Abstract
Background and purpose
Wear rates of highly crosslinked polyethylene (XLPE) acetabular components have varied considerably between different published studies. This variation is in part due to the different techniques used to measure wear and to the errors inherent in measuring the relatively low amounts of wear in XLPE bearings. We undertook a scoping review of studies that have examined the in vivo wear of XLPE acetabular components using the most sensitive method available, radiostereometric analysis (RSA).
Methods
A systematic search of the PubMed, Scopus, and Cochrane databases was performed to identify published studies in which RSA was used to measure wear of XLPE components in primary total hip arthroplasty (THA).
Results
18 publications examined 12 primary THA cohorts, comprising only 260 THAs at 2–10 years of follow-up. The mean or median proximal wear rate reported ranged from 0.00 to 0.06 mm/year. However, differences in the manner in which wear was determined made it difficult to compare some studies. Furthermore, differences in RSA methodology between studies, such as the use of supine or standing radiographs and the use of beaded or unbeaded reference segments, may limit future meta-analyses examining the effect of patient and implant variables on wear rates.
Interpretation
This scoping review confirmed the low wear rates of XLPE in THA, as measured by RSA. We make recommendations to enhance the standardization of reporting of RSA wear results, which will facilitate early identification of poorly performing implants and enable a better understanding of the effects of surgical and patient factors on wear.
Implant loosening and periprosthetic bone loss remain the most common reasons for revision of primary total hip arthroplasty (THA) in the medium to long term (AOANJRR 2013). The tissue response to polyethylene wear particles is an important cause of periprosthetic bone loss – osteolysis – behind acetabular components (Dumbleton et al. 2002). Review articles on THAs with conventional polyethylene have confirmed that the greater the amount of polyethylene wear, the higher the incidence of osteolysis (Oparaugo et al. 2001, Dumbleton et al. 2002) and that osteolysis is rare below a linear wear rate of 0.1 mm/year (Dumbleton et al. 2002). Research has therefore been focussed on improving the wear properties of polyethylene and on monitoring the in vivo wear of polyethylene liners of acetabular components of THAs by using various radiographic methods.
The introduction of XLPE
The first ultra-high-molecular-weight polyethylene (UHMWPE) that was intentionally gamma irradiated at a high level (100mRad) was first used clinically in 1971 (Oonishi et al. 2001). However, further research into the optimal dose of gamma radiation and its effect on the wear properties of conventional UHMWPE was not performed until the 1990s. Manufacturing methods were developed to crosslink polyethylene by exposing it to gamma or electron beam irradiation and then annealing or remelting the material by thermal treatments (Oonishi et al. 1997, Sun et al. 1997). In vitro hip simulator studies were able to show that highly crosslinked polyethylene (XLPE) components show significantly reduced wear compared to UHMWPE components (Kurtz et al. 1999, Muratoglu et al. 2001). Thus, XLPE components were introduced for use in THA surgery in 1998 (Kurtz et al. 2011), and by 2003 XLPE was used in two-thirds of hip arthroplasties in the USA (Kurtz 2004). More recently in Australia, the 2013 annual report of the joint replacement registry reported that XLPE was used in 94% of all primary THAs incorporating a PE bearing (AOANJRR 2013). When used in primary THA, XLPE has a lower rate of revision for any reason than conventional PE (AOANJRR 2013). Different companies continue to use different manufacturing methods for each XLPE product, aiming to balance resistance to wear, oxidation, and fatigue fracture (Pruitt et al. 2013). Ideally, as with all new prosthetic components, new XLPEs should be rigorously tested in clinical trials before being released for general use because of potential variation in manufacturing methods, which may lead to possible failure (Rohrl et al. 2005, Malchau et al. 2011).
The in vivo wear rate of XLPE acetabular components has been shown to be less than that of conventional UHMWPE components (Mu et al. 2009, Kurtz et al. 2011, Kuzyk et al. 2011). However, the wear rates reported for XLPE components have varied considerably between different published studies (Kurtz et al. 2011). For example, the mean 2D wear rate of one type of XLPE liner, using different measurement techniques after 5 years or more, has varied between 0.01 and 0.05 mm/year (Engh et al. 2006, Bitsch et al. 2008, Mutimer et al. 2010, Engh et al. 2012, Callary et al. 2013a).
Methods of wear measurement
Clinical studies of bearing surfaces use serial radiographs to measure the amount of femoral head penetration within the acetabular component as a representation of wear of the bearing surface. Traditionally, plain anteroposterior and/or lateral radiographs have been taken at regular time points postoperatively and the measurements have been made either manually (Livermore method (Livermore et al. 1990); Dorr and Wan method (Dorr and Wan 1995)) or using a software program that analyzes digitized radiographs (Martell’s “Hip Analysis Suite” (Martell and Berdia 1997); Devane’s “PolyWare” (Devane et al. 1995a, Devane et al. 1995b)). Measurements made from plain radiographs were sensitive enough to measure wear of conventional UHMWPE. However, due to the improved wear properties of XLPE, measurement of the lower amounts of in vivo wear associated with XLPE is more challenging, ideally requiring a more sensitive measurement method—namely radiostereometric analysis (RSA) (Bragdon et al. 2006a, Stilling et al. 2012) .
Radiostereometric analysis
RSA uses dual simultaneous radiographs taken over a calibration cage to calculate the 3D movement of one skeletal body segment relative to another (Karrholm et al. 2006). The traditional RSA method relies on the implantation of small spherical tantalum markers (0.8 and 1.0 mm diameter) to represent each skeletal body of interest (Karrholm et al. 1997). RSA was first used to measure polyethylene wear in 1976 (Baldursson et al. 1979). To measure polyethylene wear, tantalum markers are usually implanted in the peripheral rim of the polyethylene liner or on the back side of the cemented polyethylene components at the time of surgery, or (for a small number of studies) at the time of manufacture. The patient then undergoes consecutive radiographic examinations at set time points to measure the penetration of the femoral head within the polyethylene component. Accuracy of RSA under optimal conditions has been reported to 33, 22, 86, and 55 µm for measurement of medial, proximal, anterior, and 3D wear, respectively (Bragdon et al. 2002).
If the influences of patient and implant factors on wear rates of XLPE are to be investigated in detail, ideally it should be through meta-analysis of RSA studies. Before a meta-analysis, the published literature must be surveyed to determine whether the data reported in primary studies are sufficient to enable comparison of such factors. Scoping review is a method of inquiry similar to a systematic review but with the distinct aim of assessing the quantity and scope of the research studies conducted on a certain topic (Grant and Booth 2009, The Joanna Briggs Institute 2011). We therefore undertook a scoping review of studies on wear of XLPE acetabular components measured by RSA, using a systematic search to identify these studies.
