Abstract
Background and purpose
Background and purpose — Perioperative hyperglycemia has been associated with adverse outcomes in several fields of surgery. In this observational study, we identified factors associated with an increased risk of hyperglycemia following hip and knee replacement.
Patients and methods
Patients and methods — We prospectively monitored changes in glucose following primary hip and knee replacements in 191 patients with osteoarthritis. Possible associations of patient characteristics and operation-related factors with hyperglycemia (defined as glucose > 7.8 mmol/L in 2 consecutive measurements) and severe hyperglycemia (glucose > 10 mmol/L) were analyzed using binary logistic regression with adjustment for age, sex, operated joint, and anesthesiological risk score.
Results
Results — 76 patients (40%) developed hyperglycemia, and 48 of them (25% of the whole cohort) had severe hyperglycemia. Glycemic responses were similar following hip replacement and knee replacement. Previously diagnosed diabetes was associated with an increased risk of hyperglycemia and severe hyperglycemia, compared to patients with normal glucose metabolism, whereas newly diagnosed diabetes and milder glucose metabolism disorders had no effect. In patients without previously diagnosed diabetes, increased values of preoperative glycosylated hemoglobin (HbA1c) and fasting glucose on the day of operation were associated with hyperglycemia. Higher anesthesiological risk score—but none of the operation-related factors analyzed—was associated with an increased risk of hyperglycemia.
Interpretation
Interpretation — Perioperative hyperglycemia is common in primary hip and knee replacements. Previously diagnosed diabetes is the strongest risk factor for hyperglycemia. In patients with no history of diabetes, preoperative HbA1c and fasting glucose on the day of operation can be used to stratify the risk of hyperglycemia.
Stress-induced insulin resistance and consequent perioperative hyperglycemia have been recognized as important risk factors for adverse outcomes following major surgical interventions (Ljungqvist et al. 2007, Akhtar et al. 2010, Rizvi et al. 2010). In joint replacements, perioperative hyperglycemia is a relatively common phenomenon (Pili-Floury et al. 2009) and it has been reported be associated with an increased risk of prosthetic joint infection (PJI) (Mraovic et al. 2011) and venous thromboembolism (Cohn et al. 2012).
Although there is debate about how intensively perioperative hyperglycemia should be managed (Griesdale et al. 2009), it is reasonable to expect that controlling severe hyperglycemia would be beneficial (Ljungqvist et al. 2007, Jämsen et al. 2010). This is important because, unlike most risk factors for PJI (Jämsen et al. 2010, Bozic et al. 2012, Chen et al. 2013), hyperglycemia can be modified. Unfortunately, there is little information about factors that are predictive of hyperglycemia in orthopedic patients.
Diabetes is a well-established risk factor for PJI (Dowsey and Choong 2009, Malinzak et al. 2009, Bozic et al. 2012, Jämsen et al. 2012, Chen et al. 2013) and perioperative hyperglycemia (Akhtar et al. 2010, Masla et al. 2011, Jämsen et al. unpublished data), although it is important to note that perioperative hyperglycemia may also develop in the absence of an established glucose metabolism disorder (Ljungqvist et al. 2007, Donatelli et al. 2008, Gustafsson et al. 2009, Pili-Floury et al. 2009). Moreover, a considerable proportion of patients with type 2 diabetes are undiagnosed (Meding et al. 2007, Saaristo et al. 2008). Other studies have suggested that existing insulin resistance (Donatelli et al. 2007), metabolic syndrome (Donatelli et al. 2008), general anesthesia rather than epidural (Donatelli et al. 2007), and the severity of tissue trauma (Thorell et al. 1999) are associated with perioperative hyperglycemia. However, only 1 of these studies concerned joint replacements (Donatelli et al. 2007).
We analyzed the course of perioperative hyperglycemia in a series of 191 primary hip and knee replacements performed for osteoarthritis, and tried to identify patient characteristics and operation-related factors associated with perioperative hyperglycemia. We hypothesized that preoperative hyperglycemia and greater surgical stress (e.g. duration of surgery and amount of tissue trauma) would increase the risk of perioperative hyperglycemia.
Patients and methods
The patients for this prospective observational study were collected at a single publicly funded orthopedic hospital between December 2009 and May 2011. Patients of all ages undergoing primary hip or knee replacement for osteoarthritis were eligible for inclusion. Patients with and without diabetes were included. Exclusion criteria were regular corticosteroid treatment. Based on a previous study (Pili-Floury et al. 2009), it was estimated that a convenient sample of 200 operations would be sufficient to detect a 1.5-fold difference in the risk of hyperglycemia between different patient subgroups (each representing ≥ 20% of the material) at the 5% significance level with ≥ 80% power. A post-hoc analysis indicated that the power calculations allowed 5% loss of study patients.
Study protocol
For the detailed study protocol see Supplementary data. Briefly, the patients were recruited after they were scheduled for surgery. A research nurse registered their medical history and made anthropometric measurements. In addition to routine preoperative laboratory tests, the patients had their lipid values, fasting plasma glucose, and glycosylated hemoglobin (HbA1c) measured. Patients with no history of diabetes also underwent a 2-h 75-g oral glucose tolerance test, which is a commonly used and sensitive means of diagnosing type 2 diabetes (American Diabetes Association 2013) and other disorders of glucose metabolism (i.e. increased fasting glucose (IFG) and impaired glucose tolerance (IGT)). During the hospital stay, glucose was measured 4–6 times a day from capillary blood samples using a bedside glucose monitor (Precision Xceed; Abbott Laboratories, Abbott Park, IL), according to a predefined scheme. Short-acting insulin (Actrapid; Novo Nordisk A/S, Bagsvaerd, Denmark) was administered according to the treating anesthesiologist’s instructions to keep glucose below 10 mmol/L.
