic, have minimal impact on the normal healthy flora, and have a lower tendency to induce resistance. Furthermore, they do not exhibit any cross-resistance with antibiotics and have good cell-penetrative ability, so they can readily disrupt and lyse biofilms (Donlan 2009, Kutateladze and Adamia 2010, Loc-Carrillo and Abedon 2011). The disadvantages of bacteriophages include their narrow spectrum of activity and their ability to induce an immune response in the mammalian host after repeated exposure, which can result in their inactivation. Moreover, the life cycle, safety considerations, the ideal route of administration (for PJI management), and the side effects of bacteriophages following clinical application are yet to be fully investigated.
Enzymes
Certain enzymes act on the biofilm matrix, either by degrading extracellular polymeric substances (EPSs) or by sensitizing biofilms to eradication by other antimicrobial agents. 2 examples of such enzymes that are being explored are dispersin B (DspB) and deoxyribonuclease 1 (DNase 1).
Dispersin B: DspB is an N-acetylglucosamine enzyme produced by the Gram-negative periodontal pathogen Actinobacillus actinomycetemcomitans. It is capable of dispersing the biofilm exopolysaccharide poly-1,6-β-N-acetylglucosamine (PNAG), also known as PIA, which is required for full virulence of biofilm-forming bacteria (Kaplan et al. 2004, Vuong et al. 2004a, Arciola et al. 2011). When added to culture medium at the time of inoculation, DspB has been shown to inhibit various PNAG-producing bacteria (Kaplan 2009). In an in vitro study by Kaplan et al. (2004), DspB almost completely eradicated biofilms from the wells of a 96-well polystyrene microtiter plate after just 30 min of exposure. The authors concluded that precoating of implant surfaces with DspB may serve as an effective anti-biofilm agent.
Darouiche et al. (2009) evaluated the antimicrobial and anti-biofilm effects of vascular catheters coated with DspB and the antiseptic triclosan, against Staphylococcus aureus, Staphylococcus epidermidis, and E. coli. They demonstrated, both in vitro and in vivo (using rabbits), that the combination of DspB with triclosan resulted in a significantly greater reduction in bacterial colonization than catheters coated with chlorhexidine and silver sulphasalazine and catheters that were not coated. The use of DspB as a monotherapy or in combination with other antimicrobials is being investigated with a view to being used in wound-care gels and biomaterial coatings.
DNase 1: DNase 1 acts by degrading extracellular bacterial DNA (eDNA), thus destabilizing biofilms, and it has been shown to suppress biofilm formation in Staphylococcus aureus and Pseudomonas aeruginosa in vitro (Allesen-Holm et al. 2006, Eckhart et al. 2007). Surprisingly, the disruptive capability of DNAse 1 only appears to be effective against newly formed biofilms (approximately 6 h old) in vitro, and not against mature biofilms, but it can sensitize mature biofilms to eradication by other antimicrobial agents (Whitchurch et al. 2002, Qin et al. 2007, Izano et al. 2008, Thomas et al. 2008, Kaplan 2009).
DNase 1 is currently being used clinically in the form of an aerosol (Pulmozyme) for the treatment of Pseuomonas aeruginosa infections in cystic fibrosis, but we are not aware of its use in PJI management.
Although the full spectrum of toxicity of these enzymes in vivo is still not known, research is under way to evaluate DspB and DNase 1 as options for use as biomaterial coating agents and skin preparatory solutions, and in fluids for wound irrigation.
Surgical management of PJI
For recent guidelines for the management of PJIs as proposed by the IDSA, see Osmon et al. (2013).
Debridement and implant retention (DAIR) is usually considered for early infection with a stable prosthesis, and the reported success rates are between 14% and 100% (Marculescu et al. 2006, Byren et al. 2009, Choi et al. 2011, Engesaeter et al. 2011, Gardner et al. 2011, Kim et al. 2011, Puhto et al. 2012, Aboltins et al. 2013, Fehring et al. 2013, Lora-Tamayo et al. 2013). Patients who do not qualify for DAIR and are fit enough for surgery are usually considered for either a 1-stage or 2-stage revision procedure. Recently, Beswick et al. (2012) undertook a systematic review of unselected patients who had undergone either a 1- or 2-stage revision for an infected total hip arthroplasty. They found that there was more substantial data available for 2-stage procedures than for 1-stage procedures, probably because the 2-stage procedure has generally been more popular in previous decades. These authors concluded that there was insufficient robust evidence in the literature to ascertain which procedure was superior. Masters et al. (2013) undertook a similar systematic review of the available literature for 1- or 2-stage treatment of infected knee replacements. As with Beswick et al., they also concluded that there was insufficient evidence in the literature to determine which procedure was superior.
Prevention of PJI
General strategies
These have already been well expounded in the literature; for reviews, see Alijanipour et al. (2014), Illingworth et al. (2013), and Adeli and Parvizi (2012).
Novel strategies: Vaccines
Vaccines have been used successfully to control many infections, but they usually target single antigens and are developed for pathogens in their planktonic forms.
Brady et al. (2011) were able to generate a tetravalent vaccine using biofilm-specific antigens from Staphylococcus aureus osteomyelitis, which they subsequently administered to New Zealand white rabbits. They found that in combination with vancomycin therapy, the vaccine gave an 87.5% reduction in radiological and clinical signs of infection with Staphylococcus aureus biofilms.
Despite its success in neonate rats, clinical trials with INH-A21 (Veronate), a human immunoglobulin G with elevated levels of antibodies to the staphylococcal surface adhesin CIFA and SdrG, failed phase-III testing as it did not show any clinical benefit in neonates (Vernachio et al. 2006, DeJonge et al. 2007).
Unfortunately, the complexities of the biofilm architecture—with multiple microbiological communities and with various sites within the communities that can express different proteins required for survival—makes the development of a single, effective anti-biofilm vaccine a considerable challenge (Brady et al. 2011).
Modification of the implant surface
Implant coatings that resist biofilm-based infections fall into 2 caterories: (1) passive coatings, which impede bacterial adhesion and/or kill bacteria upon contact, and (2) active coatings, which release pre-incorporated antimicrobials to combat infection (Goodman et al. 2013).
Passive coating: Titanium-based implants are the most widely used in orthopedic practice, but they enhance protein layer formation, which in turn offers an ideal surface for bacteria to adhere to.
Polyethylene glycol (PEG) and polyethylene oxide (PEO) are highly hydrated polymer chains that can hamper protein absorption and bacterial adhesion to biomaterial surfaces (Neoh and Kang 2011). Also, chemical modification of titanium surfaces with zinc can inhibit bacterial colonization (Petrini et al. 2006).
Active coating: Antibiotics can also serve as a coating for orthopedic implants. Alt et al. (2006) inoculated the tibias of rabbits with Staphylococcus aureus, followed by implantation of either gentamicin-hydroxyapatite- (HA-) coated steel K-wires, gentamicin-RGD (arginine-glycine-aspartate)-HA-coated steel K-wires, or standard HA-coated steel K-wires. After 28 days, no infection was seen in the rabbits implanted with both types of gentamicin-coated K-wires, while infection was seen in 7 of the 8 animals with the standard HA coating. Furthermore, there was good biocompatibility and bony integration of the HA implants with the supplementary coatings, similar to that of the standard HA implants (Alt et al. 2011).
