Abstract
In two recent publications, we demonstrated that after allogeneic stimulation, regulatory T cells (Tregs) increase expression of aldehyde dehydrogenase (ALDH), the major in vivo mechanism of cyclophosphamide detoxification, thereby becoming cyclophosphamide resistant. Differential ALDH expression may explain why cyclophosphamide has pro- and anti-inflammatory effects that are temporally and contextually dependent.
Keywords: aldehyde dehydrogenase, ALDH, allogeneic, BMT, cyclophosphamide, post-transplantation cyclophosphamide, regulatory T cell, tolerance, Treg
The impact of cyclophosphamide on immunologic tolerance has been studied for over 50 years. In 1963, building on several similar studies performed earlier that year, Berenbaum and Brown reported the use of a single high-dose of cyclophosphamide (200 mg/kg) to promote skin allograft survival in mice.1 Allograft survival was prolonged only when cyclophosphamide was administered during a narrow post-transplant window; it was ineffective when given either before allograft placement or after day +4.1 Despite this tolerogenic activity of cyclophosphamide, subsequent studies showed that in different situations cyclophosphamide could exert inflammatory effects. Polak and Turk found that high-dose cyclophosphamide (250 mg/kg), when administered to guinea pigs 3 d prior to sensitization to dinitrophenyl compounds, reversed tolerance through inhibition of suppressor cell activity.2 A plethora of ensuing studies have reaffirmed this immunologic duality of cyclophosphamide wherein cyclophosphamide can function either as a pro-inflammatory or pro-tolerogenic agent in different circumstances.
The effects of cyclophosphamide in either promoting or breaking tolerance were found to involve Tregs. On one hand, Tregs could be depleted by treatment with high-dose cyclophosphamide (100 mg/kg) prior to adoptive cell transfer, thus promoting anti-tumor activity of the adoptively transferred T cells.3 Similarly, Treg depletion induced by high-dose cyclophosphamide (200 mg/kg) resulted in autoimmunity.4 By contrast, Tregs were found to be essential for maintaining allograft skin tolerance in mice induced by treatment with high-dose cyclophosphamide (200 mg/kg) 2 d after priming with spleen and bone marrow cells.5 Given clinically at 50 mg/kg/day on days +3 and +4 after allogeneic bone marrow transplantation (alloBMT), post-transplantation cyclophosphamide (PTCy) has proven successful not only in preventing acute graft-versus-host disease (GVHD), but more importantly in inducing post-transplant immunologic tolerance, manifested by quite low rates of chronic GVHD.6 These effects have been shown in human leukocyte antigen (HLA)-matched and HLA-mismatched alloBMT at multiple centers worldwide.6 In light of these clinical findings and discrepant pre-clinical data regarding cyclophosphamide's impact on Tregs and tolerance, our two recent scientific publications focused on understanding 1) the effect of PTCy on Tregs and 2) the role that Tregs play in PTCy-mediated tolerance induction.7,8