Characteristics of the study population
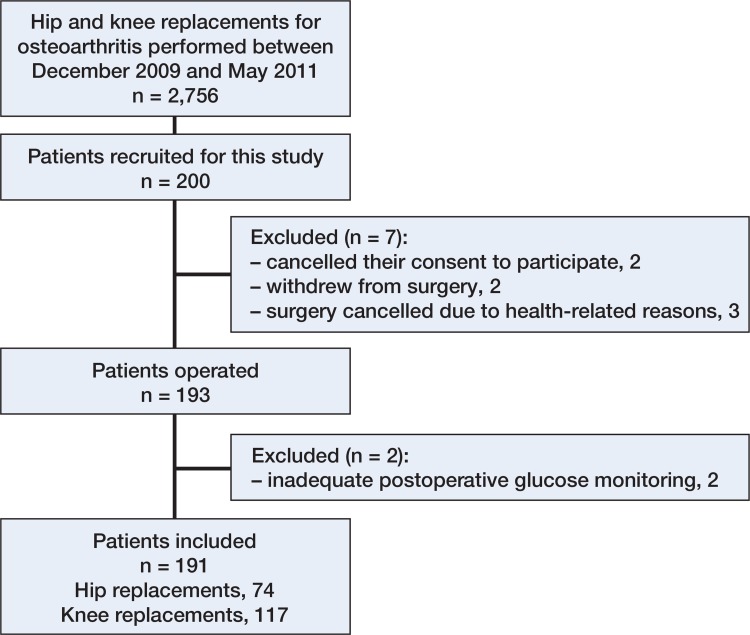
Of the 200 patients recruited initially, 191 completed the study protocol (Figure 1). The patients included were comparable to the whole population of osteoarthritis patients treated at our hospital during the same period, with respect to age (mean 66 (43–89) years in the study sample and 69 (18–93) years in all other patients; p = 0.002), sex (65% females vs. 63% females, respectively; p = 0.6), and operated joint (proportion of knee replacements: 61% vs. 57%; p = 0.3).
Figure 1.
Collection of patients.
74 study patients (39%) underwent hip replacement and 117 study patients (61%) underwent knee replacement. Patient demographics, comorbidities, and operative data are presented in Table 1. The majority of operations (89%) were unilateral, and except for 9 unicompartmental knees, a total joint replacement was implanted. A greater proportion of knee replacement recipients than hip replacement recipients were obese (BMI ≥ 30) (56% vs. 38%; p = 0.02), but the prevalences of disorders of glucose metabolism, metabolic syndrome, and self-reported comorbidities were similar (data not shown). Cemented fixation was used in the majority of knee replacements (87 of 117, 74%) whereas 55 of the 74 hip replacements (74%) were cementless. Blood loss was greater in hip replacements than in knee replacements (mean 415 (200–3100) mL vs. 50 (5–800) mL; p < 0.001), and blood transfusions were more common after hip replacement than after knee replacement (22% vs. 6%; p = 0.001).
Table 1.
Patient demographics, medical status, and operative data
| Patient demographics and comorbidity | |
| Median age at surgery (range), years | 66 (43–89) |
| Sex, no. | |
| Female | 124 (65%) |
| Male | 67 (35%) |
| Median body mass index (range), kg/m2 | 30 (21–50) |
| ASA risk score, no. | |
| I | 16 (8%) |
| II | 92 (48%) |
| III | 82 (43%) |
| IV | 1 (1%) |
| Self-reported comorbidities, n | |
| Hypertension | 106 (55%) |
| Cardiovascular disease | 42 (22%) |
| Pulmonary disease | 31 (16%) |
| Dermatological disease | 28 (15%) |
| Gastrointestinal disease | 22 (12%) |
| Genitourinary disease | 17 (9%) |
| Cancer or history of cancer | 14 (7%) |
| Neurological disease | 6 (3%) |
| Smoking, n | 22 (12%) |
| Earlier joint replacements, n | |
| None | 150 (79%) |
| Any | 41 (21%) |
| Clinical evaluation in this study | |
| State of glucose metabolism, n | |
| Normal | 91 (48%) |
| IFG and/or IGT (“pre-diabetes”) | 47 (24%) |
| Newly diagnosed diabetes | 17 (9%) |
| Previously diagnosed diabetes | 36 (19%) |
| Metabolic syndrome, n | 85 (45%) |
| Preoperative anemia, n | 10 (5%) |
| Renal function, na | |
| Normal | 100 (52%) |
| Mild insufficiency | 74 (39%) |
| Moderate insufficiency | 17 (9%) |
| Severe insufficiency | 0 |
| Operative data | |
| Simultaneous bilateral surgery, no. | 21 (11%) |
| Total joint replacement, no. | 182 (95%) |
| Prosthesis fixation, no. | |
| Cemented | 101 (53%) |
| Hybrid | 7 (4%) |
| Cementless | 83 (43%) |
| Median duration of surgery (range), min | 85 (44–225) |
| Median tourniquet time (range), min | 50 (0–129) |
| Median blood loss (range), mL | 150 (5–3100) |
| Blood transfusions, n | 24 (13%) |
| Closed suction drains, n | 114 (60%) |
Estimated, based on preoperative creatinine according to the Cockcroft-Gault formula.
SD: standard deviation; ASA: American Society of Anesthesiologists; IFG: increased fasting glucose; IGT: impaired glucose tolerance.
Operative details
Almost all the patients arrived at the hospital on the day of operation. Spinal anesthesia was used in all operations. Intraoperative fluid replacements were performed using acetated Ringer’s solution, which was changed to 5% glucose solution (to avoid hypoglycemia and catabolic metabolism) after surgery and continued until the patient resumed normal food intake. A single 3.0-g bolus of cefuroxime was used as antibiotic prophylaxis (but when contraindicated, clindamycin was used instead). Antibiotic-impregnated cement was used in all cemented joint replacements. A pneumatic tourniquet was used in all knee replacements. Closed suction drains (if used) were removed on the first postoperative day. Subcutaneous enoxaparin or oral rivaroxaban was used as thromboprophylaxis. The median length of hospital stay was 4 (1–6) days, and approximately two-thirds of the patients were discharged to home directly.
