Abstract
Myeloid-derived suppressor cells (MDSC) potently inhibit antitumor immune responses, and thereby promoti tumor progression and metastasis. However, the nature of human tumor-infiltrating MDSC remains poorly characterized. Here, we find B7-H3 is exclusively expressed on a subset of intratumoral CD14+HLA-DR−/low MDSC but absent from adjacent normal lung tissues of patients with non-small cell lung carcinoma (NSCLC). Cytokine analysis revealed that B7-H3+CD14+HLA-DR−/low MDSC (B7-H3+MDSC) produced higher levels of IL-10 and TNFα but lower levels of IL-1β and IL-6 when compared with B7-H3−CD14+HLA-DR−/low myeloid-derived suppressor cells (B7-H3−MDSC). In a murine lung cancer model, B7-H3+MDSCs were found only in the tumor microenvironment and their frequencies increased during tumor progression. Clinical data analysis indicated that a higher frequency of B7-H3+MDSCs was associated with reduced recurrence-free survival in patients with NSCLC. Taken together, we identify a novel subset of MDSCs within the tumor microenvironment that fosters tumor progression.
Keywords: B7-H3, MDSC, non-small cell lung cancer, Treg, Tumor microenvironment
Abbreviations: MDSC, Myeloid-derived suppressor cell; B7-H3+MDSC, B7-H3+CD14+HLA-DR−/low MDSC; B7-H3−MDSC, B7-H3−CD14+HLA-DR−/low MDSC; DC, dendritic cell; APC, antigen presenting cell; EAE, experimental autoimmune encephalomyelitis; NSCLC, Non-small cell lung carcinoma; FACS, Fluorescence activated cell sorter; RT-qPCR, real-time quantitative PCR; mTGFβ, membrane-bound TGFβ; LLC, Lewis Lung Carcinoma; BM, bone marrow
Introduction
Myeloid-derived suppressor cells (MDSCs) are significantly expanded in tumor-bearing animals and cancer patients, and have emerged as key immune modulators that suppress antitumor immune responses through a variety of mechanisms.1 One of the hallmarks of MDSCs are their heterogeneity, comprising precursors of macrophages, granulocytes, dendritic cells (DCs), and myeloid cells at different stages of differentiation.1 Despite the growing knowledge about MDSC development and function, many questions remain unresolved. Owing particularly to the heterogeneity of MDSCs, the different constituent subpopulations should be analyzed and characterized in more detail.
In mice, CD11b+Ly6G+Ly6Clow MDSCs have a granulocytic phenotype, whereas CD11b+Ly6G−Ly6Chigh cells display a monocytic phenotype.2,3 Human granulocytic MDSCs are negative for HLA-DR and CD14 but positive for CD15, CD66b, CD11b, and CD33, whereas monocytic MDSC are HLA-DR−/low while positive for CD14, CD11b, and CD33.1,4 MDSCs with granulocytic morphology have been reported in patients with metastatic renal cell carcinoma and non-small cell lung carcinoma.5,6 In contrast, levels of CD14+HLA-DR−/low MDSCs were found to be increased in blood from melanoma, hepatocellular carcinoma, and B-cell non-Hodgkin lymphoma patients.7-9 Despite extensive studies of peripheral blood MDSCs, relatively few studies have been done to phenotypically and functionally characterize the MDSC within the human tumor microenvironment. This is particularly important given the fact that peripheral MDSCs differ from tumor-infiltrating MDSCs in both murine and human cancer.10,11
The B7 superfamily of costimulatory molecules plays an important role in tumor immune responses. B7-H3, a member of the B7 superfamily, has been identified in both humans and mice by sharing ∼88% amino acid sequence identity.12,13 In general, B7-H3 is not expressed on freshly isolated human leukocytes but can be induced on antigen presenting cells (APCs). B7-H3 can function as a T-cell costimulator to increase T-cell proliferation and effector functions in vitro.12 In contrast, many data obtained in vivo support an immunosuppressive role for B7-H3. Blockade of B7-H3 using antibodies has been shown to increase the severity of experimental autoimmune encephalomyelitis (EAE) and allergic conjunctivitis.13,14 B7-H3 deficient mice develop severe EAE and airway inflammation.15 In human cancer, B7-H3 is detected on tumor cells arising from diverse tissues, and the expression level of B7-H3 is associated with increased disease severity.16,17 B7-H3 can therefore function as either a T-cell co-stimulator or co-inhibitor depending on the biological context.18 The exact role of B7-H3 in human cancer remains to be further delineated.
In this study, we found in non-small cell lung cancer (NSCLC) patients, B7-H3 is exclusively expressed on MDSCs present in cancerous tissues, but not on adjacent normal lung tissues or among blood cells. Likewise, we found B7-H3 to be expressed on the tumor-infiltrating Gr-1+CD11b+ subset of MDSCs in a murine lung carcinoma model and regulated by the tumor microenvironment. More importantly, we found higher frequencies of B7-H3+MDSCs correlating with poorer survival in NSCLC patients. Taken together, we have identified a novel B7-H3+ MDSC subset that resides exclusively in the tumor microenvironment, and promotes tumor progression.
Results
B7-H3 marks a novel subset of tumor -infiltrating CD14+HLA-DR−/low MDSCs
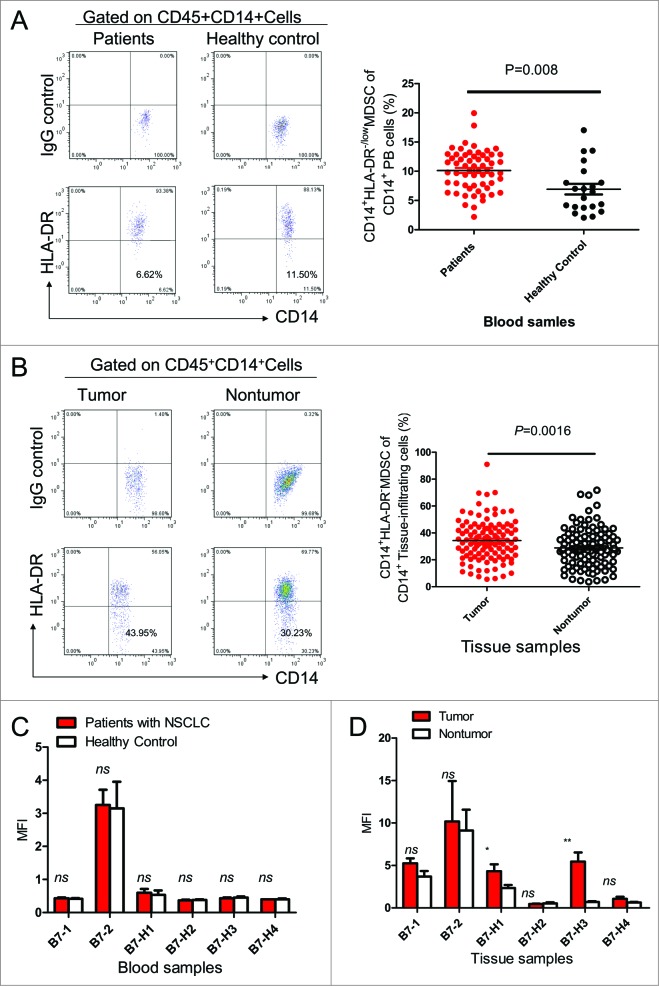
We first examined the levels of CD14+HLA-DR−/low MDSCs in circulation and in the tumor microenvironment of NSCLC patients. A significant increase in the percentage of CD14+HLA-DR−/low MDSCs was found within the CD45+CD14+ tumor-infiltrating leukocyte subset present in tumor sites as compared to adjacent normal lung tissues (Fig. 1A). Accumulation of higher frequencies of CD14+HLA-DR−/low MDSCs was also found in blood from patients with NSCLC relative to healthy donors (Fig. 1B). Theexpression level of the B7 gene family members did not differ significantly between blood derived MDSCs from cancer patients versus healthy donors (Fig. 1C). However, B7-H1 and B7-H3 were expressed at higher levels on tumor-infiltrating MDSCs than those present in normal lung tissues (Fig. 1D).