Methods
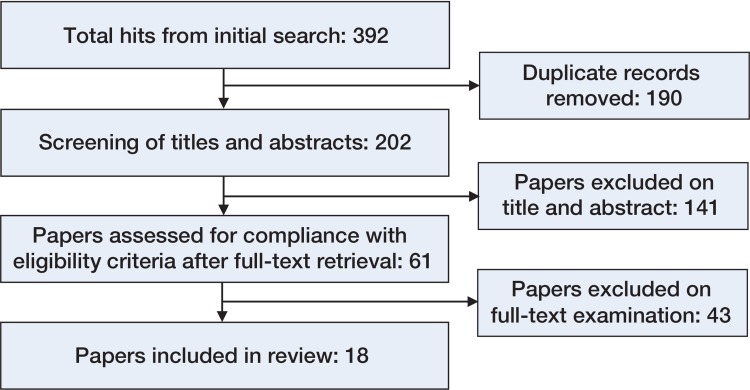
A systematic search of the published literature in PubMed, Scopus, and Cochrane databases was performed on December 19, 2013. Title, abstract, and keyword fields were queried using the following keywords and index terms in the databases where applicable: “radiostereometric” AND “wear”; “radiostereometric” AND “polyethylene”; “radiostereometry” AND “wear”; “radiostereometry” AND “polyethylene”; “rsa” AND “wear”; “rsa” AND “polyethylene”; “stereophotogrammetric” AND “wear”; “stereophotogrammetric” AND “polyethylene” (Figure 1). These search terms were chosen based on the different names used to describe RSA studies. Publications in English were included if they reported the wear of XLPE, as measured by RSA, in either cemented or uncemented acetabular components in primary THAs. All such studies were included in this review, as the aim of scoping reviews is to determine the extent of the literature on a certain topic and therefore, unlike meta-analyses or systematic reviews, exclusion based on critical appraisal of methodological quality is not required. Polyethylene components were defined as highly crosslinked when intentionally treated using a total radiation dose ranging from 50 to 105 kGy (Kurtz et al. 2002). Duplicate publications, theses, case reports, conference proceedings, and abstracts were all excluded. Data extracted from the studies included details of the patient cohort, the RSA methodology used, precision, total femoral head penetration, bedding-in/creep, and wear.
Flow chart of the systematic search performed of the PubMed, Scopus, and Cochrane databases.
Results
We found 18 publications (Table 1) that fitted the criteria (Figure), representing 12 independent cohorts of patients. 9 of the 18 publications (Digas et al. 2004, Digas et al. 2007, Rohrl et al. 2007, Glyn-Jones et al. 2008b, Thomas et al. 2011, Johanson et al. 2012, Rohrl et al. 2012, Callary et al. 2013a,b) were longer-term follow-up reports of these cohorts. The wear of 7 different XLPE components was measured, incorporating 3 designs of cemented XLPE acetabular components and 4 designs of XLPE liners of uncemented acetabular components, as detailed in Table 1. 10 of the 12 cohorts received 28-mm articulations, and 2 cohorts involved larger articulations (32-mm and 36-mm). The material of the femoral head was cobalt chromium in 8 cohorts and oxidized zirconium in one. The material was not reported for 3 cohorts.
Table 1.
Implants used in each patient cohort
| Cohort | Publication | XLPE component | Head size, mm | Head material | Femoral component |
|---|---|---|---|---|---|
| 1 | Digas et al. 2003; | Durasul (Zimmer) | 28 | CoCr | Spectron (Smith & Nephew) |
| Digas et al. 2004; | |||||
| Digas et al. 2007; | |||||
| Johanson et al. 2012 | |||||
| 2 | Digas et al. 2004; | Longevity liner within Trilogy shell (Zimmer) | 28 | CoCr | Spectron (Smith & Nephew) |
| Digas et al. 2007 | |||||
| 3 | Rohrl et al. 2005; | Osteonics Cup made of Crossfire PE (Stryker Orthopaedics) | 28 | CoCr | Exeter Femoral Stem (Stryker Orthopaedics) |
| Rohrl et a.l 2007; | |||||
| Rohrl et a.l 2012 | |||||
| 4 | Zhou, et al. 2006 | XLPE 10 within Reflection Shell (Smith and Nephew) | 28 | CoCr | Spectron (Smith & Nephew) |
| 5 | Bragdon et al. 2007 | Longevity liner within Trilogy Shell (Zimmer) | 28 | NR | NR |
| 6 | Bragdon et al. 2007 | Longevity liner within Trilogy Shell (Zimmer) | 36 | NR | NR |
| 7 | Glyn-Jones et al. 2008a; | Longevity liner within Trilogy Shell (Zimmer) | 28 | CoCr | CPT (Zimmer) |
| Glyn-Jones et al. 2008b; | |||||
| Thomas et al. 2011 | |||||
| 8 | Ayers et al. 2009 | Longevity liner within Trilogy Shell (Zimmer) | 28 | NR | ML Taper (Zimmer) |
| 9 | Campbell et al. 2010a; | Marathon liner within Pinnacle Shell (Depuy Orthopaedics) |
28 | CoCr | Corail (Depuy Orthopaedics) |
| Callary et al. 2013a | |||||
| 10 | Campbell et al. 2010b; | X3 liner within Trident Shell | 32 | CoCr | Accolade |
| Callary et al. 2013b | (Stryker Orthopaedics) | (Stryker Orthopaedics) | |||
| 11 | Kadar et al. 2011 | Reflection All-Poly XLPE (Smith & Nephew) | 28 | CoCr | Spectron EF (Smith & Nephew) |
| 12 | Kadar et al. 2011 | Reflection All-Poly XLPE (Smith & Nephew) | 28 | Oxinium | Spectron EF (Smith & Nephew) |
NR: not reported.
Collectively, RSA results have been reported for a maximum of 260 THAs (Table 2). The initial report of each cohort was published at either 2 or 3 years, and the longest follow-up was 10 years. The age of each cohort at THA varied between a mean or median of 48 and 72 years.
Table 2.