Statistics
The primary outcome was occurrence of hyperglycemia during the hospitalization. Hyperglycemia was defined as glucose > 7.8 mmol/L in 2 consecutive measurements or glucose > 10 mmol/L at any time point. The 7.8 mmol/L cutoff point was based on the definition of hyperglycemia by the American Diabetes Association (2013). Glucose > 10 mmol/L (later referred to as “severe hyperglycemia”) is considered to be the threshold for treating hyperglycemia (American Diabetes Association 2013), and it has been associated with postoperative complications in several studies (Akhtar et al. 2010). Thus, even a single occurrence of glucose exceeding 10 mmol/L was considered to be clinically significant. Occurrence of severe hyperglycemia was analyzed as a secondary outcome.
We analyzed possible associations of patient demographics, medical history, results of preoperative laboratory tests and operation-related data with hyperglycemia. Data related to medical history were based on patient reporting. Diagnoses of hypertension and (previously diagnosed) diabetes were confirmed from patient records and medication data. The American Society of Anesthesiologists (ASA) risk score (Owens et al. 1978) was used to assess comorbidity and was analyzed as a categorized variable. BMI was analyzed both as a continuous variable and a categorized variable as follows: normal (< 25.0), overweight (25–29.9), obese (30.0–34.9), severely obese (35.0–39.9), and morbidly obese (≥ 40.0).
Diabetic status was categorized as follows: normal glucose metabolism, IFG and/or IGT, and diabetes (diagnosed either previously or using OGTT in this study). HbA1c was categorized in 3 groups using 6.0% (median value for the whole series) and 6.5% (which has been accepted as a means of diagnosing diabetes by the American Diabetes Association (2013)) as cutoff points. HbA1c was analyzed also as a continuous variable. Analyses concerning HbA1c, preoperative fasting glucose, and baseline glucose (a fasting sample taken at the induction of anesthesia) were performed separately for patients with and without a previous diagnosis of diabetes. Metabolic syndrome was diagnosed according to the consensus criteria (Alberti et al. 2009). Patients whose preoperative hemoglobin was below the local laboratory reference values (i.e. < 117 g/L in women and < 136 g/L in men) were considered to have anemia. Renal function was estimated using the Cockcroft-Gault formula (Cockcroft and Gault 1976), based on preoperative creatitine, and was categorized as normal (estimated glomerular filtration rate ≥ 90 mL/min) or as mild (60–89 mL/min), moderate (30–59 mL/min), or severe (< 30 mL/min) renal insufficiency.
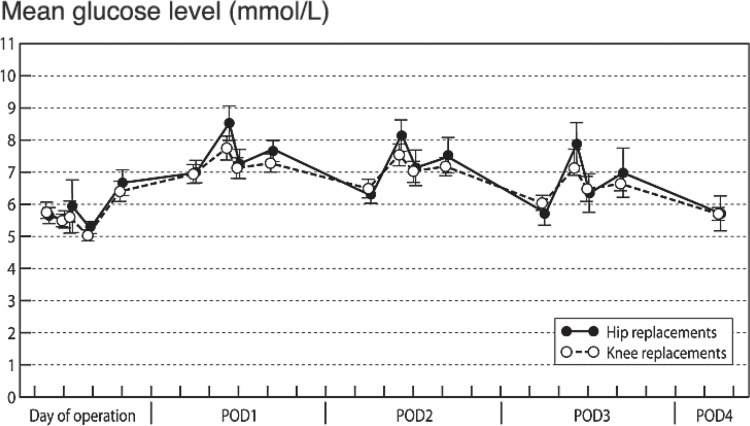
Changes in glucose after surgery were similar following hip replacement and knee replacement (Figure 2), so the materials were analyzed as a whole to maximize statistical power. In descriptive analyses, cross-tabulation and the chi-square test were used for comparison of categorical variables and independent-samples t-test or analysis of variance was used for comparison of continuous variables.
Figure 2.
Mean glucose (with 95% CIs) following primary hip and knee replacement in patients with osteoarthritis.
The rates of hyperglycemia and severe hyperglycemia are reported as patient numbers and percentages. The associations between potential risk factors for hyperglycemia were analyzed using binary logistic regression with adjustment for age, sex, operated joint (hip, knee), and ASA risk score. In the adjusted analyses, all continuous variables were treated as such, i.e. not as categorized. The variables for adjustment were selected based on clinical judgment, because they were thought to be potentially associated with both intervention and development of hyperglycemia. Furthermore, all variables were associated with the prevalence of glucose metabolism disorders, i.e. the baseline risk of hyperglycemia (data not shown). The respective results are presented as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). Because odds ratios exaggerate the increase in the risk when the initial risk is high (over 20–30%) (Davies et al. 1998), we advise the reader to look at the actual differences in the occurrence of hyperglycemia (i.e. percentages) and to consider the ORs and their CIs only as indicators of statistical significance, not as indicators of how much the risk is increased.
Ethics
This study was performed in accordance with the World Medical Association Declaration of Helsinki. The research plan was approved by the Ethics Board of Pirkanmaa Hospital District, Tampere, Finland (R09207; November 24, 2009). All patients gave informed consent to participate. The study has been registered at clinicaltrials.gov (NCT01021826).
Results
Altogether, 76 patients (40%) developed hyperglycemia and 48 of them (25%) had severe hyperglycemia. These proportions were similar in hip and knee replacements (37% vs. 45% (p = 0.3) for hyperglycemia and 30% vs. 22% (p = 0.2) for severe hyperglycemia, respectively), as was the overall glycemic response to surgery (Figure 2).