Figure 1.
Levels and characteristics of CD14+HLA-DR−/low MDSCs in NSCLC patients. (A) Representative dot plots and summarized data showing the levels of CD14+HLA-DR−/low myeloid-derived suppressor cells (MDSCs) in blood CD14+ leukocytes from healthy donors (n = 21) or non-small cell lung cancer (NSCLC) patients (n = 60). Statistical analysis was performed by Student's t-test (unpaired test) and data are presented as means ±SEM . (B) The flow cytometry gating strategy for analysis of tissue-infiltrating CD45+CD14+HLA-DR−/lowcells, representative dot plots and summarized data in paired non-tumor and tumor tissues from NSCLC patients (n=111). Statistical analysis was performed by Student's t test (paired test) and data are represented as means ±SEM . (C) B7-family costimulatory molecules were detected on CD14+HLA-DR−/low MDSC from tumor and corresponding normal lung tissues from NSCLC patients (n = 6). The representative plots from 6 patients are shown. Statistical analysis was performed by Student's t test (unpaired test) and data are represented as means ±SEM ; ns represents P > 0 .5. (D) B7-family costimulatory molecules were detected on tissue infiltrating CD45+CD14+HLA-DR−/low MDSCs from tumor sites and corresponding non-tumor tissues from NSCLC patients (n = 6). Statistical analysis was performed by Student's t test (paired test) and data are represented as means ±SEM; *P <0 .05, **P < 0 .01, ns represents P > 0 .5.
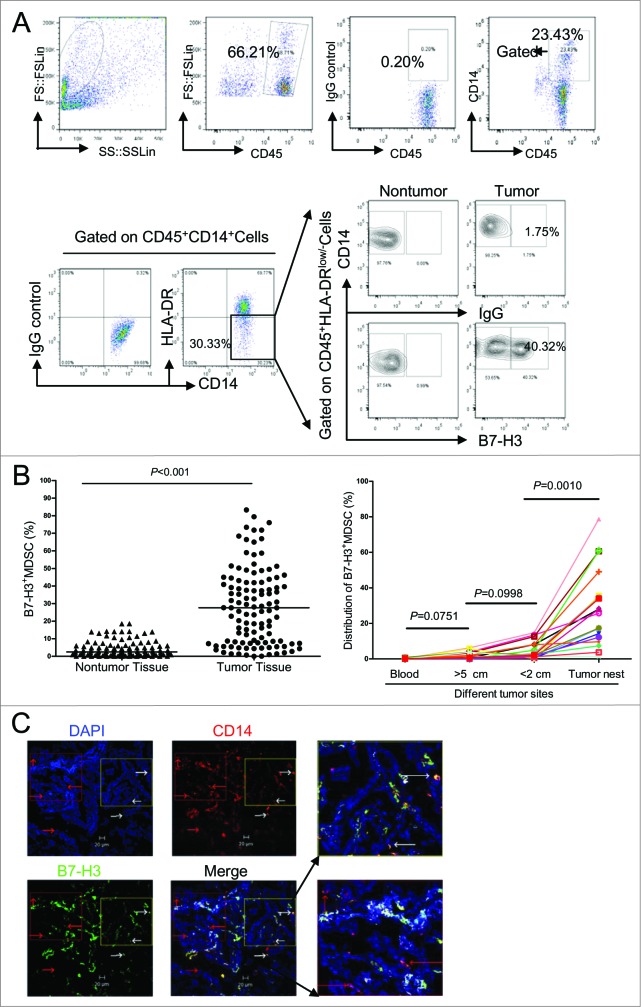
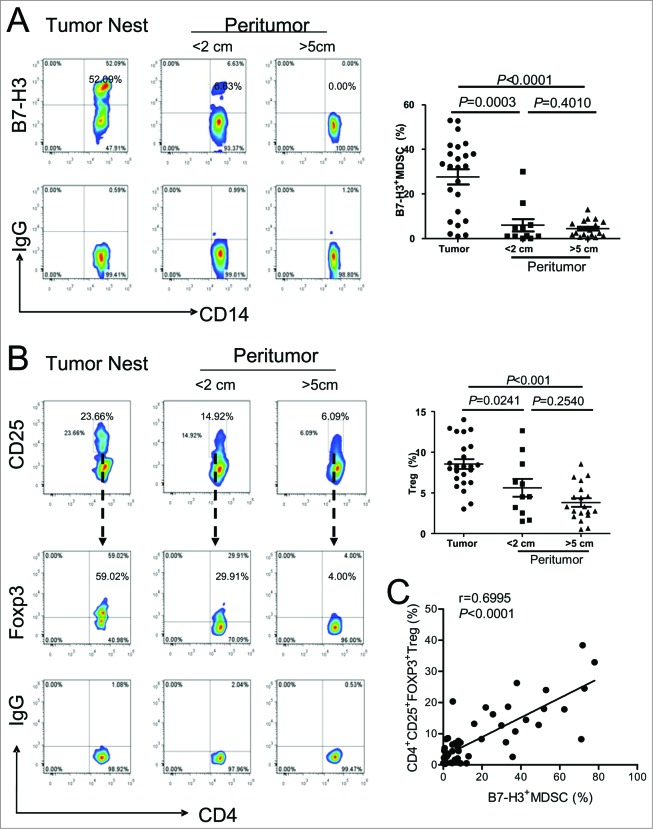
Interestingly, B7-H3 was upregulated in a portion of tumor-infiltrating CD14+HLA-DR−/low MDSCs, such that B7-H3 can be used as a marker to divide MDSC into 2 distinct subsets: B7-H3− and B7-H3+MDSCs (Fig. 2A). In order to analyze the tissue distribution of B7-H3+MDSC, we examined 111 matched non-tumor and tumor tissue samples from patients with NSCLC. The frequency of B7-H3+ MDSC in tumor tissues was much higher than those in the corresponding normal part of lung tissues (Fig. 2B). In fact, B7-H3+ MDSCs constituted approximately 30% of total CD14+HLA-DR−/low MDSC within the tumor microenvironment. In order to further determine the tumor field effect on the distribution of B7-H3+ MDSCs, matched cancer tissues, adjacent normal lung tissues resected at different distances from the tumor, and peripheral blood were collected from 17 patients. The percentages of B7-H3+MDSC were analyzed by multi-color fluorescence cytometry. The B7-H3+MDSC subset predominated among tumor-infiltrating MDSCs (Fig. 2C). The percentage of B7-H3+MDSC decreased proportional with distance from the lesion, such that these cells occurred less frequently in the peritumoral region, drastically decreased in the distal normal lung tissues, and was almost undetectable in peripheral blood. These data suggest that B7-H3 is exclusively induced in the tumor site and may serve as a unique marker for identifying tumor-infiltrating CD14+HLA-DR−/lowMDSC. We further confirmed the existence of B7-H3+ and B7-H3− subset of MDSCs in lung tumor samples (n = 5) using confocal microscopy (Fig. 2D). These data indicate that the B7-H3+MDSC subset are present predominantly within tumors, but virtually absent from corresponding normal lung tissues.
