Abstract
The expression of the immunomodulating enzyme indoleamine-2,3-dioxygenase (IDO) suppresses T-lymphocyte function, thus correlating with poor survival in a variety of cancer patients. IDO degrades the essential amino acid tryptophan leading to immunosuppressive kynurenines production. In the present study, concentrations of tryptophan, 3-hydroxykynurenine, and kynurenine were measured in pre-treatment serum samples of 251 cervical cancer patients by a mass-spectrometric method (XLC-MS/MS) and IDO activity determined by the kynurenine/tryptophan (Kyn/Trp) ratio. A low concentration of tryptophan was found to be significantly associated with tumors greater than 4 cm and lymph node metastatic spread. Furthermore, significant positive correlations were found between high concentrations of the tryptophan metabolites kynurenine and 3-hydroxykynurenine and advanced disease stage (FIGO >IIA) and lymph node metastases. High levels of kynurenine were further associated with parametrial invasion and tumor size. A high Kyn/Trp ratio was related to lymph node metastasis, FIGO stage, tumor size, parametrial invasion and poor disease-specific survival. These results suggest that IDO activation is linked to poor clinicopathological parameters and worse survival in cervical cancer, warranting the use of IDO inhibitors in future clinical trials.
Key words: cervical cancer, IDO, kynurenine, Kyn/Trp ratio, tryptophan
Abbreviations: IDO, indoleamine-2,3-dioxygenase; IFNγ, interferon γ; Kyn/Trp ratio, kynurenine/tryptophan ratio; FIGO, International Federation of Gynaecologists and Obstetricians; Gy, Gray; HPV, human papillomavirus; M0, no metastasis; NK, natural killer; SCC, squamous cell carcinoma; TDO. tryptophan-2,3-dioxygenase; TLR: toll-like receptor; Tregs, regulatory T cells; XLC-MS/MS- extraction: liquid chromatographic tandem mass spectrometry
Introduction
Cervical cancer is the fourth most common cancer in women worldwide and is typically caused by a persistent human papillomavirus (HPV) infection.1,2 Although 80% of women will be infected by HPV, the majority are able to clear the infection, indicating a crucial role for the immune system in cervical carcinogenesis and progression.3 Not surprisingly, various mechanisms are employed by malignant and premalignant cells to escape recognition and destruction by the immune system and to modulate the tumor microenvironment allowing tumor development and growth.4,5
The intracellular enzyme indoleamine-2,3-dioxygenase (IDO) plays a central role in tumor-induced immunosuppression.6,7 IDO is induced in many cell types like trophoblastic cells, dendritic cells, macrophages and tumor cells by Toll-like receptor (TLR) ligands, endotoxin and most efficiently by the T helper type 1 (Th1) cytokine, interferon γ (IFNγ).8-12 IDO provokes immune tolerance through catalysing degradation of the essential amino acid L-tryptophan. Tryptophan is used for protein and indole synthesis and is metabolized along the kynurenine pathway.13
Kynurenines, which include L-kynurenine, 3-hydroxykynurenine, 3-hydroxyanthranilic acid, and quinolinic acid, have been reported to block T-cell proliferation, resulting in growth arrest of alloreactive T cells and natural killer (NK) cells.13,14
In serum, IDO activity can be estimated by the kynurenine-to-tryptophan (Kyn/Trp) ratio, which represents the quotient of the first product of the IDO pathway (kynurenine), divided by the substrate tryptophan.15,16 In cancer patients, lower serum concentrations of tryptophan, higher kynurenine concentrations, and a higher Kyn/Trp ratio have been reported by us and others.8,16-18 Furthermore, in lung cancer patients and patients with B-cell lymphoma, a high Kyn/Trp ratio has been found to be associated with advanced disease.16,19 In addition, IDO activity has been reported to correlate with tumor progression20-22 and may facilitate tumor metastasis.23 IDO expression has also been surmised to be involved in regulatory T cell (Treg) activity.24,25
Few studies have investigated the expression of IDO in cervical cancer.26-30 Two such studies found higher levels of IDO expression in cervical cancer lesions as compared to normal cervical cells.28,29 Fotopoulou et al., described less IDO activity in primary cervical cancer in comparison to samples from healthy individuals, but this study included only 20 patients.30 A single study reported on correlation of increased IDO expression with clinical stage, lymph node metastasis, lympho-vascular space invasion and reduced disease-specific and disease-free survival.26
Here, we report for the first time on IDO metabolic activity in a large cohort of 251 cervical cancer patients by measuring tryptophan, kynurenine, 3-hydroxykynurenine and the Kyn/Trp ratio in pre-treatment serum samples, examining potential correlations between serum concentrations, clinicopathological characteristics and patient survival.