Previously diagnosed diabetes, and increased ASA score, preoperative HbA1c, and baseline glucose—but none of the operation-related factors analyzed—were associated with hyperglycemia and severe hyperglycemia in the adjusted analyses (Table 2). The incidence of both hyperglycemia and severe hyperglycemia increased with age, but after adjustments there were no statistically significant differences between the age groups (Table 2). The effect of ASA score (comparing ASA scores III–IV with I–II) was pronounced in patients with diabetes (32/38 vs. 7/15; adjusted OR = 6 (1.3–31)) and in patients with HbA1c ≥ 6.5% (24/25 vs. 3/7; adjusted OR = 10 (1.3–82)). ASA score was not associated with hyperglycemia in patients without diabetes (adjusted OR = 1.4 (0.66–3.4)) and in those with HbA1c < 6.5% (adjusted OR = 1.7 (0.66–4.5)).
Table 2.
The effects of different patient- and operation-related factors on the incidence of hyperglycemia and severe hyperglycemia. The odds ratios (ORs) were adjusted for age, sex, operated joint (hip or knee), and the American Society of Anesthesiologists risk score
| Hyperglycemia Adjusted |
Severe hyperglycemia Adjusted |
||||
|---|---|---|---|---|---|
| n | n (%) | OR (95% CI) | n (%) | OR (95% CI) | |
| Gender | |||||
| Male | 67 | 26 (39) | 1 | 17 (25) | 1 |
| Female | 124 | 50 (40) | 1.4 (0.70–2.7) | 31 (25) | 1.3 (0.60–2.6) |
| Age a | 1.0 (0.99–1.1) | 1.0 (0.97–1.1) | |||
| < 55 years | 20 | 5 (25) | 1 | 4 (20) | 1 |
| 55–64 years | 68 | 20 (29) | 0.7 (0.22–2.5) | 10 (15) | 0.4 (0.11–1.6) |
| 65–74 years | 72 | 32 (44) | 1.0 (0.79–7.3) | 20 (28) | 0.7 (0.18–2.7) |
| ≥ 75 years | 31 | 19 (61) | 1.7 (0.42–6.7) | 14 (45) | 1.2 (0.28–5.5) |
| ASA risk score | |||||
| I | 16 | 1 (6) | 1 | 1 (6) | 1 |
| II | 92 | 27 (29) | 5.0 (0.61–41) | 15 (16) | 2.6 (0.31–22) |
| III | 82 | 48 (59) | 15 (1.8–125) | 32 (39) | 8.0 (0.93–69) |
| IV | 1 | 0 (0) | - | 0 (0) | - |
| Body mass index, kg/m2 a | 1.0 (0.94–1.1) | 1.0 (0.95–1.1) | |||
| < 25.0 | 28 | 12 (43) | 1 | 9 (32) | 1 |
| 25.0–29.9 | 71 | 27 (38) | 1.0 (0.37–2.5) | 11 (16) | 0.4 (0.14–1.2) |
| 30.0–34.9 | 60 | 24 (40) | 1.0 (0.36–2.6) | 19 (32) | 1.1 (0.39–3.0) |
| 35.0–39.9 | 20 | 8 (40) | 1.0 (0.28–3.7) | 6 (30) | 1.0 (0.26–3.9) |
| ≥ 40.0 | 12 | 5 (42) | 0.8 (0.19–3.8) | 3 (25) | 0.6 (0.12–3.3) |
| State of glucose metabolism | |||||
| Normal | 91 | 20 (22) | 1 | 10 (11) | 1 |
| IFG and/or IGT | 47 | 17 (36) | 1.8 (0.79–4.1) | 7 (15) | 1.4 (0.48–4.2) |
| Newly diagnosed diabetes | 17 | 8 (47) | 2.4 (0.79–7.5) | 3 (18) | 1.5 (0.34–6.1) |
| Previously diagnosed diabetes | 36 | 31 (86) | 18 (5.7–59) | 28 (78) | 29 (9.1–97) |
| Metabolic syndrome | |||||
| Without | 106 | 31 (29) | 1 | 18 (17) | 1 |
| With | 85 | 45 (53) | 2.2 (1.1–4.2) | 30 (35) | 2.1 (1.03–4.4) |
| Smoking | |||||
| No | 169 | 67 (40) | 1 | 42 (25) | 1 |
| Yes | 22 | 9 (41) | 1.59 (0.52–4.83) | 6 (27) | 1.6 (0.52–4.8) |
| Preoperative anemia | |||||
| Without | 181 | 69 (38) | 1 | 44 (24) | 1 |
| With | 10 | 7 (70) | 3.9 (0.91–17) | 4 (40) | 2.0 (0.50–8.1) |
| Renal function | |||||
| Normal | 100 | 39 (39) | 1 | 26 (26) | 1 |
| Mild insufficiency | 74 | 29 (39) | 0.6 (0.28–1.2) | 15 (20) | 0.5 (0.22–1.2) |
| Moderate insufficiency | 17 | 8 (47) | 0.5 (0.12–1.6) | 7 (41) | 1.0 (0.27–3.7) |
| Operation-related data | |||||
| Laterality | |||||
| Unilateral | 170 | 72 (42) | 1 | 45 (27) | 1 |
| Bilateral | 21 | 4 (19) | 0.5 (0.14–1.5) | 3 (14) | 0.7 (0.17–2.5) |
| Time of induction of anesthesia | |||||
| Before 10 am | 95 | 41 (43) | 1 | 25 (26) | 1 |
| After 10 am | 96 | 35 (37) | 0.7 (0.40–1.4) | 23 (24) | 0.9 (0.43–1.7) |
| Duration of surgery × 10 min a | 191 | 0.95 (0.86–1.1) | 1.03 (0.92–1.2) | ||
| Blood loss × 100 mL a | 191 | 1.0 (0.88–1.1) | 1.0 (0.90–1.2) | ||
| Tourniquet time × 10 min (incl. knees only) a | 117 | 1.0 (0.84–1.2) | 1.2 (0.99–1.5) | ||
| Cement used for fixationb | |||||
| None | 83 | 36 (43) | 1 | 21 (25) | 1 |
| Any | 108 | 40 (37) | 0.8 (0.37–1.6) | 27 (25) | 1.3 (0.56–2.9) |
| Blood transfusion | |||||
| None | 167 | 63 (38) | 1 | 38 (23) | 1 |
| Any | 24 | 13 (54) | 1.3 (0.48–3.3) | 10 (42) | 1.8 (0.66–4.7) |
| Closed suction drains | |||||
| None | 77 | 31 (40) | 1 | 21 (27) | 1 |
| Any | 114 | 45 (40) | 2.0 (0.67–5.8) | 27 (24) | 1.4 (0.44–4.2) |
Continuous
Fully cementless vs. fully cemented and hybrid joint replacements.