Details of RSA studies
| Cohort | Age (range) | Number of patients | Follow-up, months | Report | Years of follow-up | Number of patients included in RSA results | Software | Acetabular reference | Standing/Supine |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Median 54 (35–68) | 31 | within 7 days, | 1st | 2 | (Digas et al. 2003); 23 supine, 21 standing | UmRSA | B | Supine a |
| 3, 6, 12, 24, 36 | 2nd | 3 | (Digas et al. 2004); 20 supine, 18 standing | ||||||
| 60, 84, 120 | 3rd | 5 | (Digas et al. 2007); 28 supine, 22 standing | ||||||
| 4th | 10 | (Johanson et al. 2012); 23 supine | |||||||
| 2 | Median 48 (29–70) | 32 | within 7 days, 3, | 1st | 2 | (Digas et al. 2004); 22 supine, 20 standing | UmRSA | B | Supine a |
| 6, 12, 24, 36, 60 | 2nd | 5 | (Digas et al. 2007); 19 supine, 12 standing | ||||||
| 3 | Mean 58 (49–79) | 10 | within 7 days, | 1st | 3 | (Rohrl et al. 2005); 10 | UmRSA | B | Supine |
| 2, 12, 24, 36, 60, | 2nd | 6 | (Rohrl et al. 2007); 9 | ||||||
| 72, 120 | 3rd | 10 | (Rohrl et al. 2012); 8 | ||||||
| 4 | Mean 68 (53–83) | 30 | 3 to 7 days, 2, 12, 24 |
1st | 2 | (Zhou et al. 2006); 28 | UmRSA 6.0 | B+E | Supine |
| 5 | Mean 56 (36–77) | 16 | 6 weeks, 6, 12, 24, 36 |
1st | 3 | (Bragdon et al. 2007); 16(25/30 b) | UmRSA 6.0 | B; B+ E; E |
Standing |
| 6 | Mean 56 (36–77) | 14 | 6 weeks, 6,12, 24, 36 |
1st | 3 | (Bragdon et al. 2007); 14 (25/30 b) | UmRSA 6.0 | B; B+ E; E |
Standing |
| 7 | Mean 68 (52–76) | 27 | PO, 3, 6, 12, 24, 36, 60, 84 |
1st 2nd 3rd |
2 | (Glyn-Jones et al. 2008a); 26 3 (Glyn-Jones et al. 2008b); 26 7 (Thomas et al. 2011); 22 |
Gill et al. 1998 |
Un-bedead | Standing |
| 8 | Mean 58 (SD 8) | 24 | 6 weeks, 6, 12, 24 | 1st | 2 | (Ayers et al. 2009); 24 | UmRSA | B | Standing |
| 9 | Median 72 (55–80) | 30 | 4–6 days, 6, 12, 24, 72 | 1st 2dn |
2 | (Campbell et al. 2010a); 25 6 (Callary et al. 2013a); 24 |
UmRSA 6.0 | B+E | Supine |
| 10 | Median 63 (47–76) | 21 | Within 7 days, 6, 12, 24, 60 | 1st 2nd |
2 | (Campbell et al. 2010b); 19 5 (Callary et al. 2013b); 18 |
UmRSA 6.0 | B+E | Supine |
| 11 | Mean 70 (SD 5) | 30 | 9–15 days, 3, 6, 12, 24 | 1st | 2 | (Kadar et al. 2011); 29 | UmRSA 5.0 | B | Supine |
| 12 | Mean 70 (SD 5) | 30 | 9–15 days, 3, 6, 12, 24 | 1st | 2 | (Kadar et al. 2011); 24 | UmRSA 5.0 | B | Supine |
Supine and standing from 3 months.
Combined number of patients included in wear results for cohorts 5 and 6.
B: beaded; B+E: beaded plus ellipse; E: ellipse
The specific RSA methodology used varied between cohorts (Table 2). For example, supine radiographs were used for RSA examinations in 6 cohorts, standing in 4, and a combination of both supine and standing in 2. All but 1 of the RSA studies used the UmRSA software package (RSA Biomedical, Umea, Sweden), but with different versions over time. The remaining study used software described by Gill et al. (1998). Tantalum beads were implanted within the XLPE component in 11 of 12 cohorts, to represent the acetabular segment. In most cases, RSA radiographs within the first postoperative week were used as the reference examination.
The precision of proximal wear measurements was reported for 6 cohorts and varied from 0.02 to 0.11 mm (Table 3). Both proximal and 3D head penetrations were reported for almost all cohorts, but the time over which the wear rate was calculated varied due to the time period allowed for bedding-in, which ranged from 2 to 24 months (Table 4). The proximal wear rate calculated after this period of assumed bedding-in ranged from a mean or median of 0.00 to 0.06 mm/year.
Table 3.
Precision of RSA from double examinations in each cohort
| Cohort | Publication in which precision was reported | Original calculation method | Number of double examinations | Adjusted precision |
|||
|---|---|---|---|---|---|---|---|
| x | y | z | 3D | ||||
| 1 | Digas et al. 2003 | 99% CI | 45 | 0.10 | 0.08 | 0.15 | 0.17 |
| 2 | Digas et al. 2004 | 99% CI | 45 | a | a | a | a |
| 3 | Rohrl et al. 2007 | 95% CI | 99 | b | 0.08 | b | 0.16 |
| Rohrl et al. 2012 | 1.96 × SD | b | b | 0.09 | b | 0.31 | |
| 4 | Zhou et al. 2006 | Beaded 1.96 × SD | 28 | b | 0.08 | b | 0.22 |
| Ellipse 1.96 × SD | 28 | b | 0.10 | b | 0.28 | ||
| 5 and 6 | b | b | b | b | b | b | b |
| 7 | b | b | b | b | b | b | b |
| 8 | b | b | b | b | b | b | b |
| 9 | Campbell et al. 2010a | 95% CI | 22 | 0.03 | 0.02 | 0.07 | b |
| 10 | b | b | b | b | b | b | b |
| 11 and 12 | Kadar et al. 2011 | 2.009 × SD | 50 | 0.11 | 0.11 | 0.33 | 0.21 |
Precision not specified for each axis, “between 0.07 and 0.32 mm”.
Not reported.
Table 4.
Proximal and 3D femoral head penetration, bedding-in, and wear rate reported for each cohort in each follow-up report
| Cohort | Follow-up years | Femoral head penetrationa (mm) | Bedding-inb mm | Wear ratec mm/year |
|---|---|---|---|---|
| Proximal | ||||
| 1 | 2 (Digas et al. 2003) | 0.13 (0.03 to 0.31) d | 0.1 d | 0.03 f (3–24 m) e |
| 3 (Digas et al. 2004) | 0.13 (-0.02 to 0.30) d | 0.1 d, g | 0.03 f (3–36 m) e | |
| 5 (Digas et al. 2007) | 0.15 (-0.10 to 0.86) d | 0.1 d, g | 0.02 f (3–60 m) e | |
| 10 (Johanson et al. 2012) | 0.15 g | 0.1 d, g | 0.01 (SE 0.00) (2–10 yr) d | |
| 2 | 2 (Digas et al. 2004) | 0.08 (-0.03 to 0.28) d | 0.08 d | 0.03 f (3–24 m) e |
| 5 (Digas et al. 2007) | 0.08 (-0.02 to 0.24) d | 0.08 d, g | 0.02 f(3–60 m) e | |
| 3 | 3 (Rohrl et al. 2005) | NR | 0.05 (0–2 months) | 0.01 (2–24 m) |
| 6 (Rohrl et al. 2007) | 0.08 (CI 0.02 to 0.13) | 0.06 g | 0.01 (2–72 m) | |
| 10 (Rohrl et al. 2012) | 0.07 (CI -0.02 to 0.15) | 0.06 g | 0.00 (2–120 m) | |
| 4 | 2 (Zhou et al. 2006) | 0.07 g | 0.06 g | 0.01 (2–24 m) |
| 5 | 3 (Bragdon et al. 2007) | 0.06 h (SE 0.03) | 0.06 h (SE 0.04) | 0.03 h (SE 0.02) |
| 6 | 3 (Bragdon et al. 2007) | 0.06 h (SE 0.06) | 0.07 h (SE 0.02) | 0.00 h (SE 0.06) |
| 7 | 2 (Glyn-Jones et al. 2008a) | NR | NR | 0.06 (SD 0.07) (3–24 m) |
| 3 (Glyn-Jones et al. 2008b) | NR | 0.17 g | 0.02 g | |