Results
The main clinicopathological characteristics of the 251 cervical cancer patients analysed in this study are summarized in Table 1. All additional data on the treatment is given in the Materials and Methods section.
Table 1.
Clinicopathological characteristics
| Clinicopathological parameter* | Catergory | Number of patients (%) |
|---|---|---|
| Histopathology | SCC‡ | 190 (76) |
| A(S)CC‡ | 55 (22) | |
| Age | Median | 50 |
| Range | 23-90 | |
| FIGO stage† | IAI | 2 (1) |
| IAII | 6 (2) | |
| IBI | 114 (45) | |
| IBII | 19 (8) | |
| IIA | 25 (10) | |
| IIB | 40 (16) | |
| IIIA | 5 (2) | |
| IIIB | 21 (8) | |
| IVA | 6 (2) | |
| IVB | 12 (5) | |
| Missing | 1 | |
| Lymph nodes | Negative | 165 (66) |
| Positive | 86 (34) | |
| Tumor size (mm) | <40 mm | 166 (66) |
| > = 40 mm | 82 (33) | |
| Vasoinvasion | Absent | 72 (29) |
| Present | 174 (69) | |
| Parametrium invasion | Absent | 140 (56) |
| Present | 107 (43) | |
| Type of treatment | None | 3 (1) |
| Surgery | 79 (32) | |
| Surgery + adjuvant (C)RT ≡ | 47 (19) | |
| Chemoradiation | 90 (36) | |
| Radiotherapy | 27 (11) | |
| Chemotherapy | 3 (1) | |
| NA CRT ¶ | ||
| + surgery | 2 (1) |
* For some variables, data were not available for all patients (n = 251).
† FIGO, International Federation of Gynecologists and Obstetricians.
‡ SCC: squamous and A(S)CC: adeno(squamous) cervical cancer
≡ (C)RT: (Chemo)Radiotherapy.
¶ NA CRT: Neo-adjuvant chemoradiation.
In 107 patients (43%) parametrium invasion was found and in 174 (69%) vaso-invasion was present. In one patient the operation was not complete and of 2 patients data was not found. The surgical margins of the removed tissue were negative in all patients, but 4 patients had only a margin of 1 mm, nevertheless, these 4 patients did not have disease recurrence within 5 years. Of the 90 patients who received chemoradiation, 35 (39%) had recurrent disease within 5 years, 30% of the whole cohort had disease recurrence within 5 years.
Association between tryptophan, kynurenine, 3-hydroxykynurine, Kyn/Trp ratio and clinicopathological parameters
The interquartile concentration ranges of tryptophan, kynurenine, 3-hydroxykynurenine and the Kyn/Trp ratio are presented in Table 2.
Table 2.
The concentration of the IDO pathway metabolites in cervical cancer patient serum samples as measured by the XLC-MS/MS method
| Tryptophan μmol/L | Kynurenine μmol/L | 3-Hydroxykynurenine μmol/L | Kyn/Trp ratio μmol/L | |
|---|---|---|---|---|
| 1st quartile | 10.86–41.55 | 0.22–1.33 | 3.20–26.50 | 16.20–26.53 |
| 2nd quartile | 41.56–49.26 | 1.34–1.64 | 26.51–33.90 | 26.54–33.81 |
| 3rd quartile | 49.27–55.05 | 1.65–2.03 | 33.91–47.10 | 33.82–43.54 |
| 4th quartile | 55.06–80.97 | 2.04–5.77 | 47.11–240.40 | 43.55–123.54 |
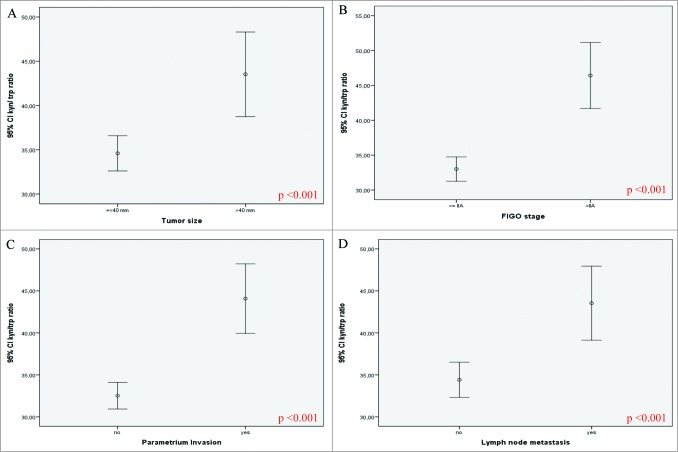