ASA: American Society of Anesthesiologists; IFG: increased fasting glucose;
IGT: impaired glucose tolerance.
None of the self-reported comorbidities were associated with an increased risk of hyperglycemia (data not shown). Patients with a history of other joint replacements (n = 41) had hyperglycemia more frequently than those having their first-ever joint replacement (n = 150) (56% vs. 35%), but after adjustments there was no statistically significant difference (adjusted OR = 1.7 (0.60-4.8)). Smoking had no effect (Table 2).
Diabetes was strongly associated with hyperglycemia (Table 2). All 9 patients who were on insulin preoperatively presented with severe hyperglycemia during the hospitalization, as compared to 19 of 27 of patients treated with oral anti-diabetic agents (p = 0.06). Patients with newly diagnosed diabetes or prediabetic states (IFG and/or IGT) were similar to the patients with normal glucose metabolism in the adjusted analyses (Table 2). Metabolic syndrome was associated with an increased risk of hyperglycemia and severe hyperglycemia (Table 2), but in the absence of co-prevalent diabetes it had no effect (13/43 vs. 24/95; adjusted OR = 1.3 (0.61–2.8) for hyperglycemia, and 4/43 vs. 13/95; adjusted OR = 0.9 (0.33–2.6) for severe hyperglycemia). BMI, preoperative anemia, and renal function were not associated with perioperative hyperglycemia (Table 2).
There was also a strong association between HbA1c and both hyperglycemia and severe hyperglycemia, independent of diabetic status (Table 3). In particular, 21 of the 22 patients with previously diagnosed diabetes and HbA1c ≥ 6.5% were hyperglycemic perioperatively and 20 of them developed severe hyperglycemia. In patients without previously diagnosed diabetes, even slightly increased HbA1c (6.1–6.4%) appeared to double the risk of hyperglycemia (Table 3), but the difference did not reach statistical significance (adjusted OR = 1.7 (0.77–3.9) compared to HbA1c ≤ 6.0%). HbA1c ≥ 6.5% instead showed a significant effect (adjusted OR = 4.3 (1.1–17).
Table 3.
Association of preoperative HbA1c and baseline glucose (on the day of operation) with occurrence of hyperglycemia in patients with and without a previous diagnosis of diabetes
| Patients with previously diagnosed diabetes Hyper-glycemia |
Patients without a previous diagnosis of diabetes Hyper-glycemia |
|||||
|---|---|---|---|---|---|---|
| n | n (%) | pa | n | n (%) | pa | |
| HbA1c | ||||||
| ≤ 6.0% | 7 | 4 (57) | - | 96 | 20 (21) | - |
| 6.1–6.4% | 7 | 6 (86) | 0.3 | 49 | 19 (39) | 0.01 |
| ≥ 6.5% | 22 | 21 (95) | 0.002 | 10 | 6 (60) | 0.04 |
| Baseline glucose | ||||||
| < 5.6 mmol/L | 6 | 4 (67) | - | 88 | 18 (20) | - |
| 5.6–6.9 mmol/L | 12 | 10 (83) | 0.4 | 60 | 22 (37) | 0.03 |
| > 6.9 mmol/L | 18 | 17 (94) | 0.1 | 5 | 4 (80) | 0.02 |
p-values are from pairwise univariate comparisons against the lowest HbA1c/glucose category.
Preoperative fasting glucose was not associated with the risk of hyperglycemia in patients with or without a previous diagnosis of diabetes (adjusted ORs: 1.7 (0.69–4.0) and 1.6 (0.92–2.7), respectively). Baseline glucose instead increased the risk of hyperglycemia in patients without a previous diagnosis of diabetes (adjusted OR = 2.4 (1.4–4.3) for an increase of 1 mmol/L). Baseline glucose ≥ 7.0 mmol/L in particular, compared to ≤ 5.5 mmol/L, markedly increased the odds of hyperglycemia (adjusted OR = 17 (1.6–178)). In patients with a previous diagnosis of diabetes, baseline glucose showed a similar, but statistically insignificant association (Table 3).
Fewer patients undergoing simultaneous bilateral surgery than those undergoing unilateral surgery had hyperglycemia (4/19 vs. 72/170) but after adjustments, there was no statistically significant difference (Table 2). None of the 9 patients undergoing unicompartmental knee replacement presented with hyperglycemia, but 40% of those who received total knee replacements did (43 of 108) (p=0.02). The other operation-related variables available (time of surgery, duration of surgery, tourniquet time, blood loss, use of bone cement for prosthesis fixation, use of closed suction drains, allogenous blood transfusion) were not associated with occurrence of hyperglycemia (Table 2). These results were similar for hip and knee replacements, and for unilateral and simultaneous bilateral operations (data not shown).