In order to better understand the impact of PTCy on human Tregs, we began by immunophenotyping peripheral blood samples from patients treated with PTCy after HLA-matched alloBMT.7 Despite expected, marked post-transplant CD4+ lymphopenia, effector Tregs recovered to donor and pretransplant levels by 30 d post-transplant. Human mixed lymphocyte reactions (MLRs) showed that Tregs were substantially more resistant to mafosfamide, an in vivo active form of cyclophosphamide, than were conventional CD4+ T cells (Tcons).7 Mafosfamide-treated Tregs maintained suppressive activity and the characteristically demethylated pattern within the Treg-specific demethylation region.7,8 The mechanism of Treg resistance to mafosfamide appeared to be via upregulation of ALDH,7,8 the major in vivo mechanism of cyclophosphamide detoxification. At baseline, human Tregs had minimal ALDH functional activity and undetectable ALDH RNA by PCR.7 However, after allogeneic stimulation in MLR for 3 d, human Tregs markedly upregulated ALDH isoforms to levels 10-fold higher than those found within Tcons, thus becoming relatively mafosfamide resistant 7 Similar findings were seen in our second publication in which ALDH expression by murine Tregs was increased after allogeneic stimulation in vitro and conferred heightened Treg resistance to mafosfamide-induced cytotoxicity.8
In both publications, Tregs were found to be necessary for PTCy-mediated GVHD prevention.7,8 Xenogeneic experiments showed that PTCy's GVHD protective effects were lost when Tregs were flow cytometrically depleted from human peripheral blood mononuclear cells allografts prior to transplantation into NOD/Lt-scid/IL-2rγnull mice.7 Using major histocompatibility antigen-matched, minor histocompatibility antigen-mismatched alloBMT murine models, removal of Tregs by flow cytometric sorting or selective in vivo depletion resulted in lethal GVHD.8 Furthermore, mice which had been treated with PTCy and then Treg depleted were rescued from lethal GVHD by the adoptive transfer of thymically derived Tregs (tTregs) from PTCy-treated mice.8 PTCy promoted both persistence of tTregs and peripheral induction of Tregs from Tcons, although tTregs constituted the more functionally active, dominant Treg population.8
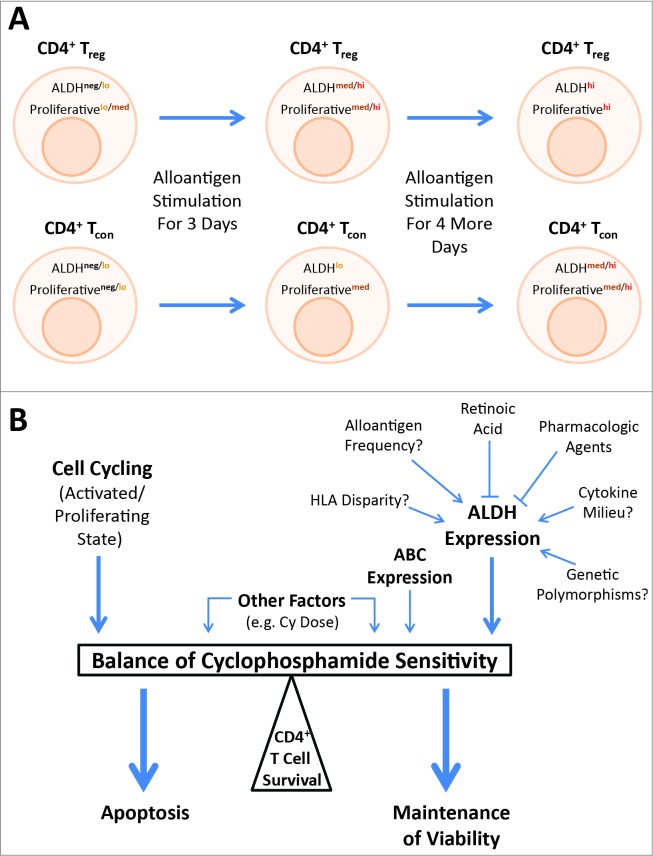
The mechanistic insights provided by these two papers may help resolve the apparent paradoxical duality of cyclophosphamide as relates to Tregs and tolerance induction (Fig. 1). At baseline, effector Tregs are the most proliferative CD4+ T cell subset.9 Thus, having low ALDH expression in the steady-state autologous setting but a relatively high proliferative rate,7,8 it is not surprising that Tregs may be highly susceptible to an alkylating agent like cyclophosphamide. However, after allogeneic or likely other types of stimulation in a lymphopenic environment, Tregs increase ALDH expression more so than CD4+ Tcons, which themselves have become more proliferative; thus Tregs become more resistant than their CD4+ Tcon counterparts.7,8 The dose of cyclophosphamide may accentuate this effect as the relative importance of ALDH in cyclophosphamide clearance is heightened with higher doses of cyclophosphamide.10 These changes in the relative resistance of Tregs and Tcons to cyclophosphamide may explain the importance of context and timing as to whether cyclophosphamide exerts a pro- or ant-inflammatory net effect (Fig. 1). Indeed, in both of our studies, differential ALDH expression was most pronounced in Tregs at MLR day 3 with more equivalent expression between Tregs and Tcons by MLR day 7,7,8 likely explaining why the window for PTCy to induce tolerance only exists within the first few days after allogeneic stimulation.1,5,6 Further work will be needed to define which clinical contexts are most conducive to Treg depletion and which to Treg preservation in order to fully exploit this delicate balance toward a clinical advantage.