Similarly, Darouiche et al. (2007) reported a significantly lower rate of Staphylococcus aureus colonization of minocyclin-rifampicin-coated titanium-alloy pins, which were implanted into rabbit femurs and left in situ for 1 week, than with uncoated implants (Darouiche et al. 2007).
Concerns over resistance may limit the use of antibiotics as implant coatings
Chitosan, silver, and antimicrobial peptides (AMPs) are alternative antimicrobials to antibiotics.
Chitosan is a natural biocompatible cationic polysaccharide that interacts with microbial cell membranes, resulting in disruption of bacterial cells (Arciola et al. 2012). Peng et al. (2011) evaluated the efficacy of hydroxypropyltrimethyl ammonium chloride chitosan (HACC)—a quaternized derivative of chitosan with different degrees of substitution (DS; referred to as HACC 6%, 18%, and 44%)—in preventing biofilm formation on titanium surfaces in vitro. They found that HACC, especially HACC 18% and 44%, significantly inhibited biofilm formation compared to the untreated control, and was effective against both new and mature biofilms on titanium surfaces.
Tan et al. (2012) observed that PMMA loaded with HACC 26% was more effective in inhibiting surface biofilm formation by staphylococci than gentamicin-loaded PMMA and regular chitosan-loaded PMMA in vitro. HACC-loaded PMMA was found to downregulate the virulence-associated gene expression of antibiotic-resistant staphylococci.
Fu et al. (2005) showed a 46–68% decrease in bacterial contact with chitosan-heparin-modified polyethylene terephthalate (PET) films, as compared to a 7% decrease in bacterial contact with untreated PET films. Only 3–8% of viable cells remained on the modified PET films after 24 h of exposure.
Silver is well known for its ability to confer good anti-adhesion properties to implant surfaces without compromising osteoblastic activities, for its broad antimicrobial spectrum, for its long-lasting antibacterial effects, and for its reduced likelihood to induce resistance (Goodman et al. 2013). Although silver has been reported to be safe for clinical use, there is still concern about the limited availability of data on its toxicity spectrum and argyria (Hardes et al. 2007).
AMPs are natural constituents of the innate immune system of all multicellular organisms. They act either by permeabilizing microbial cell membranes or by translocating across the cell membrane to attack their cytoplasmic targets (Andreu and Rivas 1998, Gordon et al. 2005, Guani-Guerra et al. 2010). Kazemzadeh-Narbat et al. (2010) coated the surface of titanium with calcium phosphate (CaP) and Tet 123 (a highly potent broad-spectrum AMP) and found a 106 times reduction in viable bacteria within 30 min of exposure to Staphylococcus aureus and Pseudomonas aeruginosa. The CaP-Tet 123 coating also provided a 92% surface inhibition of P. aeruginosa after 4 h and a 77% inhibition after 24 h. In related studies, Yoshinari et al. (2010) and Gao et al. (2011) concurred that AMP coatings on biomaterials can make the implants biofilm-resistent without being toxic to osteoblast-like cells or inducing significant activation of platelets or host complement. Thus, AMP coatings can possibly be used as an antimicrobial system on orthopedic implants.
Conclusion
Early descriptions of bacterial aggregations date back to the 1600s, when Antony van Leeuwenhoek documented the behavior of dental plaque, which he observed through a microscope (Dobell 1932, Gest 2004). Since then, there has been an ever-growing body of research dedicated to a better understanding of biofilms and their role in human infections, with a view to better diagnosis and eradication. Most of the studies on medically significant biofilms have been in vitro. This is understandable, as it would be impossible and unethical to subject humans to the levels of in vitro experimentation that have been and still are being performed on biofilms. Moreover, in vitro investigations have enabled scientists to undertake a wide variety of studies, which has resulted in a better understanding of the physiology of biofilms and has therefore been instrumental in the continuing development of biofilm management strategies. Caution should be exercised in extrapolating results of in vitro studies to in vivo scenarios, considering the fact that it is difficult to recreate the mechanism of the body’s defense system—and the normal composition of the microenvironment found in the body—in vitro. Even so, compared to the earlier years of biofilm research, more clinical studies are being carried out nowadays following the successes of in vitro, ex vivo, and animal studies and we envisage that such clinical studies will continue in even greater numbers.
This review has hopefully enlightened readers on pertinent issues of prosthetic joint infections, and especially the role of biofilms in orthopedic implant infections.
Acknowledgments
No competing interests declared.
References
- 10th Annual Report National Joint Registry for England and Wales 2013. Accessed October 2013 from http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/10th_annual_report/NJR%2010th%20Annual%20Report%202013%20B.pdf .
- Aboltins C, Dowsey MM, Peel T, Lim WK, Parikh S, Stanley P, Choong PF. Early prosthetic hip joint infection treated with debridement, prosthesis retention and biofilm-active antibiotics: functional outcomes, quality of life and complications . Intern Med J. 2013;43(7):810–5. doi: 10.1111/imj.12174. [DOI] [PubMed] [Google Scholar]
- Achermann Y, Vogt M, Leunig M, Wust J, Trampuz A. Improved diagnosis of periprosthetic joint infection by multiplex PCR of sonication fluid from removed implants . J Clin Microbiol. 2010;48(4):1208–14. doi: 10.1128/JCM.00006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adeli B, Parvizi J. Strategies for the prevention of periprosthetic joint infection . J Bone Joint Surg Br. 2012;94(11 Suppl A):42–6. doi: 10.1302/0301-620X.94B11.30833. [DOI] [PubMed] [Google Scholar]
- Ahlberg A, Carlsson AS, Lindberg L. Hematogenous infection in total joint replacement . Clin Orthop Relat Res. 1978;137:69–75. [PubMed] [Google Scholar]
- Ainscow DA, Denham RA. The risk of haematogenous infection in total joint replacements . J Bone Joint Surg Br. 1984;66(4):580–2. doi: 10.1302/0301-620X.66B4.6430907. [DOI] [PubMed] [Google Scholar]
- Alijanipour P, Heller S, Parvizi J. Prevention of periprosthetic joint infection: what are the effective strategies? . J Knee Surg. 2014;27(4):251–8. doi: 10.1055/s-0034-1376332. [DOI] [PubMed] [Google Scholar]
- Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, Tolker-Nielsen T. A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms . Mol Microbiol. 2006;59(4):1114–28. doi: 10.1111/j.1365-2958.2005.05008.x. [DOI] [PubMed] [Google Scholar]
- Alt V, Bitschnau A, Osterling J, Sewing A, Meyer C, Kraus R, Meissner SA, Wenisch S, Domann E, Schnettler R. The effects of combined gentamicin-hydroxyapatite coating for cementless joint prostheses on the reduction of infection rates in a rabbit infection prophylaxis model . Biomaterials. 2006;27(26):4627–34. doi: 10.1016/j.biomaterials.2006.04.035. [DOI] [PubMed] [Google Scholar]
- Alt V, Bitschnau A, Bohner F, Heerich KE, Magesin E, Sewing A, Pavlidis T, Szalay G, Heiss C, Thormann U, Hartmann S, Pabst W, Wenisch S, Schnettler R. Effects of gentamicin and gentamicin-RGD coatings on bone ingrowth and biocompatibility of cementless joint prostheses: an experimental study in rabbits . Acta Biomater. 2011;7(3):1274–80. doi: 10.1016/j.actbio.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Andreu D, Rivas L. Animal antimicrobial peptides: an overview . Biopolymers. 1998;47(6):415–33. doi: 10.1002/(SICI)1097-0282(1998)47:6<415::AID-BIP2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Arciola CR, Montanaro L, Costerton JW. New trends in diagnosis and control strategies for implant infections . Int J Artif Organs. 2011;34(9):727–36. doi: 10.5301/IJAO.2011.8784. [DOI] [PubMed] [Google Scholar]
- Arciola CR, Campoccia D, Speziale P, Montanaro L, Costerton JW. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials . Biomaterials. 2012;33(26):5967–82. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- Bereza PL, Ekiel A, Augusciak-Duma A, Aptekorz M, Wilk I, Kusz DJ, Wojciechowski P, Martirosian G. Identification of silent prosthetic joint infection: preliminary report of a prospective controlled study . Int Orthop. 2013;37(10):2037–43. doi: 10.1007/s00264-013-1955-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients . BMJ Open. 2012;2(1) doi: 10.1136/bmjopen-2011-000435. e000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom AW, Taylor AH, Pattison G, Whitehouse S, Bannister GC. Infection after total hip arthroplasty. The Avon experience . J Bone Joint Surg Br. 2003;85(7):956–9. doi: 10.1302/0301-620x.85b7.14095. [DOI] [PubMed] [Google Scholar]
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States . J Bone Joint Surg Am. 2009;91(1):128–33. doi: 10.2106/JBJS.H.00155. [DOI] [PubMed] [Google Scholar]
- Brady RA, O’may GA, Leid JG, Prior ML, Costerton JW, Shirtliff ME. Resolution of Staphylococcus aureus biofilm infection using vaccination and antibiotic treatment . Infect Immun. 2011;79(4):1797–803. doi: 10.1128/IAI.00451-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz HW, Engelbrecht H. Depot effects of various antibiotics mixed with Palacos resins . Chirurg. 1970;41(11):511–5. [PubMed] [Google Scholar]
- Buchholz HW, Elson RA, Engelbrecht E, Lodenkamper H, Rottger J, Siegel A. Management of deep infection of total hip replacement . J Bone Joint Surg Br. 1981;63-B(3):342–53. doi: 10.1302/0301-620X.63B3.7021561. [DOI] [PubMed] [Google Scholar]
- Byren I, Bejon P, Atkins BL, Angus B, Masters S, Mclardy-Smith P, Gundle R, Berendt A. One hundred and twelve infected arthroplasties treated with ‘DAIR’ (debridement, antibiotics and implant retention): antibiotic duration and outcome . J Antimicrob Chemother. 2009;63(6):1264–71. doi: 10.1093/jac/dkp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmen JC, Roeder BL, Nelson JL, Beckstead BL, Runyan CM, Schaalje GB, Robison RA, Pitt WG. Ultrasonically enhanced vancomycin activity against Staphylococcus epidermidis biofilms in vivo . J Biomater Appl. 2004;18(4):237–45. doi: 10.1177/0885328204040540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HR, Von Knoch F, Zurakowski D, Nelson SB, Malchau H. Can implant retention be recommended for treatment of infected TKA? . Clin Orthop Relat Res. 2011;469(4):961–969. doi: 10.1007/s11999-010-1679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christner M, Franke GC, Schommer NN, Wendt U, Wegert K, Pehle P, Kroll G, Schulze C, Buck F, Mack D, Aepfelbacher M, Rohde H. The giant extracellular matrix-binding protein of Staphylococcus epidermidis mediates biofilm accumulation and attachment to fibronectin . Mol Microbiol. 2010;75(1):187–207. doi: 10.1111/j.1365-2958.2009.06981.x. [DOI] [PubMed] [Google Scholar]
- Concensus-Report Proceedings of the International Consensus Meeting on Periprosthetic Joint Infection; 2013. from https://www.efort.org/wp-content/uploads/2013/10/Philadelphia_Consensus.pdf . [Google Scholar]
- Conrady DG, Brescia CC, Horii K, Weiss AA, Hassett DJ, Herr AB. A zinc-dependent adhesion module is responsible for intercellular adhesion in staphylococcal biofilms . Proc Natl Acad Sci U S A. 2008;105(49):19456–61. doi: 10.1073/pnas.0807717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW. Springer: 2007. The Biofilm Primer. [Google Scholar]
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms . Annu Rev Microbiol. 1995;49:711–45. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections . Science. 1999;284(5418):1318–22. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Post JC, Ehrlich GD, Hu FZ, Kreft R, Nistico L, Kathju S, Stoodley P, Hall-Stoodley L, Maale G, James G, Sotereanos N, Demeo P. New methods for the detection of orthopedic and other biofilm infections . FEMS Immunol Med Microbiol. 2011;61(2):133–40. doi: 10.1111/j.1574-695X.2010.00766.x. [DOI] [PubMed] [Google Scholar]
- Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation . Infect Immun. 1999;67(10):5427–33. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale H, Hallan G, Hallan G, Espehaug B, Havelin LI, Engesaeter LB. Increasing risk of revision due to deep infection after hip arthroplasty . Acta Orthop. 2009;80(6):639–45. doi: 10.3109/17453670903506658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darouiche RO, Dhir A, Miller AJ, Landon GC, Raad Ii, Musher DM. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria . J Infect Dis. 1994;170(3):720–3. doi: 10.1093/infdis/170.3.720. [DOI] [PubMed] [Google Scholar]
- Darouiche RO, Mansouri MD, Zakarevicz D, Alsharif A, Landon GC. In vivo efficacy of antimicrobial-coated devices . J Bone Joint Surg Am. 2007;89(4):792–7. doi: 10.2106/JBJS.F.00414. [DOI] [PubMed] [Google Scholar]
- Darouiche RO, Mansouri MD, Gawande PV, Madhyastha S. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB combination . J Antimicrob Chemother. 2009;64(1):88–93. doi: 10.1093/jac/dkp158. [DOI] [PubMed] [Google Scholar]