Discussion
One-third of the patients had hyperglycemia following routine primary hip or knee replacement for osteoarthritis. In a quarter of them, glucose exceeded 10 mmol/L—which is above the range recommended for hospitalized patients irrespective of their diabetic status (Moghissi et al. 2009, Umpierrez et al. 2012), and which is also considered the threshold for starting insulin treatment (American Diabetes Association 2013). Occurrence of hyperglycemia was strongly related to existing disorders of glucose metabolism, particularly to the presence of diabetes and to preoperative glucose control, as measured by HbA1c. None of the operation-related parameters was independently associated with hyperglycemia.
The main strengths of our study were its prospective nature and the study population, which represented the general population requiring joint replacement for osteoarthritis well. The detailed preoperative evaluation allowed detailed testing of the state of preoperative glucose metabolism and other metabolic abnormalities. At our institution, perioperative care is highly standardized and it remained essentially unchanged during the study period. The operations were performed by experienced joint replacement surgeons with high numbers of operations annually. Thus, differences in the treatment would not bias the results much. Furthermore, the coverage of glucose monitoring was good—even though ward staff performed it along with their daily work.
The key finding was the strong association between existing disorders of glucose metabolism and perioperative hyperglycemia. Our study shows that diabetes, milder disorders of glucose metabolism, and metabolic syndrome are very common in hip and knee replacement recipients. Given the high prevalence of obesity in patients who undergo joint replacement surgery (Bourne et al. 2009, Jämsen et al. 2012), this is not surprising. One-third of patients with diabetes had not been diagnosed prior to scheduling for surgery. However, hyperglycemia was less frequent in newly diagnosed diabetes patients than in previously diagnosed diabetes patients and, in the adjusted analysis, only previously diagnosed diabetes was associated with hyperglycemia.
A clinically important observation in our study was the value of HbA1c in stratifying the risk of perioperative hyperglycemia, both in patients with and in those without a previous diagnosis of diabetes. Almost all patients with diabetes and an HbA1c of ≥ 6.5% presented with hyperglycemia. Based on our figures, it can be estimated that the incidence of perioperative hyperglycemia could be reduced by almost half if patients with diabetes were treated preoperatively to HbA1c ≤ 6.0%. To our knowledge, however, there have not been any studies that have examined the effectiveness of preoperative glucose control in the prevention of perioperative hyperglycemia or postoperative complications. Moreover, in a retrospective single-center study of 4,241 hip and knee replacements (Iorio et al. 2012), HbA1c levels varied markedly in diabetic patients both with and without infection, and the average HbA1c was not significantly different between these 2 groups. This suggests that, in patients with diabetes, factors other than HbA1c also contribute to the occurrence of PJI.
Interestingly, non-diabetic HbA1c values also appeared to increase the risk of hyperglycemia in patients with no previous diagnosis of diabetes (Figure 3). Sato et al. (2010) have demonstrated the association between preoperative HbA1c and perioperative insulin resistance in cardiac operations, but in contrast to our results, such an association was not found for patients with no previous diabetes diagnosis. Altogether, HbA1c appears to be an easily applicable tool for stratifying the risk of perioperative hyperglycemia in joint replacements, irrespective of diabetic status. Given the association between baseline glucose and hyperglycemia, measuring glucose at induction of anesthesia also seems reasonable, but if one relies on this measure only, the opportunity to control hyperglycemia preoperatively is lost.
In addition to markers of disorders of glucose metabolism, the ASA score was associated with an increased risk of hyperglycemia. Importantly, the association between ASA score and perioperative hyperglycemia was seen among patients with diabetes or with a high HbA1c, so diabetes as such and glucose control do not explain the result. The occurrence of diabetes-related complications (such as nephropathy and concomitant cardiovascular disease) might be a possible explanation, but this could not be confirmed with the present data.
We hypothesized that operation-related factors such as blood loss might be measures of surgical trauma and correlate with the hyperglycemic reaction. Contrary to expectations, the effects of all the operation-related factors analyzed were slight at best and none showed a statistically significant association with hyperglycemia. The only exception—and support for our hypothesis—was the observation that none of the patients who underwent unicondylar knee replacement had hyperglycemia, but there were only 9 such patients. Patient selection probably explains the fact that the occurrence of hyperglycemia is lower in bilateral surgery than in unilateral. In our series, the operations were highly homogenous, which could explain the negative results. In revision joint replacements and trauma surgeries (Richards et al. 2012) greater hyperglycemia and more variation would be expected.
Compared to an earlier paper reporting on perioperative hyperglycemia following hip or knee replacement (Pili-Floury et al. 2009), the incidence of perioperative hyperglycemia in the present study was much lower, although we also included patients with diabetes. In the earlier study, the operations were performed under general anesthesia, leading to more difficult stress-related insulin resistance (Donatelli et al. 2007), whereas we used spinal anesthesia. In addition, Pili-Floury et al. (2009) had proportionally more postprandial glucose measurements than we did. Similarly, in a retrospective study in which only fasting samples were analyzed, only 3% of patients had glucose levels of > 11.1 mmol/L (> 200 mg/dL) (Mraovic et al. 2011). In interpreting our results, it should be acknowledged that statistical power was calculated expecting a greater incidence of hyperglycemia. Hence, the lower incidence of hyperglycemia (together with the loss of 9 of the recruited 200 patients) may have led to false-negative results in some comparisons.
