Abstract
B-precursor acute lymphoblastic leukemia (BP-ALL) patients are immunocompromised. We recently reported that functional natural killer (NK) cells can be grown from patient bone marrow and blood samples at diagnosis. Surprisingly, such NK cells exhibit cytotoxicity against autologous BP-ALL cells. Here, we outline unanswered questions, challenges and possible applications associated with these findings.
Keywords: ADCC, adoptive cell therapy, artificial antigen presenting, autologous, BP-ALL, expanded NK, pre-B ALL
BP-ALL involves massive expansion of one type of hematopoietic cell at the expense of normal hematopoiesis, which may be further compromised by chemotherapy. As a consequence, numbers of endogenous innate immune effectors, such as NK cells, are likely to be severely reduced. In addition, it is not clear if residual NK populations include cells with ability to mount spontaneous or antibody-stimulated cytotoxicity against ‘self’ BP-ALL cells.
Classical NK cells are part of the innate immune system and are generated de novo from bone marrow precursors. However, it is possible to significantly expand NK cell numbers even from peripheral blood by ex vivo co-culture with artificial antigen-presenting cells.1-3 When we found that clinical pediatric BP-ALL samples retained at least some NK cells, we asked whether it would be possible to expand their numbers through co-culture with artificial antigen-presenting K562 cl9.mbIL-21 cells. Surprisingly, CD56+ CD3-NK cells grew out from all 19 primary pre-B ALL peripheral blood or bone marrow co-culture samples. Moreover, we measured significant autologous spontaneous and antibody-stimulated activity of NK cells amplified from four pediatric BP-ALLs samples.4
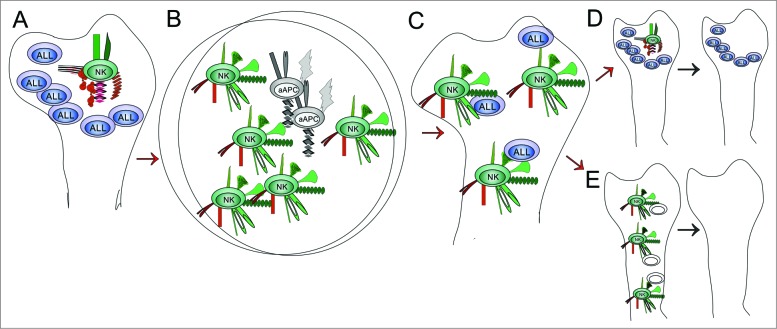
Autologous adoptive cell therapy with ex vivo expanded NK cells could have significant applications. However, many questions are raised, and answering them would seem critical to be able to formulate the most effective uses. The exact mechanisms that yield a cohort of NK cells able to lyse autologous BP-ALL cells in the presence or absence of antibodies are unknown. The initial population of NK cells used for expansion presumably lacks anti-leukemia activity (Fig. 1A). Up to 30,000 phenotypically distinct NK subsets may exist, probably generated by environmental cues.5 Thus, ‘educated’ cells within NK populations in the microenvironment of BP-ALL may express excessive inhibitory KIRs for MHC class I and ‘non-educated’ NK cells may also be present that became anergic due to chronic overstimulation by the presence of BP-ALL cells.6 Although we do not know if co-culture with K562 cl9.mbIL-21 cells in the presence of IL-2 causes selective outgrowth of a specific subset of NK cells present as a minority in the original population, it is plausible that all the NK cells expanded in the absence of autologous MHC class I are ‘uneducated’ and also express high levels of activating receptors (Fig. 1B). In view of the probable plasticity of NK cells,7 the question then arises to what extent these cytotoxic properties persist when these cells are re-introduced into the microenvironment of the BP-ALL cells (Fig. 1C). If the tumor burden is high, NK cells may be overwhelmed by frequent encounters with autologous ALL cells in the bone marrow, leading to loss of cytotoxic phenotype (Fig. 1D). This could be exacerbated by a low glucose/high lactate microenvironment modified by the presence of massive numbers of BP-ALL cells, or the lack of NK activity-promoting cytokines. Based on this, we propose that adoptive cell therapy with expanded NK cells alone or in combination with antibodies may be most effective under circumstances in which lower BP-ALL cell numbers are present and the bone marrow microenvironment is pro-inflammatory, that is, after induction therapy and upon evidence for the presence of minimal residual disease.
Figure 1.
Fate of autologous NK cells expanded ex vivo. NK cells express a number of different activating (green) or inhibitory (red) receptors. NK cells present in the bone marrow of patients with BP-ALL are presumed to not, or no longer, react to autologous leukemia cells, which should be recognized as abnormal (A). Once removed from the tumor microenvironment and exposed ex vivo to artificial antigen-presenting K562 cl9 mbIL21 cells, the NK cells encounter a novel combination of co-stimulatory signals in the absence of self MHC class I, resulting in vigorous outgrowth and increased expression of CD16 and NKG2D (B). These expanded NK cells now ‘see’ autologous BP-ALL cells as valid targets for cytotoxicity (C). The long-term persistence of this phenotype in vivo is unknown, and can result in the renewed development of ignorance/indifference regarding the presence of abnormal autologous BP-ALL cells (D) or eradication of BP-ALL (E).