Figure 2.
Identification and quantitation of a novel subset of B7-H3+MDSC in the tumor microenvironment of NSCLC patients. (A) Identification of a novel subset of B7-H3 expressing myeloid-derived suppressor cells (B7-H3+MDSCs) in the tumor microenvironment. Single cells from tumor tissues or corresponding normal lung tissues from non-small cell lung cancer (NSCLC) patients (n = 111) were stained with fluorophore conjugated anti-CD45, anti-CD14, anti-HLA-DR, and anti-B7-H3 antibodies. Cells were gated on CD45+CD14+HLA-DR−/low cells, and the levels of the B7-H3+ MDSC subset was analyzed by fluorescence cytometry. (B) The levels of B7-H3+ MDSC subset from tumor tissues or corresponding normal lung tissues from NSCLC patients (n = 111). Wilcoxon matched pairs test were performed and data are represented as median (bar). (C) Distribution of B7-H3+MDSC at different sites in the same patients with NSCLC (n = 17). Wilcoxon matched pairs test were performed between blood vs >5 cm region, >5 cm region vs < 2 cm region, and <2 cm region vs tumor nest. (D) Tissue sections from tumor samples were stained with monoclonal antibodies (mAbs) against human B7-H3 and human CD14 and counterstained with DAPI and analyzed with confocal microscopy. B7-H3, green; CD14, red; DAPI, blue. B7-H3+CD14+ cells are saffron yellow. The micrographs at higher magnification showed the B7-H3+CD14+ cells (1) and B7-H3−CD14+ cells (2). Data are representative of 5 independent experiments using samples from 5 NSCLC patients. Scale bar, 20μm.
The character of B7-H3+ and B7-H3−MDSC subset
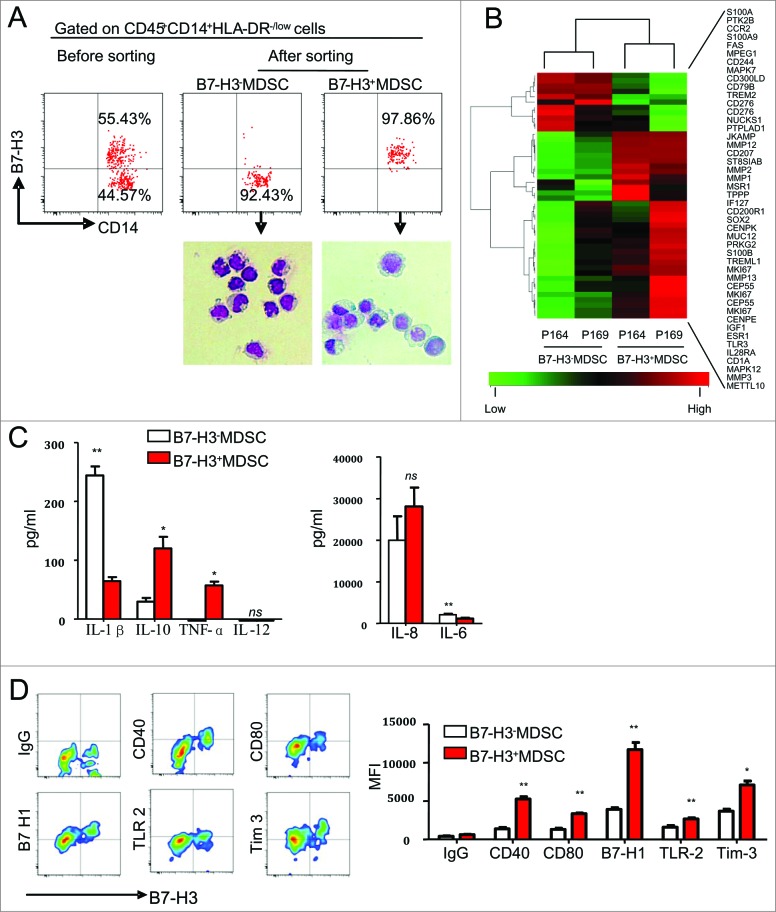
We sorted the pure B7-H3− and B7-H3+MDSCs using a fluorescence activated cell sorter (FACS) (Fig. 3A, up), after having first performed the morphologic characterization of these subsets by May-Grünwald-Giemsa staining. Both subsets were found to display monocytic morphology. We noted that the nuclear-cytoplasmic ratio of the B7-H3+MDSC subset was smaller than that of B7-H3−MDSCs (Fig. 3A, down), suggesting B7-H3+ MDSC might be more active in protein production. Additionally, real-time quantitative PCR (RT-QPCR) data showed elevated B7-H3 mRNA levels in B7-H3+MDSCs relative to B7-H3− MDSCs, indicating that B7-H3 gene expression differed between B7-H3− and B7-H3+MDSCs at the transcriptional level (data not shown). Next, we performed microarray to analyze the gene expression pattern of B7-H3− and B7-H3+MDSCs. As shown in Fig. 3B, the expression of some important adherence molecules, transcription factors (TFs) and matrix metalloproteinases (MMPs) were distinct in B7-H3− and B7-H3+ marker positive MDSCs, indicating unique functions for 2 subsets of MDSCs.
Figure 3.
Phenotypic characterization of B7-H3− and B7-H3+ MDSCs in the NSCLC tumor microenvironment. (A) Morphological characterization of purified B7-H3− and B7-H3+ myeloid derived suppressor cells (MDSCs) sorted from tumor tissues and analyzed by May-Grünwald-Giemsa staining. Representative images from 6 independent experiments are shown. (B) The gene expression profile of B7-H3+ and B7-H3−MDSCs were determined using Affymetrix oligoarrays. The selected heatmap shows the relative gene expression level. Red plots indicate genes up regulated in B7-H3+MDSC, and green plots indicate genes upregulated in B7-H3−MDSC. P164 and P169 represent 2 individual patients with NSCLC. (C) The supernatants of B7-H3− and B7-H3+ MDSC were determined for production of interleukines (IL) IL-1β, IL-6, IL-8, IL-10, IL-12p70, and tumor necrosis factor α (TNFα) using a Cytokine Bead Assay (CBA) and analyzed via flow cytometry. Statistical analysis was performed by Student's t-test (paired test) and data are presented as means ± SEM (n = 3). (D) Phenotypic analysis of different MDSC subsets. Cells were gated on B7-H3+ or B7-H3− CD45+CD14+HLA-DR−/low MDSC from NSCLC patients. One of 3 representative patient samples is shown (Left), and summarized data (Right) are shown. Statistical analysis was performed by Student's t- test (paired test) and data are presented as means ±SEM (n = 3); *P < 0 .05, **P < 0 .01.