It appears likely that the extent of surgical stress-related perioperative insulin resistance is smaller in modern hip and knee replacements than in, for example, cardiac or open abdominal surgery (Thorell et al. 1999, Donatelli et al. 2007). The optimal postoperative glucose level in orthopedic surgery is unknown. Although glucose exceeding 7.8 mmol/L is considered to be hyperglycemia, recent guidelines (American Diabetes Association 2013) and studies in the field of cardiothoracic surgery (Bhamidipati et al. 2010) suggest that keeping postoperative glucose levels between 7.8–8.1 and 10.0 mmol/L may be satisfactory. In fact, control of hyperglycemia that is too strict may be harmful—especially in patients with severe comorbidities (Griesdale et al. 2009). Thus, the clinical significance of hyperglycemia between 7.8 and 10.0 mmol/L, as defined in our study, is unclear particularly in patients without diabetes.
Higher glucose values have also been associated with surgical site infections after orthopedic procedures. In a retrospective study of 1,948 primary hip or knee replacements, postoperative fasting glucose > 200 mg/dL (11.1 mmol/L) doubled the risk of PJI (Mraovic et al. 2011). Using the same cutoff for glucose, Richards et al. (2012) demonstrated a 3-fold risk of surgical site infection following surgical treatment of orthopedic trauma. Taking into account that clinical guidelines (Moghissi et al. 2009, Umpierrez et al. 2012, American Diabetes Association 2013) suggest treatment of hyperglycemia > 10.0–11.1 mmol/L, patients with such postoperative glucose values—e.g. the patients with severe hyperglycemia in our study—should also be identified and treated after joint replacement. In addition, it should be acknowledged that in these studies as well as in ours, hyperglycemia was first treated after it had occurred (insulin was administered on a so-called sliding scale basis), which leads to inferior outcomes compared to treatment with long-acting basal-bolus insulin (Umpierrez et al. 2011).
In conclusion, postoperative hyperglycemia occurs frequently in primary hip and knee replacement, and in one-quarter of patients it requires intervention. Most patients with previously diagnosed diabetes develop hyperglycemia, which is often severe, and they therefore need close glucose monitoring during their hospital stay. In patients with no history of diabetes, preoperative HbA1c and fasting glucose on the day of surgery can be used to estimate the risk of hyperglycemia.
Supplementary data
For Study protocol, see www.actaorthop.org, identification number 7276.
Acknowledgments
EJ, PIN, JK, and TM designed the study. EJ and JK participated in collecting the materials. EJ performed statistical analysis, wrote the first draft of the manuscript, and took care of revisions. All the authors contributed to interpretation of the results and preparation of the manuscript.
We acknowledge financial support for this study from the Competitive Research Funding of Tampere University Hospital, Tampere, Finland (grants 9M026 and 9N020) (representing governmental funding) and from the Emil Aaltonen Foundation, Tampere, Finland.
AE has received lecture fees from DePuy and Stryker and congress payments from DePuy (all unrelated to the present work). TM has received lecture fees from Roche and MSD and congress payments from DePuy (all unrelated to the present work). The other authors have nothing to disclose.
References
- Akhtar S, Barash PG, Inzucchi SE. Scientific principles and clinical implications of perioperative glucose regulation and control. Anesth Analg. 2010;110(2):478–97. doi: 10.1213/ANE.0b013e3181c6be63. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr International Diabetes Federation Task Force on Epidemiology and Prevention; Hational Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(6):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes 2013 Diabetes Care. 2013;36(Suppl 1):S4–10. doi: 10.2337/dc13-S004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhamidipati CM, LaPar DJ, Stukenborg GJ, Morrison CC, Kern JA, Kron IL, Ailawadi G. Superiority of moderate control of hyperglycemia to tight control in patients undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2010;141(2):543–51. doi: 10.1016/j.jtcvs.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne R, Mukhi S, Zhu N, Keresteci M, Marin M. Role of obesity on the risk for total hip or knee arthroplasty. Clin Orthop Relat Res. 2007;465:185–8. doi: 10.1097/BLO.0b013e3181576035. [DOI] [PubMed] [Google Scholar]
- Bozic KJ, Lau E, Kurtz S, Ong K, Berry DJ. Patient-related risk factors for postoperative mortality and periprosthetic joint infection in medicare patients undergoing TKA. Clin Orthop Relat Res. 2012;470(1):130–7. doi: 10.1007/s11999-011-2043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Cui Y, Li X, Miao X, Wen Z, Xue Y, Tian J. Risk factors for deep infection after total knee arthroplasty: a meta-analysis. Arch Orthop Trauma Surg. 2013;133(5):675–87. doi: 10.1007/s00402-013-1723-8. [DOI] [PubMed] [Google Scholar]
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Cohn DM, Hermanides J, DeVries JH, Kamphuisen PW, Kuhls S, Homering M, Hoekstra JB, Lensing AW, Büller HR. Stress-induced hyperglycaemia and venous thromboembolism following total hip or total knee arthroplasty: analysis from the RECORD trials. Thromb Haemost. 2012;107(2):225–31. doi: 10.1160/TH11-07-0447. [DOI] [PubMed] [Google Scholar]
- Davies H TO, Tavakoli M, Crombie IK. When can odds ratios mislead? BMJ. 1998;316(7136):989–91. doi: 10.1136/bmj.316.7136.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatelli F, Vavassori A, Bonfanti S, Parrella P, Lorini L, Fumagalli R, Carli F. Epidural anesthesia and analgesia decrease the postoperative incidence of insulin resistance in preoperative insulin-resistant subjects only. Anesth Analg. 2007;104(6):1587–93. doi: 10.1213/01.ane.0000261506.48816.5c. [DOI] [PubMed] [Google Scholar]