Figure 1.
Model for how relative aldehyde dehydrogenase (ALDH) expression and the activation/proliferation state of the cell may interact to determine CD4+ T cell sensitivity to cyclophosphamide (Cy). (A) At baseline, CD4+ T cells have little ALDH expression and regulatory T cells (Tregs) are the most proliferative CD4+ subset, potentially explaining why Tregs can be selectively depleted by Cy in this context. However, with allogeneic stimulation, the ALDH expression and activation/proliferation states of both Tregs and conventional T cells (Tcons) change, overall favoring the survival of Tregs over Tcons when Cy is administered on day 3 of allogeneic stimulation. The narrow window to induce tolerance that is lost after day 4 of allogeneic stimulation, as previously demonstrated in murine studies, may be explained by the further dynamic changes in ALDH expression and proliferation with continued allogeneic stimulation that no longer confer a survival advantage to Tregs. As there is variability in both parameters among cells within each group (Tregs or Tcons), the general population changes are shown. (B) Whether an individual CD4+ T cell survives cyclophosphamide exposure is likely determined by a complex balance of a number of factors with ALDH expression and the cell activation/proliferation state being dominant mechanisms. Other factors such as relative expression of particular ATP-binding cassette (ABC) transporters, which can efflux cyclophosphamide from the cell, and the relative dose of cyclophosphamide likely have some impact. The mechanisms of ALDH upregulation after allogeneic stimulation require further investigation.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The work described in this article was funded by the National Institutes of Health (RO1-HL110907, RO1-CA122779, T32-HL007525) and the Conquer Cancer Foundation of the American Society of Clinical Oncology (2012 Young Investigator Award).
References
- 1. Berenbaum MC, Brown IN. Prolongation of Homograft survival in mice with single doses of cyclophosphamide. Nature 1963; 200:84; PMID:; http://dx.doi.org/ 10.1038/200084a0 [DOI] [PubMed] [Google Scholar]
- 2. Polak L, Turk JL. Reversal of immunological tolerance by cyclophosphamide through inhibition of suppressor cell activity. Nature 1974; 249:654-56; PMID:; http://dx.doi.org/ 10.1038/249654a0 [DOI] [PubMed] [Google Scholar]
- 3. North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor-induced suppressor T cells. J Exp Med 1982; 155:1063-74; PMID:; http://dx.doi.org/ 10.1084/jem.155.4.1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brode S, Raine T, Zaccone P, Cooke A. Cyclophosphamide-induced type-1 diabetes in the NOD mouse is associated with a reduction of CD4+CD25+Foxp3+ regulatory T cells. J Immunol 2006; 177:6603-12; PMID:; http://dx.doi.org/ 10.4049/jimmunol.177.10.6603 [DOI] [PubMed] [Google Scholar]
- 5. Tomita Y, Mayumi H, Eto M, Nomoto K. Importance of suppressor T cells in cyclophosphamide-induced tolerance to the non-H-2-encoded alloantigens. Is mixed chimerism really required in maintaining a skin allograft tolerance? J Immunol 1990; 144:463-73; PMID: [PubMed] [Google Scholar]
- 6. Luznik L, O'Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol 2012; 39:683-93; PMID:; http://dx.doi.org/ 10.1053/j.seminoncol.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B, Fuchs EJ, Jones RJ, Hess AD, Luznik L. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med 2013; 5:211ra157; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3006960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ganguly S, Ross DB, Panoskaltsis-Mortari A, Kanakry CG, Blazar BR, Levy RB, Luznik L. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood 2014; 124:2131-41; PMID:; http://dx.doi.org/ 10.1182/blood-2013-10-525873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009; 30:899-911; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2009.03.019 [DOI] [PubMed] [Google Scholar]
- 10. Busse D, Busch FW, Bohnenstengel F, Eichelbaum M, Fischer P, Opalinska J, Schumacher K, Schweizer E, Kroemer HK. Dose escalation of cyclophosphamide in patients with breast cancer: consequences for pharmacokinetics and metabolism. J Clin Oncol 1997; 15:1885-96; PMID: [DOI] [PubMed] [Google Scholar]