To further characterize B7-H3− and B7-H3+MDSCs, we examined the profile of cytokines produced by these 2 populations. FACS-purified B7-H3− and B7-H3+ MDSC were stimulated with lipopolysaccharide (LPS) for 24 h and supernatants were collected for quantification of the interleukins (IL) IL-1β, IL-6, IL-8, IL-10, IL-12p70 and tumor necrosis factor α (TNFα). Our data indicated that B7-H3+MDSC produced significantly higher levels of IL-10 and TNF-α, but significantly lower levels of the IL-1β and IL-6 when compared with B7-H3−MDSC (Fig. 3C).
We next compared the surface phenotype between B7-H3+ and B7-H3− tumor-infiltrating MDSC by fluorescence cytometry. We found that CD83, TLR4, TLT-2, MICA/B, membrane-bound transforming growth factor β (mTGFβ), DCsign, CD86, CD137, CD117 and AC133 were expressed at similar levels on B7-H3+ and B7-H3−MDSC (data not shown). Interestingly, B7-H3+MDSC expressed higher levels of Tim-3, B7-H1, CD40, CD80 and TLR2 than B7-H3−MDSC (Fig. 3D). In contrast, B7-H3+MDSC expressed relatively lower levels of granulocyte macrophage colony stimulating factor receptor (GM-CSFR) than B7-H3− MDSC (data not shown).
Tumor microenvironment of lung carcinoma induces B7-H3+Gr-1+CD11b+MDSC
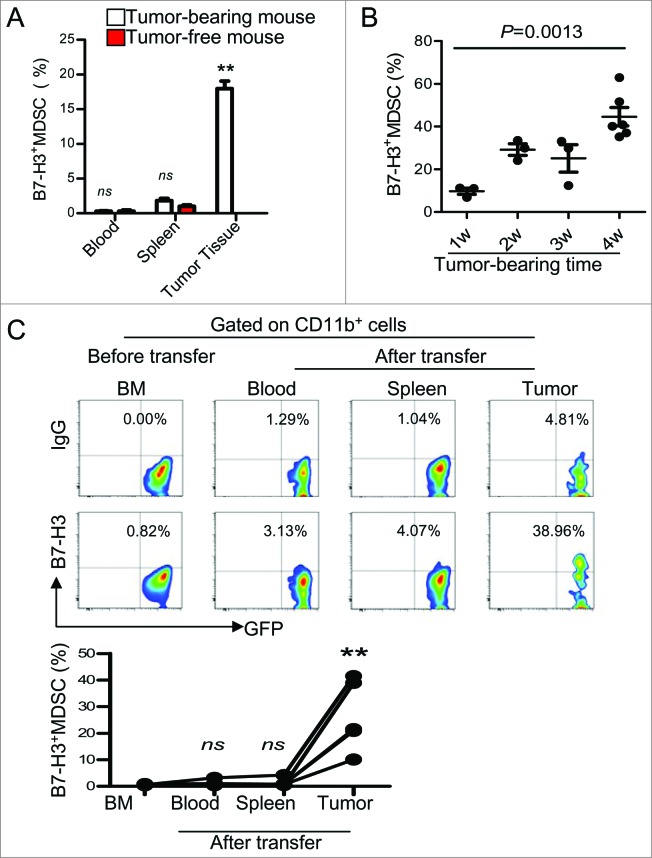
To examine the regulation of B7-H3 on MDSC in the tumor microenvironment, we established a subcutaneous Lewis Lung Carcinoma (LLC) model. Similar to human patients, a fraction of B7-H3+ cells was observed among GR-1+CD11b+MDSCs present in the tumor microenvironment (Fig. 4A). Additionally, the frequency of B7-H3+Gr-1+CD11b+ MDSCs increased coincidently with tumor growth (Fig. 4B).
Figure 4.
The tumor microenvironment promotes B7-H3+Gr-1+CD11b+MDSCs in a mouse lung carcinoma model. (A) B7-H3 expression on Gr-1+CD11b+ cells from the blood, spleen, and tumors of tumor-bearing Lewis Lung Carcinoma mice as well as tumor-free mice as determined by immunofluorescence staining and cytofluorimetric analysis.Summarized data of 3–5 mice are shown. Statistical analysis was performed by Student's t-test (between tumor-bearing mice and tumor-free mice) and data are presented as means ±SEM . For analysis of B7-H3 expression on MDSC from blood, spleen, and tumors of tumor-bearing mice, statistical analysis was performed by one-way ANOVA and data are presented as means ± SEM ; ** represents P<0 .01, not significant (ns) represents P > 0 .5. (B) Analysis of the frequency of B7-H3+ MDSC during tumor progression. Statistical analysis was performed by one-way ANOVA and data are presented as means ±SEM (n = 3–6). (C) Tumor microenvironment induced B7-H3 expression on GR-1+CD11b+MDSCs in vivo. Splenic B7-H3−Gr-1+CD11b+ cells from green fluorescence protein (GFP+) tagged transgenic mice were adoptively transferred into tumor-bearing mice via intravenous injection. One week later, B7-H3 expression on infused GFP+ cells was analyzed. Representative dot plots (up) and summarized data (down) are shown (n = 5). Statistical analysis was performed by one-way ANOVA and data are presented as means ±SEM ; ** represents P < 0 .01, not significant (ns) represents P > 0 .5.
To explore the effect of the tumor microenvironment on the induction of B7-H3+MDSCs, we adoptively transferred green fluorescence protein tagged B7-H3−MDSC (GFP+Gr-1+CD11b+cells) isolated from bone marrow (BM) of naïve C57BL/6J-EGFP+ mice into LLC tumor-bearing mice. One-week post infusion, a fraction of the adoptively transferred Gr-1+CD11b+ cells expressing B7-H3 were readily detectable in the tumor but not in the spleen or in the peripheral blood of tumor-bearing mice (Fig. 4C). These data suggest that B7-H3 expression on MDSCs is induced in the tumor microenvironment.
B7-H3+ and B7-H3−MDSCs suppress the proliferation of CD4+T cells but not CD8+T cells in vitro
To examine the suppressive effect of these 2 MDSC subsets on T-cell proliferation, B7-H3+ and B7-H3−MDSC were co-cultured with purified autologous T cells in the presence of anti-CD3 and anti-CD28 monoclonal antibodies (mAbs). Both subpopulations of MDSCs exhibited significant immunosuppressive activities on the proliferation of CD4+T cells specifically, sparing CD8+T cells. In fact, there was no significant difference in the CD4+ T-cell proliferation rate between co-cultures with B7-H3+or B7-H3−MDSCs (data not shown).