The Mann-Whitney U test was used to compare the distribution of IDO metabolite concentrations and associations with clinicopathological data. Low concentration of tryptophan was associated with tumor size greater than 4 cm (p = 0.016) and lymph node metastases (p = 0.003). A significant positive association was found between high kynurenine concentration and tumor size (p = 0.037), advanced stage of disease (FIGO >IIA) (p < 0.001), parametrial invasion (p < 0.0001) and lymph node metastases (p = 0.045). High concentration of 3-hydroxykynurenine was associated with advanced stage of disease (FIGO > IIA) (p = 0.009) and lymph node metastases (p = 0.012). A high Kyn/Trp ratio was associated with tumor size (p < 0.001; Fig. 1A), advanced stage of disease (FIGO > IIA) (p < 0.001; Fig. 1B), parametrial invasion (p < 0.001; Fig. 1C), and lymph node metastases (p < 0.001; Fig. 1D).
Figure 1.
IDO metabolites correlate with clinicopathological features of cervical cancer patients. Association between IDO activity as measured by Kyn/Trp ratio and clinicopathological parameters in cervical cancer (n = 251*). The kynurenine and tryptophan concentrations were determined by an automated online solid-phase XLC-MS/MS method with deuterated internal standards. Association of IDO activity (high Kyn/Trp ratio) and tumor size (A), FIGO stage (B), parametrium invasion (C), and lymph node metastasis (D). The non-parametric Mann-Whitney U test was used, p<0.05 was considered to be statistically significant. A high Kyn/Trp ratio was associated with tumor size (p < 0.001), advanced stage of disease (FIGO > IIA) (p < 0.001), parametrial invasion (p < 0.001) and lymph node metastases (p < 0.001). CI, confidence interval; FIGO, International Federation of Gynaecologists and Obstetricians. * For some variables, data were not available for all patients.
Disease Specific Survival in Association with IDO Metabolite Concentrations
The median follow-up time was 76 months (range, 1-118) for all patients and 88 months (range, 39-118) for patients alive at the time of data collection. Of the 84 patients who died during the follow-up period, 67 deaths could be attributed to cervical cancer and 4 patients died of another cause. For 13 patients the cause of death was unknown and for the remaining 3 patients it was unknown whether they were alive or not at time of data collection.
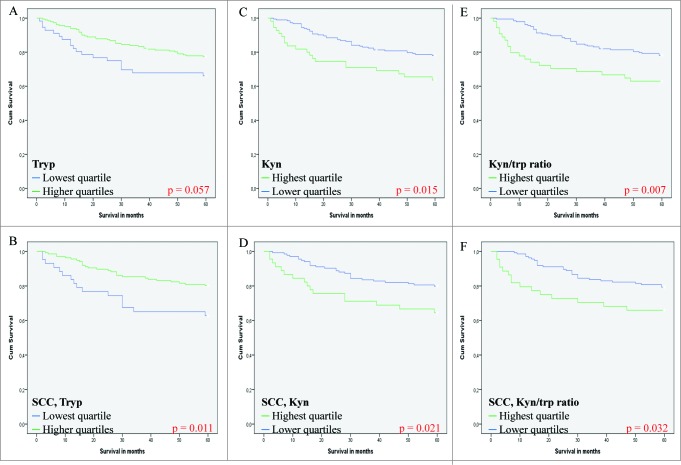
There was a trend towards worse survival for patients with the lowest quartile of tryptophan concentrations compared to higher amounts of tryptophan (p = 0.057; Fig. 2A), while there was a significant difference when solely squamous cell carcinomas (SCCs) were analyzed (p = 0.011; Fig. 2B). Patients with low amounts of kynurenine had a significantly better 5-year survival rate compared to patients with high kynurenine concentration (p = 0.015; Fig. 2C). This was also the case for squamous tumors alone (p = 0.021; Fig. 2D).There was no difference in survival rates for the total group of patients or for the SCC cases when expression of 3-hydroxykynurenine was considered (p = 0.38 and p = 0.20, respectively) (data not shown). In the complete cohort, the highest quartile of the Kyn/Trp ratio was associated with poor survival (p = 0.007; Fig. 2E) and this was also observed in the squamous cell carcinomas (p = 0.032; Fig. 2F).