- Donatelli F, Cavagna P, Di Dedda G, Catenacci A, Di Nicola M, Lorini L, Fumagalli R, Carli F. Correlation between pre-operative metabolic syndrome and persistent blood glucose elevation during cardiac surgery in non-diabetic patients. Acta Anaesthesiol Scand. 2008;52(8):1103–10. doi: 10.1111/j.1399-6576.2008.01693.x. [DOI] [PubMed] [Google Scholar]
- Dowsey MM, Choong PF. Obese diabetic patients are at substantial risk for deep infection after primary TKA. Clin Orthop Relat Res. 2009;467(6):1577–81. doi: 10.1007/s11999-008-0551-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, Dhaliwal R, Henderson WR, Chittock DR, Finfer S, Talmor D. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICE-SUGAR study data. CMAJ. 2009;180(8):821–7. doi: 10.1503/cmaj.090206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson UO, Thorell A, Soop M, Ljungqvist O, Nygren J. Haemoglobin A1c as a predictor of postoperative hyperglycaemia and complications after major colorectal surgery. Br J Surg. 2009;96(11):1358–64. doi: 10.1002/bjs.6724. [DOI] [PubMed] [Google Scholar]
- Iorio R, Williams KM, Marcantonio AJ, Specht LM, Tilzey JF, Healy WL. Diabetes mellitus, hemoglobin A1C, and the incidence of total joint arthroplasty infection. J Arthroplasty. 2012;27(5):726–9. doi: 10.1016/j.arth.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Jämsen E, Furnes O, Engesaeter LB, Konttinen YT, Odgaard A, Stefánsdóttir A, Lidgren L. Prevention of deep infection in joint replacement surgery. Acta Orthop. 2010;81(6):660–6. doi: 10.3109/17453674.2010.537805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jämsen E, Nevalainen P, Eskelinen A, Huotari K, Kalliovalkama J, Moilanen T. Obesity, diabetes, and preoperative hyperglycemia as predictors of periprosthetic joint infection: a single-center analysis of 7181 primary hip and knee replacements for osteoarthritis. J Bone Joint Surg Am. 2012;94(14):e101. doi: 10.2106/JBJS.J.01935. [DOI] [PubMed] [Google Scholar]
- Ljungqvist O, Soop M, Hedström M. Why metabolism matters in elective orthopedic surgery: a review. Acta Orthop. 2007;78(5):610–5. doi: 10.1080/17453670710014293. [DOI] [PubMed] [Google Scholar]
- Malinzak RA, Ritter MA, Berend ME, Meding JB, Olberding EM, Davis KE. Morbidly obese, diabetic, younger, and unilateral joint arthroplasty patients have elevated total joint arthroplasty infection rates. J Arthroplasty. 2009;24(6 Suppl):84–8. doi: 10.1016/j.arth.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Masla M, Gottschalk A, Durieux ME, Groves DS. HbA1c and diabetes predict perioperative hyperglycemia and glycemic variability in on-pump coronary artery bypass graft patients. J Cardiothorac Vasc Anesth. 2011;25(5):799–803. doi: 10.1053/j.jvca.2010.09.028. [DOI] [PubMed] [Google Scholar]
- Meding JB, Klay M, Healy A, Ritter MA, Keating EM, Berend ME. The prescreening history and physical in elective total joint arthroplasty. J Arthroplasty. 2007;22(Suppl 2):21–3. [Google Scholar]
- Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez G E. American Association of Clinical Endocrinologists; American Diabetes Association. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care. 2009;32(6):1119–31. doi: 10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraovic B, Suh D, Jacovides C, Parvizi J. Perioperative hyperglycemia and postoperative infection after lower limb arthroplasty. J Diabetes Sci Technol. 2011;5(2):412–8. doi: 10.1177/193229681100500231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens WD, Felts JA, Spitznagel EL., Jr ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49(4):239–43. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- Pili-Floury S, Mitifiot F, Penfornis A, Boichut N, Tripart MH, Christophe JL, Garbuio P, Samain E. Glycaemic dysregulation in nondiabetic patients after major lower limb prosthetic surgery. Diabetes Metab. 2009;35(1):43–8. doi: 10.1016/j.diabet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Richards JE, Kauffmann RM, Zuckerman SL, Obremskey WT, May AK. Relationship of hyperglycemia and surgical-site infection in orthopaedic surgery. J Bone Joint Surg Am. 2012;94(13):1181–6. doi: 10.2106/JBJS.K.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi AA, Chillag SA, Chillag KJ. Perioperative management of diabetes and hyperglycemia in patients undergoing orthopaedic surgery. J Am Acad Orthop Surg. 2010;18(7):426, 35. doi: 10.5435/00124635-201007000-00005. [DOI] [PubMed] [Google Scholar]
- Saaristo TE, Barengo NC, Korpi-Hyövälti E, Oksa H, Puolijoki H, Saltevo JT, Vanhala M, Sundvall J, Saarikoski L, Peltonen M, Tuomilehto J. High prevalence of obesity, central obesity and abnormal glucose tolerance in the middle-aged Finnish population. BMC Public Health. 2008;8:423. doi: 10.1186/1471-2458-8-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Carvalho G, Sato T, Lattermann R, Matsukawa T, Schricker T. The association of preoperative glycemic control, intraoperative insulin sensitivity, and outcomes after cardiac surgery. J Clin Endocrinol Metab. 2010;95(9):4338–44. doi: 10.1210/jc.2010-0135. [DOI] [PubMed] [Google Scholar]
- Thorell A, Nygren J, Ljungqvist O. Insulin resistance: a marker of surgical stress. Curr Opin Clin Nutr Metab Care. 1999;2(1):69–78. doi: 10.1097/00075197-199901000-00012. [DOI] [PubMed] [Google Scholar]
- Umpierrez GE, Smiley D, Jacobs S, Peng L, Temponi A, Mulligan P, Umpierrez D, Newton C, Olson D, Rizzo M. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery) Diabetes Care. 2011;34(2):256–61. doi: 10.2337/dc10-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umpierrez GE, Hellman R, Korytkowski MT, Kosiborod M, Maynard GA, Montori VM, Seley JJ, Van den Berghe G. Endocrine Society. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(1):16–38. doi: 10.1210/jc.2011-2098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.