- Dejonge M, Burchfield D, Bloom B, Duenas M, Walker W, Polak M, Jung E, Millard D, Schelonka R, Eyal F, Morris A, Kapik B, Roberson D, Kesler K, Patti J, Hetherington S. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants . J Pediatr. 2007;151(3):260–5. doi: 10.1016/j.jpeds.2007.04.060. e261. [DOI] [PubMed] [Google Scholar]
- Dobell C. Dover, New York: 1932. Antony van Leeuwenhoek his ‘little animals’. [Google Scholar]
- Donlan RM. Preventing biofilms of clinically relevant organisms using bacteriophage . Trends Microbiol. 2009;17(2):66–72. doi: 10.1016/j.tim.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Doring G, Hoiby N. Early intervention prevention of lung disease in cystic fibrosis: a European consensus . J Cyst Fibros. 2004;3(2):67–91. doi: 10.1016/j.jcf.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Dunne WM, Jr., Mason EO, Jr., Kaplan SL. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm . Antimicrob Agents Chemother. 1993;37(12):2522–2526. doi: 10.1128/aac.37.12.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhart L, Fischer H, Barken KB, Tolker-Nielsen T, Tschachler E. DNase1L2 suppresses biofilm formation by Pseudomonas aeruginosa and Staphylococcus aureus . Br J Dermatol. 2007;156(6):1342–1345. doi: 10.1111/j.1365-2133.2007.07886.x. [DOI] [PubMed] [Google Scholar]
- Ehrlich GD, Arciola CR. From Koch’s postulates to biofilm theory. The lesson of Bill Costerton . Int J Artif Organs. 2012;35(10):695–9. doi: 10.5301/ijao.5000169. [DOI] [PubMed] [Google Scholar]
- Engesaeter LB, Dale H, Schrama JC, Hallan G, Lie SA. Surgical procedures in the treatment of 784 infected THAs reported to the Norwegian Arthroplasty Register . Acta Orthop. 2011;82(5):530–7. doi: 10.3109/17453674.2011.623572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensing GT, Roeder BL, Nelson JL, Van Horn JR, Van Der Mei HC, Busscher HJ, Pitt WG. Effect of pulsed ultrasound in combination with gentamicin on bacterial viability in biofilms on bone cements in vivo . J Appl Microbiol. 2005;99(3):443–448. doi: 10.1111/j.1365-2672.2005.02643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban J, Gomez-Barrena E, Cordero J, Martin-De-Hijas NZ, Kinnari TJ, Fernandez-Roblas R. Evaluation of quantitative analysis of cultures from sonicated retrieved orthopedic implants in diagnosis of orthopedic infection . J Clin Microbiol. 2008;46(2):488–92. doi: 10.1128/JCM.01762-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban J, Alonso-Rodriguez N, Del-Prado G, Ortiz-Perez A, Molina-Manso D, Cordero-Ampuero J, Sandoval E, Fernandez-Roblas R, Gomez-Barrena E. PCR-hybridization after sonication improves diagnosis of implant-related infection . Acta Orthop. 2012;83(3):299–304. doi: 10.3109/17453674.2012.693019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelopoulos DS, Stathopoulos IP, Morassi GP, Koufos S, Albarni A, Karampinas PK, Stylianakis A, Kohl S, Pneumaticos S, Vlamis J. Sonication: a valuable technique for diagnosis and treatment of periprosthetic joint infections. Scientific World Journal. 2013;2013:375140. doi: 10.1155/2013/375140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassel TA, Edmiston CE., Jr Bacterial biofilms: strategies for preparing glycocalyx for electron microscopy . Methods Enzymol. 1999;310:194–203. doi: 10.1016/s0076-6879(99)10017-x. [DOI] [PubMed] [Google Scholar]
- Fehring TK, Odum SM, Berend KR, Jiranek WA, Parvizi J, Bozic KJ, Della Valle CJ, Gioe TJ. Failure of irrigation and debridement for early postoperative periprosthetic infection . Clin Orthop Relat Res. 2013;471(1):250–7. doi: 10.1007/s11999-012-2373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Ji J, Yuan W, Shen J. Construction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosan . Biomaterials. 2005;26(33):6684–92. doi: 10.1016/j.biomaterials.2005.04.034. [DOI] [PubMed] [Google Scholar]
- Gao G, Lange D, Hilpert K, Kindrachuk J, Zou Y, Cheng JT, Kazemzadeh-Narbat M, Yu K, Wang R, Straus SK, Brooks DE, Chew BH, Hancock RE, Kizhakkedathu JN. The biocompatibility and biofilm resistance of implant coatings based on hydrophilic polymer brushes conjugated with antimicrobial peptides . Biomaterials. 2011;32(16):3899–909. doi: 10.1016/j.biomaterials.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Gardner J, Gioe TJ, Tatman P. Can this prosthesis be saved?: implant salvage attempts in infected primary TKA . Clin Orthop Relat Res. 2011;469(4):970–6. doi: 10.1007/s11999-010-1417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan JA, Corrigan RM, Gruszka DT, Speziale P, O’gara JP, Potts JR, Foster TJ. Role of surface protein SasG in biofilm formation by Staphylococcus aureus . J Bacteriol. 2010;192(21):5663–73. doi: 10.1128/JB.00628-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gest H. The discovery of microorganisms by Robert Hooke, Antoni Van Leeuwenhoek, fellows of the Royal Society . Notes Rec R Soc Lond. 2004;58(2):187–201. doi: 10.1098/rsnr.2004.0055. [DOI] [PubMed] [Google Scholar]
- Glynn MK, Sheehan JM. An analysis of the causes of deep infection after hip and knee arthroplasties . Clin Orthop Relat Res. 1983;178:202–6. [PubMed] [Google Scholar]
- Gomez E, Cazanave C, Cunningham SA, Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, Hanssen AD, Karau MJ, Schmidt SM, Osmon DR, Berbari EF, Mandrekar J, Patel R. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication . J Clin Microbiol. 2012;50(11):3501–8. doi: 10.1128/JCM.00834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SB, Yao Z, Keeney M, Yang F. The future of biologic coatings for orthopaedic implants . Biomaterials. 2013;34(13):3174–83. doi: 10.1016/j.biomaterials.2013.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon YJ, Romanowski EG, Mcdermott AM. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs . Curr Eye Res. 2005;30(7):505–15. doi: 10.1080/02713680590968637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration . Science. 1987;237(4822):1588–95. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- Gristina AG, Costerton JW. Bacterial adherence to biomaterials and tissue. The significance of its role in clinical sepsis . J Bone Joint Surg Am. 1985;67(2):264–73. [PubMed] [Google Scholar]