On the other hand, a renewed encounter with autologous BP-ALL cells in the bone marrow may be a stimulus for persistent cytotoxicity of expanded NK cells (Fig. 1E). There is now evidence from mice that NK cells can remember and react strongly to previously encountered antigens. The 900-fold expansion of mouse NK cells when re-exposed to MCMV 8 in vivo suggests that the co-cultures used by us and others to expand NK cells may be an approximation of what happens in nature. Since K562 cl9.mbIL-21 cells are (Ph-positive) leukemia cells, and were moreover engineered to express a truncated form of the B-lineage marker CD19, NK cells co-cultured with them may have been programmed to express a combination of activating and inhibitory receptors that are particularly useful to attack BP-ALL cells.
If the activated NK cell phenotype is rapidly lost in vivo, repeated infusions of very large numbers of ex vivo-activated NK cells may be needed. Because these are autologous cell preparations, the concern for contaminating T cells that could cause GvHD does not seem to be an issue. Also, no past clinical or preclinical studies have documented any adverse effects attributable to NK cells obtained and expanded using a variety of methods, providing empirical evidence for the general ability of NK cells to distinguish ‘cancer self’ from ‘non-cancer self’. Nonetheless, it is currently not clear if the changes in activating and inhibiting receptor expression that we and others have measured by FACS on the surface of activated NK cells1-4 and that are consistent with expansion of an NK subset that is not ‘educated",6 could also result in targeted or accidental elimination of non-cancer cells such as those of the endothelium.
Specificity and effectiveness of NK cells could be enhanced by use of antibodies, such as those against the BAFF-R.9 A relatively proximal clinical application of autologous expanded NK cells could be in purging of stem cell grafts for autologous bone marrow transplants in elderly patients. The presence and carryover of possible contaminating T-cells in the NK cell preparation would not be a confounding factor, and the eradication of residual leukemia cells in this application would be relatively easy to accomplish because NK cells can be used that are freshly activated. An important question in this context is if there is a decline in replicative potential of NK cells from older, or elderly leukemia patients. Although Denman et al. 1 reported increased telomere length in NK cells from normal donors expanded on K562 cl9.mbIL-21, it is not known if replicative senescence will be reached much earlier in co-cultures with older NK cells.
As cellular anti-leukemia therapy, autologous NK cells combined with antibodies have distinct advantages compared to T-cells, including the lack of requirement for prior antigen exposure and sensitization for initial function. However, as reviewed by Bachanova and Miller,10 poor in vivo survival of NK cells is one of the limitations noted for NK cell therapies tested in the past. Thus experiments will need to be performed to address how degranulation can be prevented, and survival ensured, of these highly activated NK cells after injection into the circulation. We also need to determine if activated NK cells need to be programmed to migrate into the protective bone marrow niche where minimal residual disease BP-ALL cells can persist. Solving these problems may finally allow us to remove the word ‘potential’ associated with the term ‘NK cell therapy’.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This review was supported by grants from Alex's Lemonade Stand Foundation, the Leukemia & Lymphoma Society, the V-Foundation and PHS grant CA090321 (NH).
References
- 1. Denman CJ, Senyukov VV, Somanchi SS, Phatarpekar PV, Kopp LM, Johnson JL, Singh H, Hurton L, Maiti SN, Huls MH, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One 2012; 7:e30264; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0030264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu Y, Wu HW, Sheard MA, Sposto R, Somanchi SS, Cooper LJ, Lee DA, Seeger RC. Growth and activation of natural killer cells ex vivo from children with neuroblastoma for adoptive cell therapy. Clin Cancer Res 2013; 19:2132-43; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009; 69:4010-7; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fei F, Lim M, George AA, Kirzner J, Lee D, Seeger R, Groffen J, Abdel-Azim H, Heisterkamp N. Cytotoxicity of CD56-positive lymphocytes against autologous B-cell precursor acute lymphoblastic leukemia cells. Leukemia 2014; PMID:; http://dx.doi.org/ 10.1038/leu.2014.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, et al. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med 2013; 5:208ra145; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3006702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bessoles S, Grandclement C, Alari-Pahissa E, Gehrig J, Jeevan-Raj B, Held W. Adaptations of natural killer cells to Self-MHC class I. Front Immunol 2014; 5:349; PMID:; http://dx.doi.org/ 10.3389/fimmu.2014.00349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol 2009; 30:143-9; PMID:; http://dx.doi.org/ 10.1016/j.it.2009.01.006 [DOI] [PubMed] [Google Scholar]
- 8. Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature 2009; 457:557-61; PMID:; http://dx.doi.org/ 10.1038/nature07665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Parameswaran R, Lim M, Fei F, Abdel-Azim H, Arutyunyan A, Schiffer I, McLaughlin ME, Gram H, Huet H, Groffen J, et al. Effector-Mediated Eradication of Precursor B Acute Lymphoblastic Leukemia with a Novel Fc-Engineered Monoclonal Antibody Targeting the BAFF-R. Mol Cancer Ther 2014; 13:1567-77; PMID:; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bachanova V, Miller JS. NK cells in therapy of cancer. Crit Rev Oncog 2014; 19:133-41; PMID:; http://dx.doi.org/ 10.1615/CritRevOncog.2014011091 [DOI] [PMC free article] [PubMed] [Google Scholar]