Figure 2.
IDO metabolites correlate with survival of cervical cancer patients. Kaplan-Meier curves and log rank test analysis of IDO metabolites effect on disease-specific survival in cervical cancer patients (n = 251*). The kynurenine and tryptophan concentrations were determined by an automated online solid-phase XLC-MS/MS method with deuterated internal standards. Lowest quartile versus higher tryptophan (tryp) concentrations in the whole cohort (A) and in squamous cell carcinomas (SCC) (B), highest quartile versus lower kynurenine (kyn) concentrations in the whole cohort (C) and in SCC (D), highest quartile of the kyn/trp ratio (a surrogate for IDO activity) versus lower ratio in the whole cohort (E) and in SCC (F). Patient survival rates were analyzed by Kaplan-Meier curves and the log-rank test and p < 0.05 was considered to be statistically significant. Cum survival, cumulative survival; disease specific survival, death by cervical cancer. * For some variables, data were not available for all patients.
Because of the strong associations found with clinicopathological parameters such as FIGO stage and lymph node metastasis, the amount of the studied IDO metabolites was, although significantly associated with survival in an univariate Cox analysis (p = 0.008), not an independent prognostic factor (Table 3).
Table 3.
Univariate and multivariate Cox regression analysis IDO metabolites in serum (n = 251×)
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR∞ | 95% CI* | p value | HR∞ | 95% CI* | p value | |
| Tumor size | 2.40 | 1.44–3.98 | 0.001 | 0.88 | 0.51–1.54 | 0.66 |
| Parametrial invasion | 4.46 | 2.51–7.92 | <0.001 | 1.59 | 0.76–3.36 | 0.22 |
| FIGO stage† | 5.96 | 3.45–10.28 | <0.001 | 3.46 | 1.67–7.20 | 0.001 |
| Lymph node metastasis | 3.82 | 2.27–6.43 | <0.001 | 2.65 | 1.53–4.61 | 0.001 |
| Kyn/Trp ratio± | 2.06 | 1.20–3.52 | 0.008 | 1.16 | 0.66–2.05 | 0.61 |
×For some variables. data were not available for all patients.
† FIGO. International Federation of Gynecologists and Obstetricians.
±Highest quartile vs. rest.
∞ Hazard ratio.
*Confidence interval.
Patient survival rates were analyzed by univariate and multivariate Cox proportional hazards models. p < 0.05 was considered to be statistically significant.
The clinicopathological parameters tumor size. parametrial invasion. FIGO stage. lymph node metastasis and the Kyn/Trp ratio were all significantly associated with survival in an univariate Cox analysis. But. the amount of the studied IDO metabolites was not an independent prognostic factor in a multivariate Cox regression (p = 0.61).
Discussion
In this study, we showed for the first time that tryptophan degradation along the kynurenine pathway (the presence of active IDO) in pre-treatment serum, as measured by the Kyn/Trp ratio, is associated with detrimental clinicopathological parameters and poor survival in a large cohort of cervical cancer patients. Because of the strong associations found with FIGO stage and lymph node metastasis (p < 0.001), the Kyn/Trp ratio was, although being significantly correlated with survival in a univariate Cox proportional hazards analysis (HR 2.1, 95% CI: 1.20-3.52, p = 0.008), not an independent prognostic factor. However, the main goal of this study was to ascertain the rational for using IDO inhibitors in cervical cancer treatment; in contrast, the use of IDO activity as a prognostic marker should be analysed in comparison with serum markers already in use, such as prognostic serum biomarkers in SCC. 31
Our results are in accordance with earlier studies that have shown decreased tryptophan levels, increased kynurenine levels and a high Kyn/Trp ration in the serum of patients with various cancer types.16-19,32 In addition, we have previously described the same for endometrial, ovarian and vulvar cancer.8 These results substantiate that tryptophan catabolism can be monitored in serum samples from cancer patients and could have prognostic significance. Only in one other study has the presence of IDO metabolites in cervical cancer been analysed, but these results were unclear as a very limited number of samples was included (n = 20).30 Plasma/serum tryptophan status and the tryptophan kynurenine ratio are influenced by both IDO and the liver enzyme tryptophan 2,3-dioxygenase (TDO); however, we did not analyze the potential involvement of the latter enzyme in cervical cancer in the present study.33 IDO expression in the tumor microenvironment leads to specific in situ depletion of tryptophan and production of immune inhibitory metabolites, which subsequently inhibit T-cell proliferation and T-cell apoptosis.34 In line with these data, the