| 7 (Thomas et al. 2011) | NR | NR | 0.01 (CI ±0.03) | |
| 8 | 2 (Ayers et al. 2009) | 0.07 h (-0.04 to 0.19) | 0.07 h (-0.14 to 0.16) | 0.02 g |
| 9 | 2 (Campbell et al. 2010a) | 0.12 (-0.10 to 0.38) | 0.11 (-0.01 to 0.39) | 0.01 |
| 6 (Callary et al. 2013a) | 0.19 (0.00 to 0.51) | 0.11 (-0.01 to 0.39) | 0.01 (-0.02 to 0.06) | |
| 10 | 2 (Campbell et al. 2010b) | 0.02 h (-0.07 to 0.16) | 0.01 h (-0.09 to 0.12) | 0.02 h |
| 5 (Callary et al. 2013b) | 0.02 (-0.11 to 0.13) | 0.01 (-0.09 to 0.12) | 0.00 (-0.03 to 0.03) | |
| 11 | 2 (Kadar et al. 2011) | 0.09 (CI 0.06–0.12) | 0.06 g | 0.03 |
| 12 | 2 (Kadar et al. 2011) | 0.08 (CI 0.04–0.12) | 0.06 g | 0.02 |
| 3D | ||||
| 1 | 2 (Digas et al. 2003) | 0.18 (0.07–0.35) d | 0.15 d | 0.11 f (3–24 m) e |
| 3 (Digas et al. 2004) | 0.23 (0.04–0.41) d | 0.18 d,g | 0.09 f (3–36 m) e | |
| 5 (Digas et al. 2007) | 0.23 (0.02–0.91) d | NR | 0.04 f (3–60 m) e | |
| 10 (Johanson et al. 2012) | 0.22 d,g | 0.18 d,g | 0.01 f (SE 0.00) (2–10 yr) d | |
| 2 | 2 (Digas et al. 2004) | 0.22 (0.05–0.40) d | 0.25 (supine) | 0.19 f(3–24 m) e |
| 5 (Digas et al. 2007) | 0.20 (0.10–0.61) d | 0.24 (supine) | 0.07 f(3–60 m) e | |
| 3 | 3 (Rohrl et al. 2005) | 0.17 (CI 0.06–0.28) | NR | NR |
| 6 (Rohrl et al. 2007) | 0.23 (CI 0.10–0.35) | NR | 0.03 (2–72 m) | |
| 10 (Rohrl et al. 2012) | 0.20 (CI 0.03–0.36) | 0.19 g | 0.00 f(2–120 m) | |
| 4 | 2 (Zhou et al. 2006) | 0.19 g | 0.15 g | 0.03 f(2–24 m) |
| 5 | 3 (Bragdon et al. 2007) | NR | NR | NR |
| 6 | 3 (Bragdon et al. 2007) | NR | NR | NR |
| 7 | 2 (Glyn-Jones et al. 2008a) | 0.31 (SD 0.18) | 0.30 g | 0.06 (SD 0.06) (3–24 m) |
| 3 (Glyn-Jones et al. 2008b) | 0.35 (SD 0.14) | 0.26 (SD 0.17) | 0.03 (SD 0.06) | |
| 7 (Thomas et al. 2011) | 0.33 (CI ± 0.10) | 0.29 (95% CI ±0.07) | 0.01 (CI ± 0.02) | |
| 8 | 2 (Ayers et al. 2009) | NR | NR | NR |
| 9 | 2 (Campbell et al. 2010a) | 0.23 (0.02–0.84) | 0.23 (0.06 to 0.93) | 0.00 f |
| 6 (Callary et al. 2013a) | 0.32 (0.05–0.60) | 0.23 (0.06 to 0.93) | 0.018 (-0.11 to 0.08) | |
| 10 | 2 (Campbell et al. 2010b) | 0.16 h (0.07–0.26) | 0.16 h (0.02 to 0.32) | -0.04 h |
| 5 (Callary et al. 2013b) | 0.15 (0.04–0.32) | 0.19 (0.02 to 0.32) | -0.01 (-0.06 to 0.04) | |
| 11 | 2 (Kadar et al. 2011) | 0.19 (CI 0.15–0.23) | NR | NR |
| 12 | 2 (Kadar et al. 2011) | 0.18 (CI 0.13–0.22) | NR | NR |
CI: 95% confidence interval
NR: not reported.
Initial to final follow-up unless otherwise noted; mean (range).
Initial examination to 1-year follow-up unless otherwise noted; mean (range).
Annual rate from 1-year follow-up to final follow-up unless otherwise noted; mean (range).
Supine
Standing
Manually calculated to be rate/year from a reported value given after bedding-in.
Visualized from graph
Median
Discussion
New materials, such as XLPE components used in THA, need to be closely monitored as part of their stepwise introduction into clinical use (Malchau 1995, Malchau et al. 2011). The wear rates reported for XLPE components varied between studies. Some of this variation is likely to be due to the different measurement methods used (Kurtz et al. 2011). However, some variation could also be due to variables that include patient factors such as BMI and activity; implant factors such as femoral head material, liner thickness, and manufacturing methods for XLPE; and surgical factors such as inclination angle of the acetabular component. Thus, while the wear rate of XLPE acetabular components has been shown to be substantially less than that of conventional UHMWPE components (Kurtz et al. 2011, Kuzyk et al. 2011, Mu et al. 2009), the possible influence of the above variables remains unclear. Although a meta-analysis would be required to investigate the influence of these variables on wear rates of XLPE, the number of patients required for a meta-analysis would greatly exceed that included in the current literature, due to the low wear of XLPE and the relatively weak effect of such variables on wear. Our scoping review identified a relatively small number of studies that had measured the wear of XLPE components using the most sensitive measure available, namely RSA. Overall, the studies examined 12 cohorts involving only 260 THAs. By recommending further guidelines to standardize the reporting of RSA wear studies, we hope that this will assist retrospective analysis of the influence of these factors in the future.
Methodology of RSA studies
Cohort size
All of the cohorts had a sample size of between 10 and 32 at recruitment. As RSA has been demonstrated to have high sensitivity (Bragdon et al. 2002), statistical power can be achieved with fewer observations and therefore a small sample size is not, in itself, necessarily a methodological limitation. However, most studies had decreasing sample sizes over time. Missing or poor-quality RSA examinations further reduced the size of the originally recruited cohort, after exclusions for death and other reasons for loss to follow-up. This common problem of decreasing availability of RSA data over time must be considered when designing RSA-based clinical trials, especially if longer-term follow-up is required.
Follow-up time points
Follow-up time points within the first year varied between the RSA studies, thereby potentially influencing the amount of femoral head penetration recorded. The first reference RSA examination was usually performed within the first week postoperatively. However, some studies used 11–15 days, 2 weeks, or 6 weeks as their baseline examination. This may influence both bedding-in and femoral head penetration measurements. How bedding-in and wear varies between different types of XLPE components remains unknown. The amount of initial plastic (permanent) deformation of the polyethylene liner may differ due to design, manufacturing error, fit of the liner within the shell, elasticity of the metal shell, and surface of the inner shell. Bedding-in may also differ between cemented XLPE components and XLPE liners within uncemented metal shells.