- Guani-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Teran LM. Antimicrobial peptides: general overview and clinical implications in human health and disease . Clin Immunol. 2010;135(1):1–11. doi: 10.1016/j.clim.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Hagihara M, Crandon JL, Nicolau DP. The efficacy and safety of antibiotic combination therapy for infections caused by Gram-positive and Gram-negative organisms . Expert Opin Drug Saf. 2012;11(2):221–33. doi: 10.1517/14740338.2012.632631. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media . JAMA. 2006;296(2):202–11. doi: 10.1001/jama.296.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Stoodley P, Kathju S, Hoiby N, Moser C, Costerton JW, Moter A, Bjarnsholt T. Towards diagnostic guidelines for biofilm-associated infections . FEMS Immunol Med Microbiol. 2012;65(2):127–45. doi: 10.1111/j.1574-695X.2012.00968.x. [DOI] [PubMed] [Google Scholar]
- Hardes J, Ahrens H, Gebert C, Streitbuerger A, Buerger H, Erren M, Gunsel A, Wedemeyer C, Saxler G, Winkelmann W, Gosheger G. Lack of toxicological side-effects in silver-coated megaprostheses in humans . Biomaterials. 2007;28(18):2869–75. doi: 10.1016/j.biomaterials.2007.02.033. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis . Mol Microbiol. 1996;20(5):1083–91. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Hussain M, Peters G, Gotz F. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface . Mol Microbiol. 1997;24(5):1013–24. doi: 10.1046/j.1365-2958.1997.4101774.x. [DOI] [PubMed] [Google Scholar]
- Hoiby N, Frederiksen B, Pressler T. Eradication of early Pseudomonas aeruginosa infection . J Cyst Fibros. 2005;4(Suppl 2):49–54. doi: 10.1016/j.jcf.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Hoiby N, Ciofu O, Johansen HK, Song ZJ, Moser C, Jensen PO, Molin S, Givskov M, Tolker-Nielsen T, Bjarnsholt T. The clinical impact of bacterial biofilms . Int J Oral Sci. 2011;3(2):55–65. doi: 10.4248/IJOS11026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holinka J, Pilz M, Hirschl AM, Graninger W, Windhager R, Presterl E. Differential bacterial load on components of total knee prosthesis in patients with prosthetic joint infection . Int J Artif Organs. 2012;35(10):735–41. doi: 10.5301/ijao.5000152. [DOI] [PubMed] [Google Scholar]
- Hussain M, Herrmann M, Von Eiff C, Perdreau-Remington F, Peters G. A. 140-kilodalton extracellular protein is essential for the accumulation of Staphylococcus epidermidis strains on surfaces . Infect Immun. 1997;65(2):519–24. doi: 10.1128/iai.65.2.519-524.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth KD, Mihalko WM, Parvizi J, Sculco T, Mcarthur B, Bitar YE, Saleh KJ. How to minimize infection and thereby maximize patient outcomes in total joint arthroplasty: a multicenter approach: AAOS Exhibit Selection . J Bone Joint Surg Am. 2013;95(8):e501–13. doi: 10.2106/JBJS.L.00596. [DOI] [PubMed] [Google Scholar]
- Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms . Appl Environ Microbiol. 2008;74(2):470–6. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamsen E, Huhtala H, Puolakka T, Moilanen T. Risk factors for infection after knee arthroplasty. A register-based analysis of 43,149 cases . J Bone Joint Surg Am. 2009;91(1):38–47. doi: 10.2106/JBJS.G.01686. [DOI] [PubMed] [Google Scholar]
- Janz V, Wassilew GI, Hasart O, Matziolis G, Tohtz S, Perka C. Evaluation of sonicate fluid cultures in comparison to histological analysis of the periprosthetic membrane for the detection of periprosthetic joint infection . Int Orthop. 2013a;37(5):931–6. doi: 10.1007/s00264-013-1853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janz V, Wassilew GI, Hasart O, Tohtz S, Perka C. Improvement in the detection rate of PJI in total hip arthroplasty through multiple sonicate fluid cultures . J Orthop Res. 2013b;31(12):2021–4. doi: 10.1002/jor.22451. [DOI] [PubMed] [Google Scholar]
- John AK, Baldoni D, Haschke M, Rentsch K, Schaerli P, Zimmerli W, Trampuz A. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin . Antimicrob Agents Chemother. 2009;53(7):2719–24. doi: 10.1128/AAC.00047-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CW, Smolinski D, Keogh A, Kirk TB, Zheng MH. Confocal laser scanning microscopy in orthopaedic research . Prog Histochem Cytochem. 2005;40(1):1–71. doi: 10.1016/j.proghi.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Joseph TN, Chen AL, Di Cesare PE. Use of antibiotic-impregnated cement in total joint arthroplasty . J Am Acad Orthop Surg. 2003;11(1):38–47. doi: 10.5435/00124635-200301000-00006. [DOI] [PubMed] [Google Scholar]
- Kachlany SC, Levery SB, Kim JS, Reuhs BL, Lion LW, Ghiorse WC. Structure and carbohydrate analysis of the exopolysaccharide capsule of Pseudomonas putida G7 . Environ Microbiol. 2001;3(12):774–84. doi: 10.1046/j.1462-2920.2001.00248.x. [DOI] [PubMed] [Google Scholar]
- Kaplan JB. Therapeutic potential of biofilm-dispersing enzymes . Int J Artif Organs. 2009;32(9):545–54. doi: 10.1177/039139880903200903. [DOI] [PubMed] [Google Scholar]
- Kaplan JB, Ragunath C, Velliyagounder K, Fine DH, Ramasubbu N. Enzymatic detachment of Staphylococcus epidermidis biofilms . Antimicrob Agents Chemother. 2004;48(7):2633–6. doi: 10.1128/AAC.48.7.2633-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemzadeh-Narbat M, Kindrachuk J, Duan K, Jenssen H, Hancock RE, Wang R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections . Biomaterials. 2010;31(36):9519–26. doi: 10.1016/j.biomaterials.2010.08.035. [DOI] [PubMed] [Google Scholar]
- Khoury AE, Lam K, Ellis B, Costerton JW. Prevention and control of bacterial infections associated with medical devices . ASAIO J. 1992;38(3):M174–8. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- Kim YH, Choi Y, Kim JS. Treatment based on the type of infected TKA improves infection control . Clin Orthop Relat Res. 2011;469(4):977–84. doi: 10.1007/s11999-010-1425-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Oethinger M, Tuohy MJ, Procop GW, Bauer TW. Improved detection of biofilm-formative bacteria by vortexing and sonication: a pilot study . Clin Orthop Relat Res. 2009;467(5):1360–4. doi: 10.1007/s11999-008-0609-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz SM, Ong KL, Schmier J, Mowat F, Saleh K, Dybvik E, Karrholm J, Garellick G, Havelin LI, Furnes O, Malchau H, Lau E. Future clinical and economic impact of revision total hip and knee arthroplasty . J Bone Joint Surg Am. 2007;89(Suppl 3):144–51. doi: 10.2106/JBJS.G.00587. [DOI] [PubMed] [Google Scholar]
- Kutateladze M, Adamia R. Bacteriophages as potential new therapeutics to replace or supplement antibiotics . Trends Biotechnol. 2010;28(12):591–5. doi: 10.1016/j.tibtech.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Lawrence JR, Neu TR. Confocal laser scanning microscopy for analysis of microbial biofilms . Methods Enzymol. 1999;310:131–44. doi: 10.1016/s0076-6879(99)10011-9. [DOI] [PubMed] [Google Scholar]