B7-H3+MDSCs induce Treg expansion in vitro and the frequency of B7-H3+ MDSCs correlates with that of Tregs within tumor microenviroment
To investigate the effect of B7-H3+MDSC on the expansion of regulatory T cells (Tregs), we first examined the frequency of Tregs and B7-H3+ MDSC in the tumor nest and matched normal tissues. We found B7-H3+MDSCs primarily accumulated in the tumor nest (Fig. 5A). This distribution pattern resembled that of CD4+CD25+FOXP3+ that also occur at markedly elevated levels in the tumor microenvironment than in either adjacent or distal normal lung tissues (Fig. 5B). Based on this observation, we subsequently analyzed the association between the frequency of tumor-infiltrating Tregs and B7-H3+MDSCs. We found a significant correlation between the occurrence of B7-H3+MDSCs and CD4+CD25+FOXP3+Tregs in NSCLC patient tumors (Fig. 5C). Thus, our data support the notion that one possible mechanism by which B7-H3+MDSCs promote cancer progression is through the induction of Tregs in the tumor microenvironment.
Figure 5.
Analysis of B7-H3+MDSC and Treg in tumor and normal lung tissue from patients of NSCLC. (A)The distribution of B7-H3+ CD14+HLA-DR−/lowMDSC and, (B) CD4+CD25+FoxP3+Tregs in tumor nest (n = 24), boundary (<2 cm, n = 11) and distant site of peritumoral tissues (>5 cm, n = 19). Statistical analysis was performed by Kruskal-Wallis test and data are presented as the median. Representative flow histogram (left) and summarized data (right) are shown respectively. (C) Correlation between the percentage of Tregs and B7-H3+MDSCs in tumor tissues (n = 54). Statistical analysis was performed by nonparametric correlation (Spearman).
Next, we sought to examine the effect of B7-H3+MDSCs on Treg expansion. Our data indicate that Treg co-cultures with B7-H3+MDSCs produce more CD4+FOXP3+Tregs than those cultured with either B7-H3−MDSCs or medium alone, suggesting that B7-H3+MDSCs can stimulate greater Treg expansion in vitro (Fig. 6A). As shown in Fig. 3C, we found that B7-H3+MDSC secreted higher levels of IL-10 as compared to corresponding B7-H3−MDSCs, consistent with the proposed role of IL-10 in promoting Treg expansion.20 Indeed, by co-culturing in the presence of anti-IL-10 blocking antibody, we found that the induction of Tregs by B7-H3+MDSC was at least partly dependent upon IL-10 (Fig. 6B).
Figure 6.
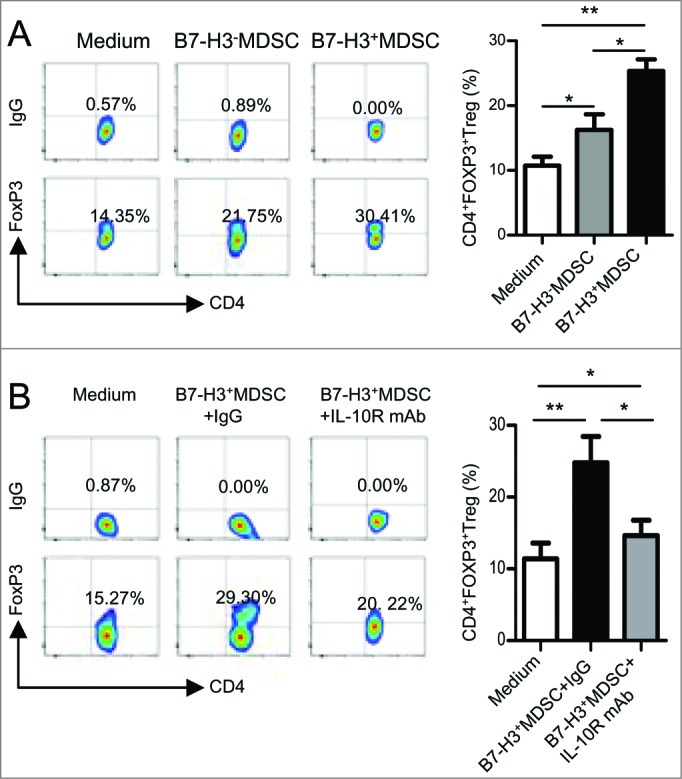
B7-H3+ MDSC more potently induced Treg in vitro. (A) The expansion of CD4+FOXP3+ Treg induced by B7-H3− or B7-H3+MDSC in vitro was examined by flow cytometric analysis. Cumulative results of 4 independent experiments are shown and data are presented as means ±SEM . One-way ANOVA was performed. *P < 0 .05, **P < 0 .01. (B) Induction of Treg by B7-H3+ MDSC was partly dependent on IL-10. The frequency of CD4+FOXP3+ Treg was examined by flow cytometric analysis. Cumulative results of 4 independent experiments are shown and data are presented as means ±SEM . Statistical analysis was performed by one-way ANOVA;*P < 0 .05, **P < 0 .01.
The frequency of B7-H3+ MDSCs correlates with lung cancer progression in NSCLC patients
To determine the clinical significance of B7-H3+MDSC, 111 NSCLC patients were enrolled and divided into 2 groups according to the frequency of B7-H3+MDSCs. For the negative or low group, the frequency of B7-H3+MDSCs was defined as less than or equal to 10%, whereas for the high group, the frequency of intratumoral B7-H3+MDSCs was greater than 10%. We found that the higher frequency of B7-H3+MDSC was associated with tumor invasion, tumor stage, and nodal metastasis (Table 1). Additionally, 61 patients, who had received surgical operation and had 2-year follow-up data available, were further subjected to survival analysis. Our data indicated that patients belonging to the high group of B7-H3+MDSCs had significantly shorter recurrence-free survival than those in the negative/low group of B7-H3+MDSCs (Fig. 7). These results suggest that the increase in the frequency of B7-H3+MDSC subset correlates with malignant disease progression of NSCLC. Thus, the frequency of B7-H3+MDSCs might serve as a poor prognostic marker for the survival of patients afflicted with NSCLC.
Table 1.
Clinical significance of tumor-infiltrating B7-H3+ MDSC in patients with NSCLC
| Tumor-infiltrating B7-H3+MDSC | |||||
|---|---|---|---|---|---|
| Characteristics | N | % | Negative/Lowa | Highb | P |
| Sex | |||||
| Male | 89 | 80.2 | 29 | 60 | 0.3691c |
| Female | 22 | 19.8 | 5 | 17 | |
| Age(year) | |||||
| ≥60 | 70 | 63.1 | 25 | 45 | 0.129c |
| <60 | 41 | 36.9 | 9 | 32 | |
| Histologic subtype | |||||
| Adenocarcinoma | 46 | 41.4 | 14 | 32 | 0.1309c |
| Squamous cell carcinoma | 38 | 34.2 | 8 | 30 | |
| Others | 27 | 24.3 | 12 | 15 | |
| Tumor differentiation | |||||
| Moderate | 74 | 66.7 | 23 | 51 | 0.9359d |
| Poor | 15 | 13.5 | 4 | 11 | |
| Unknown | 22 | 19.8 | 7 | 15 | |
| T factor | |||||
| 1+2 | 80 | 72.1 | 33 | 47 | < 0.0001d |
| 3+4 | 31 | 27.9 | 1 | 30 | |
| N factor | |||||
| 0 | 49 | 44.1 | 28 | 21 | < 0.0001c |
| ≥1 | 62 | 55.9 | 6 | 56 | |
| Tumor stage | |||||
| I | 33 | 29.7 | 24 | 9 | < 0.0001d |
| II | 30 | 27 | 6 | 24 | |
| III+IV | 48 | 43.2 | 4 | 44 | |
aB7-H3+MDSC≤10 %, n = 34.
bB7-H3+MDSC>10%, n = 77.
cChi-square or dFisher's exact test.