RSA software and acetabular reference segment
The specific manner in which RSA was undertaken also varied between the studies, and may therefore also affect the outcome of meta-analyses. Early versions of the UmRSA software required the implantation of tantalum markers in the polyethylene, and subsequently measured the movement of the center of the femoral head within the rigid body defined by the markers in the polyethylene. A recent modification to the UmRSA software allows metal-backed hemispherical acetabular components to be measured using an ellipse algorithm (Borlin et al. 2006). Therefore, the movement of the femoral head can be measured within the ellipse of the metal acetabular components or by using beads in the liner, or by using a combination of both methods (beaded plus ellipse) (Borlin et al. 2006). The study that used Gill’s software (Gill et al. 1998) used the known dimensions of the prostheses and measured the femoral head penetration relative to the center of the metal acetabular component, this approach being similar to the ellipse-only method. Studies that do not use beads have the potential to save time and money, and also eliminate safety concerns relating to the implantation of beads. Furthermore, there is no exclusion of patients due to insufficiently marked components, which is a common reason for exclusion of hips in beaded analysis (Borlin et al. 2006). RSA wear measurements in the proximal direction using the ellipse algorithm alone are less precise than those using a beaded reference segment: 0.10 mm and 0.08 mm, respectively (Zhou et al. 2006). A combination of beads and the ellipse algorithm was found to have the smallest error (Borlin et al. 2006) and the least amount of variance (Bragdon et al. 2007). To date, only 1 study has presented the results of all 3 different reference segments (beaded, beaded plus ellipse, and ellipse only), and showed only slight variation in the results (Bragdon et al. 2007). However, these different representations of the acetabular reference segment may influence the measurement of early creep and bedding-in if there is early movement between the liner and the metal-backed shell.
Patient positioning
Another methodological difference between studies was the use of standing and/or supine positioning during RSA examinations (Table 2). Standing radiographs are thought to position the femoral head in the deepest part of its wear track within the polyethylene liner. However, standing radiographs may have poorer image quality due to different soft tissue exposure (stomach overhang) and different pelvic positioning. Patients have also reported that standing examinations caused discomfort at the initial postoperative examination (Digas et al. 2003). 3 RSA studies have investigated the differences in measurements made using standing and supine radiographs. Specifically, von Schewelov et al. (2006) reported that 3D wear measurements made from supine and standing (i.e. weight-bearing) examinations taken on the same day had a high correlation and there was no difference in the magnitude of penetration. Digas et al. (2004) also found no difference in the proximal head penetration recorded, while Bragdon et al. (2006b) found small differences in some wear measurements between standing and supine examinations, but the occurrence was low and did not affect the average results.
Precision reported in RSA studies
Given the typically low amounts of XLPE wear reported in RSA clinical studies, determination of the precision of the RSA method is important. Despite the RSA-reporting guidelines recommending the inclusion of precision measurements in clinical studies (Valstar et al. 2005), double examinations to determine precision were undertaken for only 6 of the 12 cohorts. While double examinations give a slight increase in radiation exposure of patients, the precision of RSA measurements cannot be determined using a phantom model (Borlin et al. 2006). Proximal wear measurements were more precise (range: 0.02–0.11 mm) than 3D wear measurements (range: 0.16–0.28 mm). The RSA method is more precise in the x- and y-axes relative to the z-axis because the latter measurements are made “out of plane” in the uniplanar setup (Karrholm et al. 1997). This will in turn affect the precision of the 3D measurement, namely the vectorial sum of all 3 axes.
Reporting of RSA wear results
To summarize the wear rate derived from studies identified in this review, the reported results were described using 3 terms: (1) “femoral head penetration” (initial examination to latest follow-up), (2) “bedding-in” (initial examination to the 1-year examination), and (3) “wear rate” (the annual wear rate between the 1-year examination and latest follow-up). RSA provides measurements in 3 axes: proximal-distal, medial-lateral, and anterior-superior. Proximal and 3D (vectorial sum) femoral head penetration and wear rates were most commonly reported, although the axis of measurement was not defined in some publications. Interpretation of the results was further complicated by the use of a number of different terms to denote the same concept. For example, proximal measurements were variously referred to as superior, longitudinal, or linear—and 3D measurements as total, linear, or maximum total point motion (MTPM). Interestingly, the 2D wear rates, which allow comparison of results to those of studies using less sophisticated techniques and plain radiographs, were only reported in 2 cohorts (Callary et al. 2013a, Callary et al. 2013b).
Mean and median values were commonly reported, but for some publications these figures had to be estimated from graphs or calculated using other data provided in the publication (Table 4). Within any one cohort, varying numbers of patients were often included at different follow-up time points, possibly affecting the reported mean wear rate, particularly if patients with wear rates at either end of the range were differentially represented over time. The mean annual proximal wear rate did not exceed 0.06 mm/year for any cohort. Only 2 publications reported mean 3D wear rates above 0.06 mm/year (Digas et al. 2003, Digas et al. 2004). However, because the wear rate in both of these cohorts was calculated between 3 months and the latest follow-up, some of the penetration attributed to wear may in fact have been due to bedding-in. This is supported by the finding that a much lower mean 3D wear rate of 0.005 mm/year was reported for the same cohort between 2 and 10 years (Rohrl et al. 2012). It is therefore important to emphasize that if wear rate is calculated using a reference time point within the bedding-in phase, the reported rate may be an overestimation of the true wear rate. Although the majority of studies used 1 year as the baseline reference for wear rate calculations, the assumed end of bedding-in/creep and the beginning of wear has varied in the literature, ranging from 2 months to 2 years (McCalden et al. 2005).
Studies of UHMWPE identified that an annual wear rate exceeding 0.1 mm/year was associated with an increased risk of developing osteolysis (Dumbleton et al. 2002), and an increased risk of revision surgery due to loosening or lysis. This suggests that the percentage of THAs with wear exceeding certain thresholds is, in fact, of more clinical importance than a mean or median wear rate. It is important to emphasize that the threshold of XLPE wear possibly associated with osteolysis is unknown. Therefore, presentation of scatter plots of individual wear rates, coupled with long-term clinical follow-up of patients, will facilitate a better understanding of the relationship between XLPE wear and subsequent development of osteolysis. Only 4 publications in the current review have reported percentages of patients exceeding specified thresholds (Digas et al. 2007, Thomas et al. 2011, Callary et al. 2013a, Callary et al. 2013b). Specifically, 3 reported no patients with a wear rate greater than 0.1 mm/year (Thomas et al. 2011, Callary et al. 2013a, Callary et al. 2013b) and 1 reported that 24 of 28 patients in cohort 1 had a wear rate of less than 0.05 mm/year and that all patients in cohort 2 had a wear rate below 0.05 mm/year (Digas et al. 2007).