- Lee B, Schjerling CK, Kirkby N, Hoffmann N, Borup R, Molin S, Hoiby N, Ciofu O. Mucoid Pseudomonas aeruginosa isolates maintain the biofilm formation capacity and the gene expression profiles during the chronic lung infection of CF patients . APMIS. 2011;119(4)(5):263–74. doi: 10.1111/j.1600-0463.2011.02726.x. [DOI] [PubMed] [Google Scholar]
- Legeay G, Poncin-Epaillard F, Arciola CR. New surfaces with hydrophilic/hydrophobic characteristics in relation to (no)bioadhesion . Int J Artif Organs. 2006;29(4):453–61. doi: 10.1177/039139880602900416. [DOI] [PubMed] [Google Scholar]
- Levy PY, Fenollar F. The role of molecular diagnostics in implant-associated bone and joint infection . Clin Microbiol Infect. 2012;18(12):1168–75. doi: 10.1111/1469-0691.12020. [DOI] [PubMed] [Google Scholar]
- Lewis K. Riddle of biofilm resistance . Antimicrob Agents Chemother. 2001;45(4):999–1007. doi: 10.1128/AAC.45.4.999-1007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidwell OM, Lowbury EJ, Whyte W, Blowers R, Stanley SJ, Lowe D. Bacteria isolated from deep joint sepsis after operation for total hip or knee replacement and the sources of the infections with Staphylococcus aureus . J Hosp Infect. 1983;4(1):19–29. doi: 10.1016/0195-6701(83)90061-0. [DOI] [PubMed] [Google Scholar]
- Loc-Carrillo C, Abedon ST. Pros and cons of phage therapy . Bacteriophage. 2011;1(2):111–4. doi: 10.4161/bact.1.2.14590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lora-Tamayo J, Murillo O, Iribarren JA, Soriano A, Sanchez-Somolinos M, Baraia-Etxaburu JM, Rico A, Palomino J, Rodriguez-Pardo D, Horcajada JP, Benito N, Bahamonde A, Granados A, Del Toro MD, Cobo J, Riera M, Ramos A, Jover-Saenz A, Ariza J, and Infection R G F T S OP A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention . Clin Infect Dis. 2013;56(2):182–94. doi: 10.1093/cid/cis746. [DOI] [PubMed] [Google Scholar]
- Mack D, Haeder M, Siemssen N, Laufs R. Association of biofilm production of coagulase-negative staphylococci with expression of a specific polysaccharide intercellular adhesin . J Infect Dis. 1996;174(4):881–4. doi: 10.1093/infdis/174.4.881. [DOI] [PubMed] [Google Scholar]
- Maderazo EG, Judson S, Pasternak H. Late infections of total joint prostheses. A review and recommendations for prevention . Clin Orthop Relat Res. 1988;229:131–42. [PubMed] [Google Scholar]
- Mah TF, O’Toole GA. Mechanisms of biofilm resistance to antimicrobial agents . Trends Microbiol. 2001;9(1):34–9. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Marculescu CE, Berbari EF, Hanssen AD, Steckelberg JM, Harmsen SW, Mandrekar JN, Osmon DR. Outcome of prosthetic joint infections treated with debridement and retention of components . Clin Infect Dis. 2006;42(4):471–8. doi: 10.1086/499234. [DOI] [PubMed] [Google Scholar]
- Masters JP, Smith NA, Foguet P, Reed M, Parsons H, Sprowson AP. A systematic review of the evidence for single stage and two stage revision of infected knee replacement . BMC Musculoskelet Disord. 2013;14(222) doi: 10.1186/1471-2474-14-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean RJ, Lam JS, Graham LL, Training the Biofilm Generation--a tribute to J. W. Costerton . J Bacteriol. 2012;194(24):6706–11. doi: 10.1128/JB.01252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan J, Jamali AA, Nguyen H. Prophylactic antibiotics in hip and knee arthroplasty . J Bone Joint Surg Am. 2009;91(10):2480–90. doi: 10.2106/JBJS.H.01219. [DOI] [PubMed] [Google Scholar]
- Merino N, Toledo-Arana A, Vergara-Irigaray M, Valle J, Solano C, Calvo E, Lopez JA, Foster TJ, Penades JR, Lasa I. Protein A-mediated multicellular behavior in Staphylococcus aureus . J Bacteriol. 2009;191(3):832–43. doi: 10.1128/JB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsen T, Lovgren E, Widerstrom M, Wallinder L. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections . J Clin Microbiol. 2009;47(8):2496–501. doi: 10.1128/JCM.02316-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanaro L, Speziale P, Campoccia D, Ravaioli S, Cangini I, Pietrocola G, Giannini S, Arciola CR. Scenery of Staphylococcus implant infections in orthopedics . Future Microbiol. 2011;6(11):1329–49. doi: 10.2217/fmb.11.117. [DOI] [PubMed] [Google Scholar]
- Neoh KG, Kang ET. Combating bacterial colonization on metals via polymer coatings: relevance to marine and medical applications . ACS Appl Mater Interfaces. 2011;3(8):2808–19. doi: 10.1021/am200646t. [DOI] [PubMed] [Google Scholar]
- Neut D, Van Horn JR, Van Kooten TG, Van Der Mei HC, Busscher HJ. Detection of biomaterial-associated infections in orthopaedic joint implants . Clin Orthop Relat Res. 2003;413:261–8. doi: 10.1097/01.blo.0000073345.50837.84. [DOI] [PubMed] [Google Scholar]
- Neut D, Hendriks JG, Van Horn JR, Kowalski RS, Van Der Mei HC, Busscher HJ. Antimicrobial efficacy of gentamicin-loaded acrylic bone cements with fusidic acid or clindamycin added . J Orthop Res. 2006;24(2):291–9. doi: 10.1002/jor.20058. [DOI] [PubMed] [Google Scholar]
- Nickel JC, Ruseska I, Wright JB, Costerton JW. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material . Antimicrob Agents Chemother. 1985;27(4):619–24. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski RJ, Gillespie RJ, Shishani Y, Cohen B, Walch G, Gobezie R. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders . J Shoulder Elbow Surg. 2012;21(3):324–8. doi: 10.1016/j.jse.2011.08.072. [DOI] [PubMed] [Google Scholar]
- O’Neill E, Pozzi C, Houston P, Humphreys H, Robinson DA, Loughman A, Foster TJ, O’Gara JP. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB . J Bacteriol. 2008;190(11):3835–50. doi: 10.1128/JB.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole GA. Microbiology: a resistance switch . Nature. 2002;416(6882):695–6. doi: 10.1038/416695a. [DOI] [PubMed] [Google Scholar]
- Osmon DR, Berbari EF, Berendt AR, Lew D, Zimmerli W, Steckelberg JM, Rao N, Hanssen A, Wilson W R, and Infectious Diseases Society Of A Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America . Clin Infect Dis. 2013;56(1):e1–e25. doi: 10.1093/cid/cis803. [DOI] [PubMed] [Google Scholar]
- Pamp SJ, Gjermansen M, Johansen HK, Tolker-Nielsen T. Tolerance to the antimicrobial peptide colistin in Pseudomonas aeruginosa biofilms is linked to metabolically active cells, and depends on the pmr and mexAB-oprM genes . Mol Microbiol. 2008;68(1):223–40. doi: 10.1111/j.1365-2958.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- Parvizi J, Gehrke T, Chen AF. Proceedings of the international consensus on periprosthetic joint infection . Bone Joint J. 2013;95-B(11):1450–52. doi: 10.1302/0301-620X.95B11.33135. [DOI] [PubMed] [Google Scholar]