Figure 7.
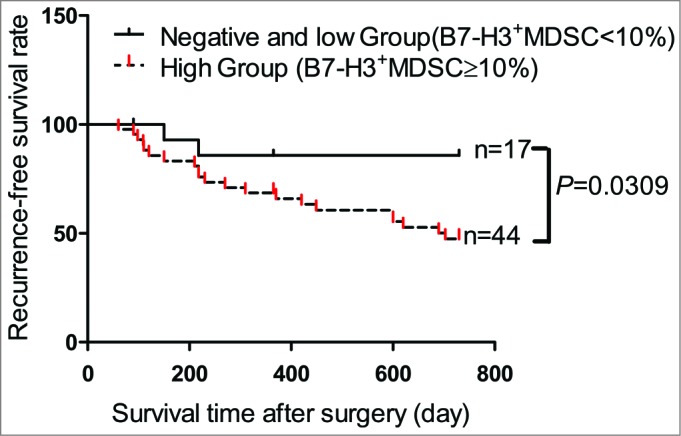
B7-H3+ MDSC serves as a poor prognostic marker for the survival of patients with NSCLC. The recurrence-free survival rate analysis of 61 patients with non-small cell lung cancer (NSCLC) who underwent surgical resection, according to the frequency of B7-H3 myeloid-derived suppressor cells (B7-H3+MDSCs) in tumor nests. The patients were divided into 2 groups according to the levels of B7-H3+MDSCs: dotted line, the patient group with negative or low levels of B7-H3 (the frequency of B7-H3 ≤10 %, n = 17); continuous line, the patient group with high levels B7-H3 (the frequency of B7-H3>10 %, n = 44). Statistical analysis was performed by Log-rank (Mantel-Cox) Test.
Discussion
The immune system is known to play a pivotal role in the development, progression and metastasis of human NSCLC. Although the precise mechanisms by which this pro-tumorigenic activity occurs remains elusive, clinical studies have shown that MDSCs are critical tumor-promoting immune cells correlating with poor clinical outcomes, including tumor metastasis and survival.19 Most of previous studies have focused on the prevalence and functional activity of these cells in the periphery, MDSCs within the human tumor microenvironment have yet to be well characterized.1 In this study, we used matched blood and tissue samples from same NSCLC patients to investigate the phenotype of MDSCs from blood, lung cancer lesions and matched normal lung tissues. We discovered a novel subset of human MDSCs that express high levels of the costimulatory molecule B7-H3 and reside primarily within the tumor tissue. More importantly, the frequency of B7-H3+MDSCs correlates with worse clinical and pathological parameters of lung cancer (versus low expressers) and is a poor prognostic marker for recurrence-free survival of NSCLC patients.
The expression and biological role of costimulatory molecules, such as CD40, CD80 and DC-sign, on MDSCs has attracted a great deal of attention in recent years.21-24 In this study, we found that B7-H3 was almost exclusively expressed on tumor-infiltrating MDSCs and largely devoid of expression among peripheral blood MDSCs in lung cancer patients. Our data suggest that the tumor microenvironment is causal, inducing a portion of MDSCs to express B7-H3. In support, the closer the sampled tissues were to the tumor, the higher the frequency of B7-H3+MDSCs. In vivo, B7-H3−MDSC can be converted into B7-H3+MDSC primarily within the tumor and not in the blood or spleen, suggesting that the tumor microenvironment context is crucial to induce B7-H3 expression on MDSCs. Although the exact immunological function of B7-H3 remains to be defined, our current study demonstrates that B7-H3 may serve as a marker expressed on a subset of tumor-infiltrating MDSCs and reflecting the unique immunomodulatory effects of the tumor on MDSCs. We also made attempts to determine which factor(s) is important for B7-H3 expression on human intratumoral MDSCs. Using sorted B7-H3−CD14+HLA-DR−/low from PB, we found that IL-6, IL-15, LPS and malignant pleural fluid could induce B7-H3 expression. However, these factors also stimulate HLA-DR high expression (data not shown). Thus, it remains unclear as to whether these factors are indeed responsible for inducing B7-H3 expression on CD14+HLA-DR−/low MDSCs in vivo. Of note, using sorted mouse B7-H3−CD11b+Gr-1+MDSCs, we found that LPS, IFNγ, and tumor infiltrated supernatants (TIS) induced B7-H3 expression on Gr-1+CD11b+ cells in vitro (data not shown).
To examine the suppressive effect of these 2 MDSC subsets on T-cell proliferation, B7-H3+ and B7-H3−MDSC were co-cultured with purified autologous T cells in the presence of anti-CD3 and anti-CD28 mAbs. Both subpopulations of MDSCs exhibited significant suppressive activities on the proliferation of CD4+T cells but not CD8+T cells (data not shown). In addition, there was no significant difference in CD4+ T cell proliferation between cultures containing either, or both, B7-H3+ and B7-H3−MDSCs (data not shown).
Although mouse models have established that the induction of Tregs is a routeby which MDSCs suppress T-cell functions, 22,25 results from human studies have so far been inconsistent.8,11,22 Hoechst et al. reported that CD14+HLA-DR−/low MDSC from hepatocellular carcinoma patients induced Treg expansion in vitro.8 In contrast, Poschke and colleagues did not observe Treg induction after co-culturing blood-derived MDSCs with autologous T cells.22 In addition, a correlation between blood MDSCs and Treg frequency was not apparent in patients with advanced malignant melanoma.11 Therefore, the exact MDSC subsets that are responsible for the induction of Tregs in human cancer remain to be fully established. In the current study, we find the levels of tumor-infiltrating B7-H3+MDSCs correlate significantly with the frequency of CD4+Foxp3+T cells present in the tumors (Fig. 5C). Further in vitro studies confirm that B7-H3+MDSCs significantly enhanced expansion of CD4+Foxp3+T cells (Fig. 6A), consistent with the idea that B7-H3+MDSCs are involved in Treg induction in the tumor. Therefore, our study identifies a unique subset of MDSCs, characterized by their expression of B7-H3 as well as their intratumoral location, which seems to be primarily responsible for the induction of Tregs in human lung cancer within the tumor microenvironment.
Cytokine analysis showed that B7-H3+MDSCs produce more IL-10 as compared to B7-H3−MDSCs. Of particular relevance, IL-10 has been previously shown to play a key role in the MDSC-solicited induction of Tregs.20 Huang et al. and Pan et al. also reported the induction of Treg by MDSC required the activation of tumor-specific T cells and the presence of IFNγ and IL-10.23,25 Our in vitro data further support the premise that IL-10 is a major factor involved in Treg induction by B7-H3+MDSCs (Fig. 6B), although other factors may also contribute to tumor-infiltrating Treg induction. For example,we found that the co-stimulatory molecule CD40 is highly expressed on B7-H3+MDSCs (Fig. 3D), a factor previously shown to be involved in Treg induction in other cancer contexts.23 CD40 likely has similar Treg regulatory effects in lung cancer, and future studies are needed to further address this question in detail.