numbers of CD3+ and CD8+ T cells have been found to decrease with IDO activity increase.10,35,36 Kynurenine has also been implicated in the direct inhibition of T-cell function.37 Furthermore, IDO has been shown to induce immune tolerance through modulating inhibitory dendritic cells in mouse models and cancer patients38 and by suppressing TCR-mediated activation of T cells via blocking multiple downstream kinases, such as the mitogen activated protein kinase family members ERK and MAPK, and the nuclear factor κB inhibitory enzyme IκB.39,40 In mouse models of lung and breast carcinoma, it has been previously reported that IDO deficiency reduces tumor burden and improves survival. Interestingly, during tumor and metastasis outgrowth, interleukin (IL)-6 induction was found to be significantly impaired in IDO deficient mice, causing a substantial diminishing of pro-tumorigenic myeloid-derived suppressor cells (MDSC).41 These findings have been substantiated by a study in human breast cancer, showing that IDO is linked to higher MDSC numbers and signal transducer and activator of transcription 3 (STAT3)-dependent IDO expression mediates the immunosuppressive effects of MDSCs.42
Moreover, suppressive action of IDO can also be exerted by the induction and expansion of Tregs by an IDO-dependent mechanism.24,25,43,44 In cervical cancer, we have previously shown that the presence of high intraepithelial numbers of CD4+FoxP3+ Tregs is associated with poor survival.45 It will be of interest to further evaluate additional links between the IDO pathway and other important immunological parameters in the tumor microenvironment in cervical cancer patients.
As we have shown, the activity of IDO can be easily measured in pre-treatment serum of cancer patients. This will allow the selection of patients who might benefit from treatment with IDO inhibitors. Various IDO inhibitors have been described and have already gone through Phase I cancer clinical trials, including 1-Methyl-D-tryptophan (Indoximod).46-49 Further studies with IDO inhibitors INCB024360, Indoximod and NLG919 are on-going and the use such inhibitors as sole treatment or in combination with chemotherapy in various advanced tumours is promising (www.clinicaltrials.gov; NCT02048709).
We believe the data presented here underscore the clinical potential of applying IDO inhibitors as a treatment modality for cervical cancer in the future.
Material and Methods
Patients
For measuring IDO activity, available pre-surgical treatment serum samples from all patients treated for cervical cancer between 2003 and 2008 at the Academic Medical Centre (AMC), Centre for Gynaecologic Oncology Amsterdam, The Netherlands were included (n = 251). None of the patients received chemo(radio)therapy before the serum was obtained. The main clinicopathological characteristics of the study cohort are summarized in Table 1.
Patient with early stage cervical cancer had radical hysterectomy plus lymph node resection by Okabayashi's modification of the Wertheim procedure, with complete intent. Patients with extended ‘inoperable’ cervical cancer (≥bulky stage IIA or IIB, but M0), received external beam radiotherapy to the pelvis (46 Gray (Gy) in fractions of 2.0 Gy) or extended ‘chimney’ fields included the para-aortic lymph node region (50.4 Gy in 1.8 Gy fractions) plus weekly concurrent chemotherapy (cisplatin, 40 mg/m2/week).
Patients with parametrial tumor invasion and/or lymph node metastases received an external radiotherapy boost up to 60 Gy.
Patient samples were handled according to the medical ethical guidelines described in the Code of Conduct for Proper Secondary Use of Human Tissue of the Dutch Federation of Biomedical Scientific Societies.
Measuring IDO Activity in Serum Samples
The concentration of tryptophan, kynurenine and 3-hydroxykynurenine was determined by an automated online solid-phase extraction-liquid chromatographic tandem mass spectrometric (XLC-MS/MS) method with deuterated internal standards exactly as described previously.8,50 Briefly, 50 μL plasma equivalent was pre-purified by automated on-line solid-phase extraction, using strong cation exchange (PRS, propylsulphonic) cartridges. Chromatographic separation of the analytes and deuterated analogues occurred by C18 reversed phase chromatography. Mass spectrometric detection was performed in the multiple reaction-monitoring mode using a quadrupole tandem mass spectrometer with positive electrospray ionization. Serum samples from 251 patients were analyzed. The Kyn/Trp ratio was calculated by dividing the amounts of the two metabolites. The concentrations of the different metabolites were measured in μmol/L.