Recommendations to improve reporting of RSA wear results
13 guidelines were described by Valstar et al (2005) for standardization of RSA of implants. These guidelines have recently been incorporated within the ISO for measuring migration with RSA (ISO 16087:2013 (E)). The findings of the present scoping review have led to further recommendations of important items that should be included when reporting RSA wear results (Table 5). Standardization of the manner in which RSA wear results are presented will enable a better understanding of the effects of surgical and patient factors on wear. Most importantly, such standardization is also likely to facilitate early identification of poorly performing implants.
Table 5.
Recommendations to enhance reporting of RSA wear results
| Recommendations to enhance reporting of RSA wear results |
|---|
| Methodology |
| 1 Components used (femoral head size and material; description of XLPE component) |
| 2 Patient positioning (supine or standing) |
| 3 Software and acetabular reference segment used |
| Results |
| 4 Allow one year for bedding-in and creep, and report results using the terms: – femoral head penetration (initial examination to latest follow-up) – bedding-in (initial examination to the one-year examination) – wear rate (the annual wear rate between the one-year examination and latest follow-up) |
| 5 Report axis of measurement (x, y, z, 2D or 3D) |
| 6 Use scatter plots of wear results to allow identification of outliers |
Future studies on wear using RSA
Our review has identified that the wear rates reported for XLPE components are low, which is encouraging for continued clinical use. With 1 exception, the mean proximal and 3D annual wear rates decreased when the length of follow-up increased. In the cohort in which this was not the case (Campbell et al. 2010a, Callary et al. 2013a), the liner was manufactured using an irradiation dose at the lower end of the range included as XLPE (50 kGy). Thus, new designs of XLPE components need to be monitored prospectively. Second-generation XLPEs are being introduced rapidly internationally and differ from first-generation XLPEs by being either sequentially irradiated and annealed, mechanically deformed or compressed, or diffused with vitamin E (Dumbleton et al. 2006). However, we identified only 1 cohort in which the bedding-in and wear of a second-generation XLPE liner had been investigated (Campbell et al. 2010b, Callary et al. 2013b). In this cohort, the mean proximal bedding-in was lower than that of all first-generation XLPE components (0.007 mm vs. 0.06–0.17 mm).
The low wear rates reported for XLPE have also encouraged the use of larger articulations, which have been shown to reduce the incidence of dislocation within the first year after THA (Howie et al. 2012). In Australia, head sizes of 32 mm or more have been increasingly used over the last 5 years in primary THAs with XLPE components (AOANJRR 2013). However, the effect of articulation size on XLPE wear rates is poorly understood. To date, only 1 RSA study has compared the wear rates of 28- and 36-mm articulations. Although that study reported no difference at 3 years (Bragdon et al. 2007), it is important to note that this non-randomized comparison included only 25 hips.
Identification of any potential association between patient-related factors such as age, sex, weight, or activity on the one hand and wear of XLPE on the other is desirable. However, such studies require relatively large samples, given the variability in these factors between patients. Individual RSA studies are limited in this regard due to the costly specialized equipment and analysis required, and to the need for prospective radiographs above a calibration cage. Conversely, although other measurement techniques, such as Martell’s Hip Analysis Suite (Martell and Berdia 1997) and PolyWare (Devane et al. 1995a,b), are able to measure the wear rates of larger cohorts retrospectively using plain radiographs, they are limited by their lack of sensitivity. In future, meta-analyses of data pooled from existing RSA studies may provide a means of examining not only the effect of patient-related factors on XLPE wear, but also the relationship between XLPE wear and osteolysis.
Conclusion
Despite the almost universal acceptance of the use of XLPE in acetabular components, to date the publications reporting in vivo wear of XLPE measured using RSA have been based on 12 small cohorts covering only 260 hips. The present scoping review has identified variation in both the methodology and the manner of reporting results of RSA studies. We have made a number of recommendations to enhance the reporting of RSA-based wear results. Longer-term studies are required to determine whether the low wear of XLPE identified in the short term does indeed translate to a low incidence of osteolysis in the medium to long term and, importantly, to a reduction in the need for revision surgery.
Acknowledgments
SC: performed scoping review, collected data from publications included, and wrote the manuscript. LS: assisted with collection of data and proofread the manuscript. OH: analyzed the review data and contributed to the manuscript. DC: planned the study and proofread the manuscript. ZM: planned systematic search criteria and assisted with the scoping review. DH: planned the study and contributed to the manuscript.
No funding was received.
No competing interests declared.
References
- AOANJRR. Annual Report. Adelaide: AOA; 2013. Australian Orthopaedic Association National Joint Replacement Registry. [Google Scholar]
- Ayers DC, Hays PL, Drew JM, Eskander MS, Osuch D, Bragdon CR. Two-year radiostereometric analysis evaluation of femoral head penetration in a challenging population of young total hip arthroplasty patients . J Arthroplasty. 2009;24(6 Suppl):9–14. doi: 10.1016/j.arth.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Baldursson H, Egund N, Hansson LI, Selvik G. Instability and wear of total hip prostheses determined with roentgen stereophotogrammetry . Arch Orthop Trauma Surg. 1979;95(4):257–63. doi: 10.1007/BF00389695. [DOI] [PubMed] [Google Scholar]
- Bitsch RG, Loidolt T, Heisel C, Ball S, Schmalzried TP. Reduction of osteolysis with use of Marathon cross-linked polyethylene. A concise follow-up, at a minimum of five years, of a previous report . J Bone Joint Surg Am. 2008;90(7):1487–91. doi: 10.2106/JBJS.F.00991. [DOI] [PubMed] [Google Scholar]
- Borlin N, Rohrl SM, Bragdon CR. RSA wear measurements with or without markers in total hip arthroplasty . J Biomech. 2006;39(9):1641–50. doi: 10.1016/j.jbiomech.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Bragdon CR, Malchau H, Yuan X, Perinchief R, Karrholm J, Borlin N, et al. Experimental assessment of precision and accuracy of radiostereometric analysis for the determination of polyethylene wear in a total hip replacement model . J Orthop Res. 2002;20(4):688–95. doi: 10.1016/S0736-0266(01)00171-1. [DOI] [PubMed] [Google Scholar]