We also sought to determine the clinical significance of B7-H3+MDSCs in NSCLC patients in our study. We found that a higher frequency of this novel MDSC subset correlated with a poor TNM stage and tumor metastasis (Table 1). Survival analysis also indicated that the higher percentage of B7-H3+MDSC may serve as a poor predictor for recurrence-free survival of patients with NSCLC. Taken together, these results suggest that B7-H3+MDSC play an important role in NSCLC malignant disease progression.
Based on the current findings, we propose a novel mechanism of tumor-mediated immune evasion. Tumor cells secrete a number of factors that stimulate MDSC accumulation in the periphery and within the neoplasm. In the complex signaling environment of the tumor milieu, a subset of intratumoral B7-H3 expressing MDSCs is induced, an immunosuppressive immune cell subset that promotes tumor progression. These tumor-associated B7-H3+MDSCs may further stimulate tumor metastasis, ultimately leading to patient mortality. Taken together, our findings identify B7-H3+ MDSCs as a novel immunomodulatory cell subset with the potential to enhance human cancer progression via mechanisms that remain to be fully elucidated.
Material and Methods
Patients and specimens
Fresh tumor tissues and corresponding non-tumor tissues were obtained from 111 untreated patients with pathologically confirmed NSCLC at the Department of Thoracic Surgery, the First Affiliated Hospital of Soochow University. Lobectomy with radical mediastinal and hilar lymphadenectomy was performed on all patients. Cancer staging was based on TNM of the International Union against Cancer. Blood samples were collected by venipuncture technique from patients before operation and from healthy donors who underwent physical examination. Tumor tissues were taken from areas of solid tumor tissues lacking the gross aspect of massive necrosis. The tumor-free normal lung tissue samples were taken at least 5 cm away from the tumor margin; the tumor-boundary lung tissue samples were taken at most 2 cm away from the tumor margin. Fresh tumors and corresponding normal tissues were used for the isolation of tissue-infiltrating leukocytes. The clinical characteristics of all patients are summarized in Table 1. This study was approved by the ethics committee of the First Affiliated Hospital of Soochow University (Suzhou, China) for clinical investigation, and written informed consent was obtained from patients or their relatives before enrollment.
Preparation of single cell suspensions
Single cell suspensions of lung tumor samples were obtained after digestion according to our previous report.19
Flow cytometry and antibodies
Blood leukocytes and tissue-infiltrating leukocytes were stained with fluorochrome-conjugated mAbs and then analyzed by multicolor cytofluorimetry (Beckman-Coulter, Altra, USA). Isotype-matched antibodies were used with all the samples as controls. The fluorophore-conjugated mAbs used in this study are as follows: anti-human CD14-PC5 (IM2640U), CD14-ECD (IM2707U), CD25-PC5 (IM2646U), CD80 (B7–1)-PE (IM1976U), CD83-PE (IM2218U), HLA-DR-ECD (IM3636), DC-sign-PE (IM2218U) were from Beckman Coulter (USA); CD14-PC7 (325618), CD86 (B7–2)-PE (305438), B7-H1-PE (329706), B7-H3-PE (331606), TIM-3-PE (345005), CD40-PE (334307), CD45-PC5 (304010), CD4-PC7 (317414), CD3-PC5 (300310), FOXP3-PE (320108), CD25-PC5 (302607) were from Biolegend (USA); B7-H3-FITC (FAB1027F), B7-H3-PE (FAB1027P), GM-CSFR-PE (FAB706P), were from R&D system (USA); TLR2-PE (12–9024), B7-H4-PE (12–5949–71), CD40-FITC (12–0409), B7-H2-PE (12–5889) were from eBioscience. TLT-2-PE (563661) was from BD Bioscience (USA). Anti-human B7-H3 (2E6) was produced by our laboratory and labeled with Fluorescein Labeling Kit-NH2 (Dojindo Molecular Technologies, Inc.); anti-Mouse CD11b-PC7 (101215), Gr-1-FITC (108405), Gr-1-PC5 (108409), B7-H1-PE (124307), B7-H4-PE (128107) were from Biolegend (USA); B7-H3-PE (12–5973; eBioscience). The purified anti-human CD210 (IL-10R) antibody (308805) for blocking of IL-10 binding to IL-10 receptor was from Biolegend (USA). The cells were subject to analysis at the Flow Cytometry Facility (Beckman-Coulter, Altra, USA) and the FLOWJO software (version 7.6.5) was used to process the data.
Cell purification
For human samples, CD3+ and CD4+ T cells were magnetically enriched using the EasySep™ Human T Cell Enrichment Kit (19051, STEMCELL Technologies) and StemSep™ Human CD4 Positive Selection Kit (18052, STEMCELL Technologies) respectively from the peripheral blood mononuclear cells (PBMCs) of patients with NSCLC. B7-H3+ and B7-H3−MDSCs were purified from tumor-infiltrating leukocytes using the Beckman Coulter MoFlowTM cell sorting system (Beckman Coulter), and the purity of the cells used for the study was more than 90%.
For mouse samples, MDSCs were purified by Myeloid-Derived Suppressor Cell Isolation Kit (130–094–538, Miltenyi Biotec) from spleen or bone marrow. The purity of Gr-1+CD11b+ cells used for the experiments was more than 90%.
Immunostaining and microscopy
Fixed tumor tissue sections (10 μm) were stained first with antibodies against human CD14 and then with secondary antibody anti-mouse IgG labeled with Cy3 (red). After thorough washing, anti-B7-H3-FITC (green) was used to stain the section. DAPI (Blue) DNA staining was then performed in the analysis of tissue-infiltrated CD14+ cells. Confocal microscopy was performed on an Axiovert.200M inverted microscope using a Zeiss LSM 510 Meta scan head (Carl Zeiss, Göttingen, Germany). To limit the nonspecific staining, HLA-DR molecule was not analyzed in the same section.
Morphological characteristics of B7-H3− and B7-H3+MDSC
The two MDSC subsets were sorted from tumor tissue-infiltrating leukocytes as described above. The morphology of these cells was analyzed by May–Grünwald-Giemsa staining and imaged via microscopy (200×) and the photos were then acquired.
Affymetric Genechip Assay
The sorted 2 × 105 B7-H3− versus B7-H3+ MDSCs were collected and resuspended in Trizol (15596–026, Invitrogen). The samples were stored in −80°C for Affimetrix oligonucleotide genechip assay. The microarray was done by CapitalBio Corporation (Beijing, china). Data were analyzed using CapitalBio Molecule Annotation System V3.0 (MAS3.0).
Cytokine analysis
The FACS-sorted B7-H3− and B7-H3+MDSC (1 × 105 /ml) from the same tumor sample of each NSCLC patient (n = 3) were stimulated by 1 μg/mL, LPS (L5293, Sigma) for 24 h. Cell free supernatants were collected for the cytokine analysis. To measure cytokines such as IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNFα, a cytokine bead assay (CBA) was performed using a CBA immunoassay kit (CBA Human Inflammatory Cytokines Kit, 551811, BD Biosciences) according to the manufacturer's instructions. Data were acquired using a flow cytometer (FC500; Beckman-Coulter, Altra, USA).