Statistics
To determine whether serum concentrations of tryptophan, kynurenine, 3-hydroxykynurenine and the Kyn/Trp ratio were significantly different between groups, the non-parametric Mann-Whitney U test was used. Disease specific survival was defined as death by cervical cancer, while disease-free survival was defined as the time until disease recurrence. Patient survival rates were analyzed by Kaplan-Meier curves and the log-rank test and univariate/multivariate Cox proportional hazards models.
All tests were 2-sided, and p < 0.05 was considered to be statistically significant. Analyses were performed using SPSS, version 21.0 for Windows (SPSS, Inc, Chicago, IL).
Acknowledgements
We would like to thank Dr. Claude van der Ley and Dr. Renske de Jong from the UMCG and Wouter Opdam from the AMC for excellent technical assistance. Furthermore, we would like to thank Dr. Lukas Stalpers from the AMC for helping with the patient treatment description.
Disclosure of Potential Conflicts of Interests
No potential conflicts of interest were disclosed.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11-30. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC cancerbase No. 11. Lyon, France: international agency for research on cancer.; 2013 [April 8, 2014]; Available from: http://globocan.iarc.fr [Google Scholar]
- 3. Munoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, Snijders PJ, Meijer CJ. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518-27. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539-45. [DOI] [PubMed] [Google Scholar]
- 5. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74. [DOI] [PubMed] [Google Scholar]
- 6. Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013 Mar;34(3):137-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prendergast GC, Smith C, Thomas S, Mandik-Nayak L, Laury-Kleintop L, Metz R, Muller AJ. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother. 2014 Jul; 63(7):721-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. de Jong RA, Nijman HW, Boezen HM, Volmer M, Ten Hoor KA, Krijnen J, van der Zee AG, Hollema H, Kema IP. Serum Tryptophan and Kynurenine Concentrations as Parameters for Indoleamine 2,3-Dioxygenase Activity in Patients With Endometrial,Ovarian, and Vulvar Cancer. Int J Gynecol Cancer 2011; 21:1320-7. [DOI] [PubMed] [Google Scholar]
- 9. Uyttenhove C, Pilotte L, Théate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoural immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003; 10:1269-74. [DOI] [PubMed] [Google Scholar]
- 10. Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Göbel G, Margreiter R, Königsrainer A, Fuchs D, Amberger A. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumour-infiltrating T cells. Clin Cancer Res 2006; 12:1144-51. [DOI] [PubMed] [Google Scholar]
- 11. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 1991; 5:2516-22. [PubMed] [Google Scholar]
- 12. Corm S, Berthon C, Imbenotte M, Biggio V, Lhermitte M, Dupont C, Briche I, Quesnel B. Indoleamine 2,3-dioxygenase activity of acute myeloid leukemia cells can be measured from patients’ sera by HPLC and is inducible by IFN-y. Leukemia Res 2009; 33:490-4. [DOI] [PubMed] [Google Scholar]
- 13. Prendergast GC. Immune escape as a fundamental trait of cancer: focus on IDO. Oncogene 2008; 27:3889-900. [DOI] [PubMed] [Google Scholar]
- 14. Kolodziej LR, Paleolog EM, Williams RO. Kynurenine metabolism in health and disease. Amino Acids 2011; 41:1173-83. [DOI] [PubMed] [Google Scholar]
- 15. Creelan BC, Antonia S, Bepler G, Garrett TJ, Simon GR, Soliman HH. Indoleamine 2,3-dioxygenase activity and clinical outcome following induction chemotherapy and concurrent chemoradiation in Stage III non-small cell lung cancer. Oncoimmunol 2013; 3:e23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suzuki Y, Suda T, Furuhashi K, Suzuki M, Fujie M, Hahimoto D, Nakamura Y, Inui N, Nakamura H, Chida K. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 2010; 67:361-5. [DOI] [PubMed] [Google Scholar]