- Bragdon CR, Martell JM, Greene ME, Estok DM, 2nd, Thanner J, Karrholm J, et al. Comparison of femoral head penetration using RSA and the Martell method . Clin Orthop Relat Res. 2006a;448:52–7. doi: 10.1097/01.blo.0000224018.88410.83. [DOI] [PubMed] [Google Scholar]
- Bragdon CR, Thanner J, Greene ME, Malchau H, Digas G, Harris WH, et al. Standing versus Supine Radiographs in RSA Evaluation of Femoral Head Penetration . Clin Orthop Relat Res. 2006b;448:46–51. doi: 10.1097/01.blo.0000224012.50292.67. [DOI] [PubMed] [Google Scholar]
- Bragdon CR, Greene ME, Freiberg AA, Harris WH, Malchau H. Radiostereometric analysis comparison of wear of highly cross-linked polyethylene against 36- vs 28-mm femoral heads . J Arthroplasty. 2007;22(6 Suppl 2):125–9. doi: 10.1016/j.arth.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Callary SA, Campbell DG, Mercer G, Nilsson KG, Field JR. Wear of a 5 megarad cross-linked polyethylene liner: a 6-year RSA study . Clin Orthop Relat Res. 2013a;471:2238–44. doi: 10.1007/s11999-013-2789-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callary SA, Field JR, Campbell DG. Low wear of a second-generation highly crosslinked polyethylene liner: a 5-year radiostereometric analysis study . Clin Orthop Relat Res. 2013b;471:3596–600. doi: 10.1007/s11999-013-3188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D, Mercer G, Nilsson K, Wells V, Field JR, Callary SA. Wear of a highly cross-linked polyethylene liner: a preliminary RSA study. Eur J Orthop Surg Traumatol. 2010a;20(1):23–7. [Google Scholar]
- Campbell DG, Field JR, Callary SA. Second-generation highly cross-linked X3 polyethylene wear: a preliminary radiostereometric analysis study . Clin Orthop Relat Res. 2010b;468:2704–9. doi: 10.1007/s11999-010-1259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane PA, Bourne RB, Rorabeck CH, Hardie RM, Horne JG. Measurement of polyethylene wear in metal-backed acetabular cups. I. Three-dimensional technique . Clin Orthop Relat Res. 1995a;319:303–16. [PubMed] [Google Scholar]
- Devane PA, Bourne RB, Rorabeck CH, MacDonald S, Robinson EJ. Measurement of polyethylene wear in metal-backed acetabular cups. II. Clinical application . Clin Orthop Relat Res. 1995b;319:317–26. [PubMed] [Google Scholar]
- Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. Highly cross-linked polyethylene in cemented THA: randomized study of 61 hips . Clin Orthop Relat Res. 2003;417:126–38. doi: 10.1097/01.blo.0000096802.78689.45. [DOI] [PubMed] [Google Scholar]
- Digas G, Karrholm J, Thanner J, Malchau H, Herberts P. Highly cross-linked polyethylene in total hip arthroplasty: randomized evaluation of penetration rate in cemented and uncemented sockets using radiostereometric analysis . Clin Orthop Relat Res. 2004;429:6–16. [PubMed] [Google Scholar]
- Digas G, Karrholm J, Thanner J, Herberts P. 5-year experience of highly cross-linked polyethylene in cemented and uncemented sockets: two randomized studies using radiostereometric analysis . Acta Orthop. 2007;78(6):746–54. doi: 10.1080/17453670710014518. [DOI] [PubMed] [Google Scholar]
- Dorr LD, Wan Z. Ten years of experience with porous acetabular components for revision surgery . Clin Orthop Relat Res. 1995;319:191–200. [PubMed] [Google Scholar]
- Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty . J Arthroplasty. 2002;17(5):649–61. doi: 10.1054/arth.2002.33664. [DOI] [PubMed] [Google Scholar]
- Dumbleton JH, D’Antonio JA, Manley MT, Capello WN, Wang A. The basis for a second-generation highly cross-linked UHMWPE . Clin Orthop Relat Res. 2006;453:265–71. doi: 10.1097/01.blo.0000238856.61862.7d. [DOI] [PubMed] [Google Scholar]
- Engh CA, Jr., Stepniewski AS, Ginn SD, Beykirch SE, Sychterz-Terefenko CJ, Hopper RH, Jr., et al. A randomized prospective evaluation of outcomes after total hip arthroplasty using cross-linked marathon and non-cross-linked Enduron polyethylene liners . J Arthroplasty. 2006;21(6 Suppl 2):17–25. doi: 10.1016/j.arth.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Engh CA, Jr., Hopper RH, Jr., Huynh C, Ho H, Sritulanondha S, Engh CA., Sr A prospective, randomized study of cross-linked and non-cross-linked polyethylene for total hip arthroplasty at 10-year follow-up . J Arthroplasty. 2012;27(8 Suppl):2–7. doi: 10.1016/j.arth.2012.03.048. e1. [DOI] [PubMed] [Google Scholar]
- Gill HS, Alfaro J, Murray DW, Marks BE. A new hybrid video/digitiser based roentgen stereometric measurement system. J Biomech. 1998;31:88. [Google Scholar]
- Glyn-Jones S, Isaac S, Hauptfleisch J, McLardy-Smith P, Murray DW, Gill HS. Does highly cross-linked polyethylene wear less than conventional polyethylene in total hip arthroplasty? A double-blind, randomized, and controlled trial using roentgen stereophotogrammetric analysis . J Arthroplasty. 2008a;23(3):337–43. doi: 10.1016/j.arth.2006.12.117. [DOI] [PubMed] [Google Scholar]
- Glyn-Jones S, McLardy-Smith P, Gill HS, Murray DW. The creep and wear of highly cross-linked polyethylene: A three-year randomised, controlled trial using radiostereometric analysis . J Bone Joint Surg Br. 2008b;90(5):556–61. doi: 10.1302/0301-620X.90B5.20545. [DOI] [PubMed] [Google Scholar]
- Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies . Health Info Libr J. 2009;26(2):91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- Howie DW, Holubowycz OT, Middleton R. Large Articulation Study Group. Large femoral heads decrease the incidence of dislocation after total hip arthroplasty: a randomized controlled trial . J Bone Joint Surg Am. 2012;94(12):1095–102. doi: 10.2106/JBJS.K.00570. [DOI] [PubMed] [Google Scholar]
- Johanson PE, Digas G, Herberts P, Thanner J, Karrholm J. Highly crosslinked polyethylene does not reduce aseptic loosening in cemented THA 10-year findings of a randomized study . Clin Orthop Relat Res. 2012;470:3083–93. doi: 10.1007/s11999-012-2400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadar T, Hallan G, Aamodt A, Indrekvam K, Badawy M, Skredderstuen A, et al. Wear and migration of highly cross-linked and conventional cemented polyethylene cups with cobalt chrome or Oxinium femoral heads: a randomized radiostereometric study of 150 patients . J Orthop Res. 2011;29(8):1222–9. doi: 10.1002/jor.21389. [DOI] [PubMed] [Google Scholar]
- Karrholm J, Herberts P, Hultmark P, Malchau H, Nivbrant B, Thanner J. Radiostereometry of hip prostheses. Review of methodology and clinical results . Clin Orthop Relat Res. 1997;344:94–110. [PubMed] [Google Scholar]