- Patti JM, Allen BL, Mcgavin MJ, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- Peersman G, Laskin R, Davis J, Peterson M. Infection in total knee replacement: a retrospective review of 6489 total knee replacements . Clin Orthop Relat Res. 2001;392:15–23. [PubMed] [Google Scholar]
- Peng ZX, Tu B, Shen Y, Du L, Wang L, Guo SR, Tang TT. Quaternized chitosan inhibits icaA transcription and biofilm formation by Staphylococcus on a titanium surface . Antimicrob Agents Chemother. 2011;55(2):860–6. doi: 10.1128/AAC.01005-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival SL, Hill KE, Williams DW, Hooper SJ, Thomas DW, Costerton JW. A review of the scientific evidence for biofilms in wounds . Wound Repair Regen. 2012;20(5):647–57. doi: 10.1111/j.1524-475X.2012.00836.x. [DOI] [PubMed] [Google Scholar]
- Periasamy S, Joo HS, Duong AC, Bach TH, Tan VY, Chatterjee SS, Cheung GY, Otto M. How Staphylococcus aureus biofilms develop their characteristic structure . Proc Natl Acad Sci U S A. 2012;109(4):1281–86. doi: 10.1073/pnas.1115006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini P, Arciola CR, Pezzali I, Bozzini S, Montanaro L, Tanzi MC, Speziale P, Visai L. Antibacterial activity of zinc modified titanium oxide surface . Int J Artif Organs. 2006;29(4):434–42. doi: 10.1177/039139880602900414. [DOI] [PubMed] [Google Scholar]
- Piper KE, Jacobson MJ, Cofield RH, Sperling JW, Sanchez-Sotelo J, Osmon DR, Mcdowell A, Patrick S, Steckelberg JM, Mandrekar JN, Fernandez Sampedro M, Patel R. Microbiologic diagnosis of prosthetic shoulder infection by use of implant sonication . J Clin Microbiol. 2009;47(6):1878–84. doi: 10.1128/JCM.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portillo ME, Salvado M, Sorli L, Alier A, Martinez S, Trampuz A, Gomez J, Puig L, Horcajada JP. Multiplex PCR of sonication fluid accurately differentiates between prosthetic joint infection and aseptic failure. J Infect. 2012;65(6):541–8. doi: 10.1016/j.jinf.2012.08.018. [DOI] [PubMed] [Google Scholar]
- Post JC, Preston RA, Aul JJ, Larkins-Pettigrew M, Rydquist-White J, Anderson KW, Wadowsky RM, Reagan DR, Walker ES, Kingsley LA, Magit AE, Ehrlich GD. Molecular analysis of bacterial pathogens in otitis media with effusion . JAMA. 1995;273(20):1598–604. [PubMed] [Google Scholar]
- Psaltis AJ, Ha KR, Beule AG, Tan LW, Wormald PJ. Confocal scanning laser microscopy evidence of biofilms in patients with chronic rhinosinusitis . Laryngoscope. 2007;117(7):1302–1306. doi: 10.1097/MLG.0b013e31806009b0. [DOI] [PubMed] [Google Scholar]
- Puhto AP, Puhto T, Syrjala H. Short-course antibiotics for prosthetic joint infections treated with prosthesis retention . Clin Microbiol Infect. 2012;18(11):1143–1148. doi: 10.1111/j.1469-0691.2011.03693.x. [DOI] [PubMed] [Google Scholar]
- Qin Z, Ou Y, Yang L, Zhu Y, Tolker-Nielsen T, Molin S, Qu D. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis . Microbiology. 2007;153(Pt 7):2083–92. doi: 10.1099/mic.0.2007/006031-0. [DOI] [PubMed] [Google Scholar]
- Raad I, Hanna H, Jiang Y, Dvorak T, Reitzel R, Chaiban G, Sherertz R, Hachem R. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm . Antimicrob Agents Chemother. 2007;51(5):1656–60. doi: 10.1128/AAC.00350-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KC, Mann EE, Endres JL, Weiss EC, Cassat JE, Smeltzer MS, Bayles KW. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. Proc Natl Acad Sci U S A. 2007;104(19):8113–8118. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochford ET, Richards RG, Moriarty TF. Influence of material on the development of device-associated infections . Clin Microbiol Infect. 2012;18(12):1162–7. doi: 10.1111/j.1469-0691.2012.04002.x. [DOI] [PubMed] [Google Scholar]
- Rohde H, Burdelski C, Bartscht K, Hussain M, Buck F, Horstkotte MA, Knobloch JK, Heilmann C, Herrmann M, Mack D. Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases . Mol Microbiol. 2005;55(6):1883–95. doi: 10.1111/j.1365-2958.2005.04515.x. [DOI] [PubMed] [Google Scholar]
- Rupp ME, Ulphani JS, Fey PD, Bartscht K, Mack D. Characterization of the importance of polysaccharide intercellular adhesin/hemagglutinin of Staphylococcus epidermidis in the pathogenesis of biomaterial-based infection in a mouse foreign body infection model . Infect Immun. 1999a;67(5):2627–32. doi: 10.1128/iai.67.5.2627-2632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp ME, Ulphani JS, Fey PD, Mack D. Characterization of Staphylococcus epidermidis polysaccharide intercellular adhesin/hemagglutinin in the pathogenesis of intravascular catheter-associated infection in a rat model . Infect Immun. 1999b;67(5):2656–9. doi: 10.1128/iai.67.5.2656-2659.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Ray P, Das A, Sharma M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms . J Antimicrob Chemother. 2010;65(9):1955–8. doi: 10.1093/jac/dkq257. [DOI] [PubMed] [Google Scholar]
- Sorli L, Puig L, Torres-Claramunt R, Gonzalez A, Alier A, Knobel H, Salvado M, Horcajada JP. The relationship between microbiology results in the second of a two-stage exchange procedure using cement spacers and the outcome after revision total joint replacement for infection: the use of sonication to aid bacteriological analysis . J Bone Joint Surg Br. 2012;94(2):249–53. doi: 10.1302/0301-620X.94B2.27779. [DOI] [PubMed] [Google Scholar]
- Speziale P, Pietrocola G, Rindi S, Provenzano M, Provenza G, Di Poto A, Visai L, Arciola CR. Structural and functional role of Staphylococcus aureus surface components recognizing adhesive matrix molecules of the host . Future Microbiol. 2009;4(10):1337–52. doi: 10.2217/fmb.09.102. [DOI] [PubMed] [Google Scholar]
- Stewart PS, Davison WM, Steenbergen JN. Daptomycin rapidly penetrates a Staphylococcus epidermidis biofilm . Antimicrob Agents Chemother. 2009;53(8):3505–7. doi: 10.1128/AAC.01728-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P, Nistico L, Johnson S, Lasko LA, Baratz M, Gahlot V, Ehrlich GD, Kathju S. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. A case report . J Bone Joint Surg Am. 2008;90(8):1751–8. doi: 10.2106/JBJS.G.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley P, Ehrlich GD, Sedghizadeh PP, Hall-Stoodley L, Baratz ME, Altman DT, Sotereanos NG, Costerton JW, Demeo P. Orthopaedic biofilm infections . Curr Orthop Pract. 2011;22(6):558–63. doi: 10.1097/BCO.0b013e318230efcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan H, Peng Z, Li Q, Xu X, Guo S, Tang T. The use of quaternised chitosan-loaded PMMA to inhibit biofilm formation and downregulate the virulence-associated gene expression of antibiotic-resistant staphylococcus. Biomaterials. 2012;33(2):365–77. doi: 10.1016/j.biomaterials.2011.09.084. [DOI] [PubMed] [Google Scholar]