- 17. Chuang SC, Fanidi A, Ueland PM, Relton C, Midttun O, Vollset SE, Gunter MJ, Seckl MJ, Travis RC, Wareham N, Trichopoulou A, Lagiou P, Trichopoulos D, Peeters PH, Bas Bueno-de-Mesquita H, Boeing H, Wientzek A, Kuehn T, Kaaks R, Tumino R, Agnoli C, Palli D, Naccarati A, Aicua EA, Sánchez MJ, Quirós JR, Chirlaque MD, Agudo A, Johansson M, Grankvist K, Boutron-Ruault MC, Clavel-Chapelon F, Fagherazzi G, Weiderpass E, Riboli E, Brennan PJ, Vineis P, Johansson M. Circulating biomarkers of tryptophan and the kynurenine pathway and lung cancer risk. Cancer Epidemiol Biomarkers Prev 2014; 23:461-8. [DOI] [PubMed] [Google Scholar]
- 18. Sperner-Unterweger B, Neurauter G, Klieber M, Kurz K, Meraner V, Zeimet A, Fuchs D. Enhanced tryptophan degradation in patients with ovarian carcinoma correlates with several serum soluble immune activation markers. Immunobiol 2011; 216:296-301. [DOI] [PubMed] [Google Scholar]
- 19. Yoshikawa T, Hara T, Tsurumi H, Goto N, Hoshi M, Kitagawa J, Kanemura N, Kasahara S, Ito H, Takemura M, Saito K, Seishima M, Takami T, Moriwaki H. Serum concentration of L-kynurenine predicts the clinical outcome of patients with diffuse large B-cell lymphoma treated with R-CHOP. Eur J Haematol 2010; 84:304-9. [DOI] [PubMed] [Google Scholar]
- 20. Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005; 11:312-9. [DOI] [PubMed] [Google Scholar]
- 21. Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA, 3rd, Kahler DJ, Pihkala J, Soler AP, Munn DH, Prendergast GC, Mellor AL. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci USA. 2008; 105:17073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muller AJ, DuHadaway JB, Chang MY, Ramalingam A, Sutanto-Ward E, Boulden J, Soler AP, Mandik-Nayak L, Gilmour SK, Prendergast GC. Non-hematopoietic expression of IDO is integrally required for inflammatory tumor promotion. Cancer Immunol Immunother. 2010; 59:1655-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu J, Sun J, Wang S, Li H, Cao S, Cong Y, Liu J, Ren X. Upregulated expression of indoleamine 2,3-dioxygenase in primary breast cancer correlates with increase of infiltrated regulatory T cells in situ and lymph node metastasis. Clin Dev Immunol 2011;AI469135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chung DJ, Rossi M, Romano E, Ghith J, Yuan J, Munn DH, Young JW. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood 2009; 114:555-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jurgens B, Hainz U, Fuchs D, Felzmann T, Heitger A. Interferon-gamma-triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood 2009; 114:3235-43. [DOI] [PubMed] [Google Scholar]
- 26. Inaba T, Ino K, Kajiyama H, Shibata K, Yamamoto E, Kondo S, Umezu T, Nawa A, Takikawa O, Kikkawa F. Indoleamine 2,3-dioxygenase expression predicts impaired survival of invasive cervical cancer patients treated with radical hysterectomy. Gynecol Oncol 2010; 117:423-8. [DOI] [PubMed] [Google Scholar]
- 27. Sato N, Saga Y, Mizukami H, Wang D, Takahashi S, Nonaka H, Fujiwara H, Takei Y, Machida S, Takikawa O, Ozawa K, Suzuki M. Downregulation of indoleamine-2,3-dioxygenase in cervical cancer cells suppresses tumour growth by promoting natural killer cell accumulation. Oncol Rep 2012; 28:1574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamura T, Shima T, Saeki A, Hidaka T, Nakashima A, Takikawa O, Saito S. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci 2007; 98:874-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kobayashi A, Weinberg V, Darragh T, Smith-McCune K. Evolving immunosuppressive microenvironment during human cervical carcinogenesis. Mucosal Immunol 2008; 1:412-20. [DOI] [PubMed] [Google Scholar]
- 30. Fotopoulou C, Sehouli J, Pschowski R, von Haehling S, Domanska G, Braicu El, Fusch G, Reinke P, Schefold JC. Systemic changes of tryptophan catabolites via the indoleamine-2,3-dioxygenase pathway in primary cervical cancer. Anticancer Res 2011; 31:2629-35. [PubMed] [Google Scholar]
- 31. Li J, Cheng H, Zhang P, Dong Z, Tong HL, Han JD, Guo F, Tian YP. Prognostic value of combined serum biomarkers in predicting outcomes in cervical cancer patients. Clin Chim Acta. 2013 Sep 23; 424:292-7. [DOI] [PubMed] [Google Scholar]