- Karrholm J, Gill RH, Valstar ER. The history and future of radiostereometric analysis . Clin Orthop Relat Res. 2006;448:10–21. doi: 10.1097/01.blo.0000224001.95141.fe. [DOI] [PubMed] [Google Scholar]
- Kurtz SM. Elevier Academic Press; 2004. The UHMWPE handbook. [Google Scholar]
- Kurtz SM, Muratoglu OK, Evans M, Edidin AA. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty . Biomaterials. 1999;20(18):1659–88. doi: 10.1016/s0142-9612(99)00053-8. [DOI] [PubMed] [Google Scholar]
- Kurtz SM, Manley M, Wang A, Taylor S, Dumbleton J. Comparison of the properties of annealed crosslinked (Crossfire) and conventional polyethylene as hip bearing materials . Bull Hosp Jt Dis. 2002;61(1)(2):17–26. [PubMed] [Google Scholar]
- Kurtz SM, Gawel HA, Patel JD. History and systematic review of wear and osteolysis outcomes for first-generation highly crosslinked polyethylene . Clin Orthop Relat Res. 2011;469:2262–77. doi: 10.1007/s11999-011-1872-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzyk PR, Saccone M, Sprague S, Simunovic N, Bhandari M, Schemitsch EH. Cross-linked versus conventional polyethylene for total hip replacement: a meta-analysis of randomised controlled trials . J Bone Joint Surg Br. 2011;93(5):593–600. doi: 10.1302/0301-620X.93B5.25908. [DOI] [PubMed] [Google Scholar]
- Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component . J Bone Joint Surg Am. 1990;72(4):518–28. [PubMed] [Google Scholar]
- Malchau H. Goteborg: Goteborg University; 1995. On the importance of stepwise introduction of new hip implant technology. In: Assessment of total hip replacement using clinical evaluation, radiostereometry, digitised radiography and a national hip registry. [Google Scholar]
- Malchau H, Bragdon CR, Muratoglu OK. The stepwise introduction of innovation into orthopedic surgery: the next level of dilemmas . J Arthroplasty. 2011;26(6):825–31. doi: 10.1016/j.arth.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Martell JM, Berdia S. Determination of polyethylene wear in total hip replacements with use of digital radiographs . J Bone Joint Surg Am. 1997;79(11):1635–41. doi: 10.2106/00004623-199711000-00004. [DOI] [PubMed] [Google Scholar]
- McCalden RW, Naudie DD, Yuan X, Bourne RB. Radiographic methods for the assessment of polyethylene wear after total hip arthroplasty . J Bone Joint Surg Am. 2005;87(10):2323–34. doi: 10.2106/JBJS.E.00223. [DOI] [PubMed] [Google Scholar]
- Mu Z, Tian J, Wu T, Yang J, Pei F. A systematic review of radiological outcomes of highly cross-linked polyethylene versus conventional polyethylene in total hip arthroplasty . Int Orthop. 2009;33(3):599–604. doi: 10.1007/s00264-008-0716-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratoglu OK, Bragdon CR, O’Connor DO, Jasty M, Harris WH. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties . J Arthroplasty. 2001;16(2):149–60. doi: 10.1054/arth.2001.20540. [DOI] [PubMed] [Google Scholar]
- Mutimer J, Devane PA, Adams K, Horne JG. Highly crosslinked polyethylene reduces wear in total hip arthroplasty at 5 years . Clin Orthop Relat Res. 2010;468:3228–33. doi: 10.1007/s11999-010-1379-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oonishi H, Kuno M, Tsuji E, Fujsiawa A. The optimum dose of gamma radiation-heavy doses to low wear polyethylene in total hip prostheses. Materials Science: Materials in Medicine. 1997;8:11–8. doi: 10.1023/a:1018582027349. [DOI] [PubMed] [Google Scholar]
- Oonishi H, Clarke IC, Masuda S, Amino H. Study of retrieved acetabular sockets made from high-dose, cross-linked polyethylene . J Arthroplasty. 2001;16(8 Suppl 1):129–33. doi: 10.1054/arth.2001.28371. [DOI] [PubMed] [Google Scholar]
- Oparaugo PC, Clarke IC, Malchau H, Herberts P. Correlation of wear debris-induced osteolysis and revision with volumetric wear-rates of polyethylene: a survey of 8 reports in the literature . Acta Orthop Scand. 2001;72(1):22–8. doi: 10.1080/000164701753606644. [DOI] [PubMed] [Google Scholar]
- Pruitt LA, Ansari F, Kury M, Mehdizah A, Patten EW, Huddlestein J, et al. Clinical trade-offs in cross-linked ultrahigh-molecular-weight polyethylene used in total joint arthroplasty . J Biomed Mater Res B Appl Biomater. 2013;101(3):476–84. doi: 10.1002/jbm.b.32887. [DOI] [PubMed] [Google Scholar]
- Rohrl S, Nivbrant B, Mingguo L, Hewitt B. In vivo wear and migration of highly cross-linked polyethylene cups a radiostereometry analysis study . J Arthroplasty. 2005;20(4):409–13. doi: 10.1016/j.arth.2004.09.040. [DOI] [PubMed] [Google Scholar]
- Rohrl SM, Li MG, Nilsson KG, Nivbrant B. Very low wear of non-remelted highly cross-linked polyethylene cups: an RSA study lasting up to 6 years . Acta Orthop. 2007;78(6):739–45. doi: 10.1080/17453670710014509. [DOI] [PubMed] [Google Scholar]
- Rohrl SM, Nivbrant B, Nilsson KG. No adverse effects of submelt-annealed highly crosslinked polyethylene in cemented cups: an RSA study of 8 patients 10 years after surgery . Acta Orthop. 2012;83(2):148–52. doi: 10.3109/17453674.2011.652889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilling M, Kold S, de Raedt S, Andersen NT, Rahbek O, Soballe K. Superior accuracy of model-based radiostereometric analysis for measurement of polyethylene wear: A phantom study . Bone Joint Res. 2012;1(8):180–91. doi: 10.1302/2046-3758.18.2000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun DC, Schmidig G, Yau SS, Jeanty M, Wang A, Stark C, et al. Correlation between oxidation, cross linking, and wear performance of UHMWPE. 43rd Annual Meeting, Orthopaedic Research Society; San Francisco, CA: 1997. p. 783. [Google Scholar]
- The Joanna Briggs Institute Joanna Briggs Institute Reviewers’ Manual: 2011 edition
- Thomas GE, Simpson DJ, Mehmood S, Taylor A, McLardy-Smith P, Singh Gill H, et al. The seven-year wear of highly cross-linked polyethylene in total hip arthroplasty: a double-blind, randomized controlled trial using radiostereometric analysis . J Bone Joint Surg Am. 2011;93(8):716–22. doi: 10.2106/JBJS.J.00287. [DOI] [PubMed] [Google Scholar]
- Valstar ER, Gill R, Ryd L, Flivik G, Borlin N, Karrholm J. Guidelines for standardization of radiostereometry (RSA) of implants . Acta Orthop. 2005;76(4):563–72. doi: 10.1080/17453670510041574. [DOI] [PubMed] [Google Scholar]
- von Schewelov T, Onsten I, Markusson P, Carlsson A. Weight bearing radiographs are not necessary for measurement of polyethylene penetration in total hip prostheses: a radiostereometric study of 111 patients examined in weight-bearing and supine position . Acta Orthop. 2006;77(1):104–8. doi: 10.1080/17453670610045768. [DOI] [PubMed] [Google Scholar]
- Zhou ZK, Li MG, Borlin N, Wood DJ, Nivbrant B. No increased migration in cups with ceramic-on-ceramic bearing: an RSA study . Clin Orthop Relat Res. 2006;448:39–45. doi: 10.1097/01.blo.0000223999.10389.c9. [DOI] [PubMed] [Google Scholar]