- Thomas VC, Thurlow LR, Boyle D, Hancock LE. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J Bacteriol. 2008;190(16):5690–8. doi: 10.1128/JB.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trampuz A, Zimmerli W. Antimicrobial agents in orthopaedic surgery: Prophylaxis and treatment . Drugs. 2006;66(8):1089–105. doi: 10.2165/00003495-200666080-00005. [DOI] [PubMed] [Google Scholar]
- Trampuz A, Zimmerli W. Diagnosis and treatment of implant-associated septic arthritis and osteomyelitis . Curr Infect Dis Rep. 2008;10(5):394–403. doi: 10.1007/s11908-008-0064-1. [DOI] [PubMed] [Google Scholar]
- Trampuz A, Osmon DR, Hanssen AD, Steckelberg JM, Patel R. Molecular and antibiofilm approaches to prosthetic joint infection . Clin Orthop Relat Res. 2003;414:69–88. doi: 10.1097/01.blo.0000087324.60612.93. [DOI] [PubMed] [Google Scholar]
- Trampuz A, Murphy CK, Rothstein DM, Widmer AF, Landmann R, Zimmerli W. Efficacy of a novel rifamycin derivative, ABI-0043, against Staphylococcus aureus in an experimental model of foreign-body infection . Antimicrob Agents Chemother. 2007a;51(7):2540–5. doi: 10.1128/AAC.00120-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trampuz A, Piper KE, Jacobson MJ, Hanssen AD, Unni KK, Osmon DR, Mandrekar JN, Cockerill FR, Steckelberg JM, Greenleaf JF, Patel R. Sonication of removed hip and knee prostheses for diagnosis of infection . N Engl J Med. 2007b;357(7):654–63. doi: 10.1056/NEJMoa061588. [DOI] [PubMed] [Google Scholar]
- Tunney MM, Patrick S, Gorman SP, Nixon JR, Anderson N, Davis RI, Hanna D, Ramage G. Improved detection of infection in hip replacements. A currently underestimated problem . J Bone Joint Surg Br. 1998;80(4):568–72. doi: 10.1302/0301-620x.80b4.8473. [DOI] [PubMed] [Google Scholar]
- Valerius NH, Koch C, Hoiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment . Lancet. 1991;338(8769):725–6. doi: 10.1016/0140-6736(91)91446-2. [DOI] [PubMed] [Google Scholar]
- Van Belkum A, Tassios PT, Dijkshoorn L, Haeggman S, Cookson B, Fry NK, Fussing V, Green J, Feil E, Gerner-Smidt P, Brisse S, Struelens M. Guidelines for the validation and application of typing methods for use in bacterial epidemiology . Clin Microbiol Infect. 2007;13(Suppl 3):1–46. doi: 10.1111/j.1469-0691.2007.01786.x. European Society of Clinical M and Infectious Diseases Study Group on Epidemiological M. [DOI] [PubMed] [Google Scholar]
- Vernachio JH, Bayer AS, Ames B, Bryant D, Prater BD, Syribeys PJ, Gorovits EL, Patti JM. Human immunoglobulin G recognizing fibrinogen-binding surface proteins is protective against both Staphylococcus aureus and Staphylococcus epidermidis infections in vivo . Antimicrob Agents Chemother. 2006;50(2):511–8. doi: 10.1128/AAC.50.2.511-518.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong C, Saenz HL, Gotz F, Otto M. Impact of the agr quorum-sensing system on adherence to polystyrene in Staphylococcus aureus . J Infect Dis. 2000;182(6):1688–93. doi: 10.1086/317606. [DOI] [PubMed] [Google Scholar]
- Vuong C, Gerke C, Somerville GA, Fischer ER, Otto M. Quorum-sensing control of biofilm factors in Staphylococcus epidermidis . J Infect Dis. 2003;188(5):706–18. doi: 10.1086/377239. [DOI] [PubMed] [Google Scholar]
- Vuong C, Kocianova S, Voyich JM, Yao Y, Fischer ER, Deleo FR, Otto M. A crucial role for exopolysaccharide modification in bacterial biofilm formation, immune evasion, and virulence . J Biol Chem. 2004a;279(52):54881–6. doi: 10.1074/jbc.M411374200. [DOI] [PubMed] [Google Scholar]
- Vuong C, Kocianova S, Yao Y, Carmody AB, Otto M. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo . J Infect Dis. 2004b;190(8):1498–505. doi: 10.1086/424487. [DOI] [PubMed] [Google Scholar]
- Walker JT, Verran J, Boyd RD, Percival S. Microscopy methods to investigate structure of potable water biofilms . Methods Enzymol. 2001;337:243–55. doi: 10.1016/s0076-6879(01)37018-0. [DOI] [PubMed] [Google Scholar]
- Watnick P, Kolter R. Biofilm, city of microbes . J Bacteriol. 2000;182(10):2675–9. doi: 10.1128/jb.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb JC, Spencer RF. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery . J Bone Joint Surg Br. 2007;89(7):851–7. doi: 10.1302/0301-620X.89B7.19148. [DOI] [PubMed] [Google Scholar]
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295(5559):1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- Williams DL, Costerton JW. Using biofilms as initial inocula in animal models of biofilm-related infections . J Biomed Mater Res B Appl Biomater. 2012;100(4):1163–9. doi: 10.1002/jbm.b.31979. [DOI] [PubMed] [Google Scholar]
- Wittebole X, De Roock S, Opal SM. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens . Virulence. 2014;5(1):226–35. doi: 10.4161/viru.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolcott RD, Ehrlich GD. Biofilms and chronic infections . JAMA. 2008;299(22):2682–4. doi: 10.1001/jama.299.22.2682. [DOI] [PubMed] [Google Scholar]
- Worthington RJ, Melander C. Combination approaches to combat multidrug-resistant bacteria . Trends Biotechnol. 2013;31(3):177–84. doi: 10.1016/j.tibtech.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz C, Colak M, Yilmaz BC, Ersoz G, Kutateladze M, Gozlugol M. Bacteriophage therapy in implant-related infections: an experimental study . J Bone Joint Surg Am. 2013;95(2):117–25. doi: 10.2106/JBJS.K.01135. [DOI] [PubMed] [Google Scholar]
- Yoshinari M, Kato T, Matsuzaka K, Hayakawa T, Shiba K. Prevention of biofilm formation on titanium surfaces modified with conjugated molecules comprised of antimicrobial and titanium-binding peptides . Biofouling. 2010;26(1):103–10. doi: 10.1080/08927010903216572. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Stewart PS. Penetration of rifampin through Staphylococcus epidermidis biofilms . Antimicrob Agents Chemother. 2002;46(3):900–3. doi: 10.1128/AAC.46.3.900-903.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli W. Orthopaedic device-associated infection . Clin Microbiol Infect. 2012;18(12):1160–1. doi: 10.1111/j.1469-0691.2012.04011.x. [DOI] [PubMed] [Google Scholar]
- Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections . N Engl J Med. 2004;351(16):1645–54. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]