Treg induction assay
Purified CD4+T cells (1×105 /well) were co-cultured with either tumor-infiltrating B7-H3+ or B7-H3−MDSCs (2.5×104 /well) and Tregs were induced using human Treg Expansion Kit (130–095–345, Miltenyi biotec.) plus 50 unit/mL rhIL-2 ( 202-IL-010, R&D Systems) for 5d. In blocking experiments, 1 μg/mL anti-IL-10R mAb was added to B7-H3+CD14+HLA-DR−/low cells. The proportion of CD4+FOXP3+ Tregs was assessed by flow cytometry.
Mice and tumor model
C57BL/6J and C57BL/6J-EGFP+ mice were purchased from Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). Mouse lung cancer cell line LLC were originally obtained from American Type Culture Collection (ATCC, Rockville, MD). All animal experiments were approved by the IACUC (Institutional Animal Care and Use Committee) of Soochow University.
Mouse LLC cells (1 × 106) were injected subcutaneously in 100 μL phosphate buffered saline (PBS) in the left flank of 6-week-old mice. Blood, spleen and tumor tissues from tumor-bearing mice or tumor-free mice were collected for analysis of expression of B7-H3 on Gr-1+CD11b+ MDSC. To analyze B7-H3 induction in vivo, Gr-1+CD11b+ cells isolated from bone marrow of EGFP-transgenic mice (C57BL/6J-EGFP+) were injected into tumor-bearing mice via caudal vein (1×106 /mouse). The infused GFP+MDSC were collected 7 d post transfer, and B7-H3 expression was subsequently examined by flow cytometry.
Statistical analysis
Statistical analysis for normally distributed values was performed using Student's t test or ANOVA. Non-normally distributed values, as assessed by the Kolmogorov-Smirnov test, were analyzed by the Mann-Whitney U test. Correlations between MDSCs and Tregs were assessed by Spearman's correlation test. Patient survival was analyzed by Log-rank (Mantel-Cox) test. P values <0 .05 were considered statistically significant. Statistical analysis was done with GraphPad Prism 5.0 software (version 5.01).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the National Natural Science Foundation of China (Grants 81372276 to G.Z., 31320103918 to X.Z., 30930085 to J.H., 31170866 to Y.Z., 31770834 to C.L.), National Basic Research Development Program of China (973 program, 2013CB53051 to X.Z.) and by Natural Science Foundation of Jiangsu (BK20131158 to G.Z.).
References
- 1. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181:5791-802; PMID:; http://dx.doi.org/ 10.4049/jimmunol.181.8.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu B, Kennedy JK, Wang Y, Sandoval-Garcia C, Cao L, Xiao S, Wu C, Elyaman W, Khoury SJ. Plasticity of Ly-6C(hi) myeloid cells in T cell regulation. J Immunol 2011; 187:2418-32; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1100403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brandau S, Moses K, Lang S. The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: Cousins, siblings or twins? Semin Cancer Biol 2013; 23:171-82; PMID:; http://dx.doi.org/ 10.1016/j.semcancer.2013.02.007 [DOI] [PubMed] [Google Scholar]
- 5. Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res 2005; 65:3044-8; PMID: [DOI] [PubMed] [Google Scholar]
- 6. Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14⁻/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2010; 136:35-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol 2007; 25:2546-53; PMID:; http://dx.doi.org/ 10.1200/JCO.2006.08.5829 [DOI] [PubMed] [Google Scholar]
- 8. Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008;135:234-43; PMID:; http://dx.doi.org/ 10.1053/j.gastro.2008.03.020 [DOI] [PubMed] [Google Scholar]
- 9. Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood 2011;117:872-81; PMID:; http://dx.doi.org/ 10.1182/blood-2010-05-283820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 2010; 207:2439-53; PMID:; http://dx.doi.org/ 10.1084/jem.20100587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA. Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin Cancer Res 2012; 18:5212-5223; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol 2001; 2:269-74; PMID:; http://dx.doi.org/ 10.1038/85339 [DOI] [PubMed] [Google Scholar]
- 13. Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, Dong C. Murine B7-H3 Is a Negative Regulator of T Cells. J Immunol 2004; 173:2500-6; PMID:; http://dx.doi.org/ 10.4049/jimmunol.173.4.2500 [DOI] [PubMed] [Google Scholar]
- 14. Fukushima A, Sumi T, Fukuda K, Kumagai N, Nishida T, Yamazaki T, Akiba H, Okumura K, Yagita H, Ueno H. B7-H3 regulates the development of experimental allergic conjunctivitis in mice. Immunol Lett 2007; 113:52-7; PMID:; http://dx.doi.org/ 10.1016/j.imlet.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 15. Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol 2003; 4:899-06; PMID:; http://dx.doi.org/ 10.1038/ni967 [DOI] [PubMed] [Google Scholar]
- 16. Lemke D, Pfenning PN, Sahm F, Klein AC, Kempf T, Warnken U, Schnölzer M, Tudoran R, Weller M, Platten M, et al. Costimulatory Protein 4IgB7H3 Drives the Malignant Phenotype of Glioblastoma by Mediating Immune Escape and Invasiveness. Clin Cancer Res 2012; 18:105-17; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-0880 [DOI] [PubMed] [Google Scholar]
- 17. Arigami T, Narita N, Mizuno R, Nguyen L, Ye X, Chung A, Giuliano AE, Hoon DS. B7-h3 ligand expression by primary breast cancer and associated with regional nodal metastasis. Annals of surgery 2010; 252:1044-51; PMID:; http://dx.doi.org/ 10.1097/SLA.0b013e3181f1939d [DOI] [PubMed] [Google Scholar]
- 18. Hofmeyer KA, Ray A, Zang X. The contrasting role of B7-H3. Proc Natl Acad Sci U S A 2008;105:10277-8; PMID:; http://dx.doi.org/ 10.1073/pnas.0805458105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B. TIM-3 Expression Characterizes Regulatory T Cells in Tumor Tissues and Is Associated with Lung Cancer Progression. PLoS ONE. 2012; 7(2): e30676; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0030676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ma G, Pan PY, Eisenstein S, Divino CM, Lowell CA, Takai T, Chen SH. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity 2011; 34:385-95; PMID:http://dx.doi.org/ 10.1016/j.immuni.2011.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011; 60:1419-30; PMID:; http://dx.doi.org/ 10.1007/s00262-011-1028-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res 2010; 70:4335-45; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3767 [DOI] [PubMed] [Google Scholar]
- 23. Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, Chen SH. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res 2010; 70:99-108; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fujimura T, Ring S, Umansky V, Mahnke K, Enk AH. Regulatory T Cells Stimulate B7-H1 Expression in Myeloid-Derived Suppressor Cells in ret Melanomas. J Invest Dermatol 2012; 132:1239-46; PMID:; http://dx.doi.org/ 10.1038/jid.2011.416 [DOI] [PubMed] [Google Scholar]
- 25. Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006; 66:1123-31; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-1299 [DOI] [PubMed] [Google Scholar]