- 32. Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology 2007; 214:8-14. [DOI] [PubMed] [Google Scholar]
- 33. Pantouris G, Mowat CG. Antitumour agents as inhibitors of tryptophan 2,3-dioxygenase. Biochem Biophys Res Commun 2014; 443:28-31. [DOI] [PubMed] [Google Scholar]
- 34. Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, Mellor AL. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity 2005; 22:633-42. [DOI] [PubMed] [Google Scholar]
- 35. Inaba T, Ino K, Kajiyama H, Yamamoto E, Shibata K, Nawa A, Nagasaka T, Akimoto H, Takikawa O, Kikkawa F. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol Oncol 2009; 115:185-92. [DOI] [PubMed] [Google Scholar]
- 36. Ino K, Yamamoto E, Shibata K, Kajiyama H, Yoshida N, Terauchi M, Nawa A, Nagasaka T, Takikawa O, Kikkawa F. Inverse correlation between tumoural indoleamine 2,3-dioxygenase expression and tumour-infiltrating lymphocytes in endometrial cancer: its association with disease progression and survival. Clin Cancer Res 2008; 14:2310-7. [DOI] [PubMed] [Google Scholar]
- 37. Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med 2002; 196:459-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol 2009; 182:1818-28. [DOI] [PubMed] [Google Scholar]
- 39. Li R, Wei F, Yu J. IDO inhibits T-cell function through suppressing Vav1 expression and activation. Cancer Biol Ther 2009; 8:1402-8. [DOI] [PubMed] [Google Scholar]
- 40. Sun J, Yu J, Li H, Yang L, Wei F, Yu W, Liu J, Ren X. Upregulated expression of indoleamine 2, 3-dioxygenase in CHO cells induces apoptosis of competent T cells and increases proportion of Treg cells. J Exp Cliun Canc Res 2011; 30:82-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smith C, Chang MY, Parker KH, Beury DW, DuHadaway JB, Flick HE, Boulden J, Sutanto-Ward E, Soler AP, Laury-Kleintop LD, Mandik-Nayak L, Metz R, Ostrand-Rosenberg S, Prendergast GC, Muller AJ. IDO is a nodal pathogenic driver of lung cancer and metastasis development. Cancer Discov. 2012. Aug; 2(8):722-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013. Apr 1; 190(7):3783-97. [DOI] [PubMed] [Google Scholar]
- 43. Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 2006; 176:6752-61. [DOI] [PubMed] [Google Scholar]
- 44. Baban B, Chandler PR, Sharma M, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol 2009; 183:2475-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jordanova ES, Gorter A, Ayachi O, Prins F, Durrant LG, Kenter GG, van der Burg SH, Fleuren GJ. Human leukocyte antigen class I, MHC class I chain-related molecule A, and CD8+/regulatory T-cell ratio: which variable determines survival of cervical cancer patients? Clin Cancer Res. 2008. Apr 1;14(7):2028-35. [DOI] [PubMed] [Google Scholar]
- 46. Fatokun AA, Hunt NH, Ball HJ. Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease. Amino Acids 2013; 45:1319-29. [DOI] [PubMed] [Google Scholar]
- 47. Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, Link CJ, Prendergast GC. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: a novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunol 2012; 1:1460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koblish HK, Hansbury MJ, Bowman KJ, Yang G, Neilan C, Haley PJ, Burn T, Waeltz P, Sparks RB, Yue EW, Combs AP, Scherle P, Vaddi K, Fridman JS. Hydroxyamidine Inhibitors of Indoleamine-2,3-dioxygenase Potently Suppress Systemic Tryptophan Catabolism and the Growth of IDO-Expressing tumours. Mol Cancer Ther 2010; 9:489-98. [DOI] [PubMed] [Google Scholar]
- 49. Dolušić E, Frédérick R. Indoleamine 2,3-dioxygenase inhibitors: a patent review (2008–2012). Expert Opin Ther Pat 2013; 23:1367-81. [DOI] [PubMed] [Google Scholar]
- 50. de Jong WH, Smit R, Bakker SJ, et al. Plasma tryptophan, kynurenine and 3-hydroxykynurenine measurement using automated on-line solid-phase extraction HPLC-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009; 877(7):603-9. [DOI] [PubMed] [Google Scholar]