Abstract
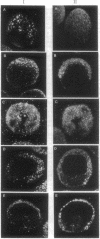
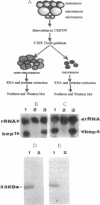
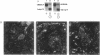
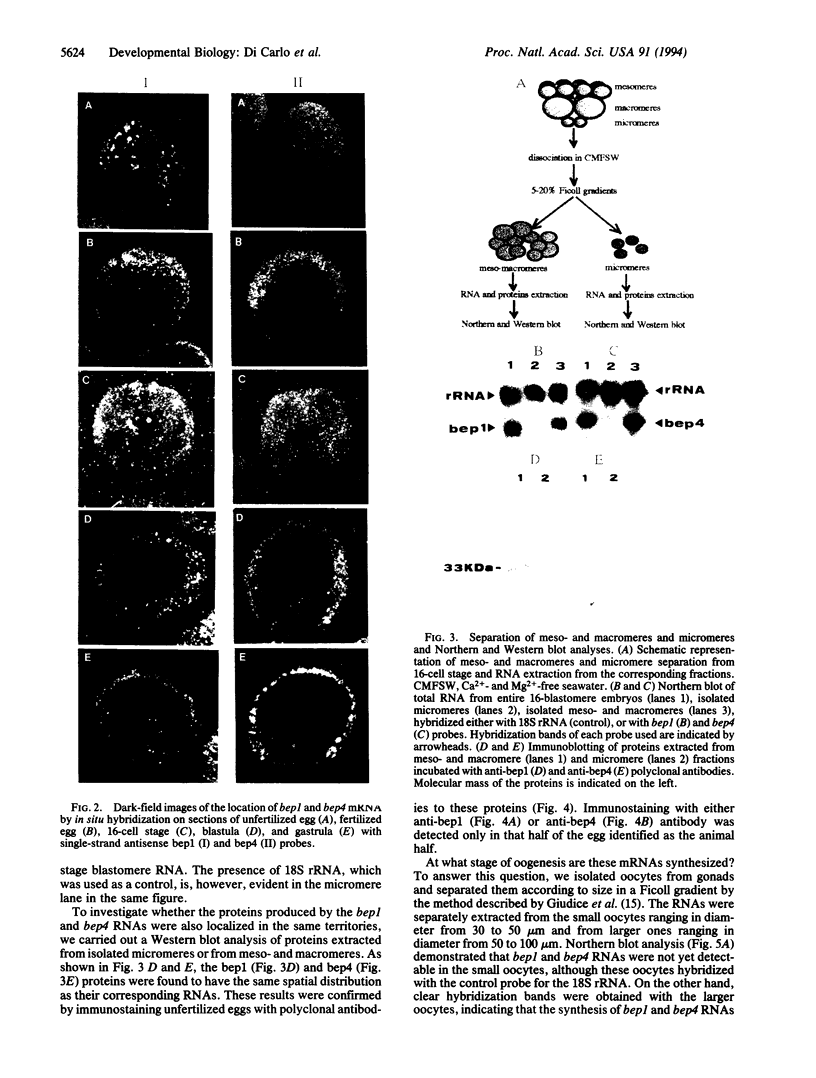
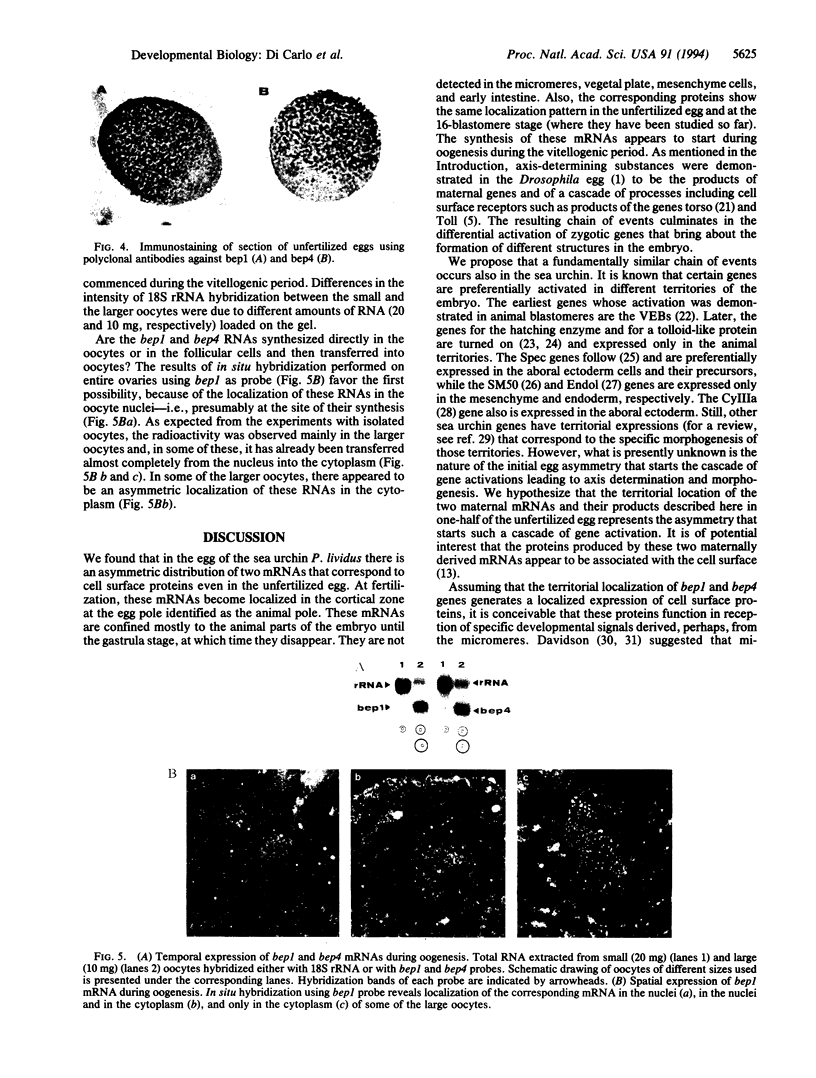
We demonstrated that two mRNAs that are synthesized during the vitellogenic period of oogenesis and that code for cell surface proteins are asymmetrically distributed in the unfertilized egg of Paracentrotus lividus. At fertilization, these RNAs rapidly localize in the cortical zone at the animal pole of the egg. They are then detected in the mesomeres and the macromeres, but not in the micromeres, and thereafter are found in the ectoderm but not in the vegetal plate, mesenchyme cells, or early intestine. They disappear in late gastrula. The proteins synthesized by these mRNAs show the same territorial location during the period examined here, which included the unfertilized egg and the 16-blastomere stage. These conclusions were reached on the basis of in situ hybridization and immunostaining experiments, as well as Northern and Western blot analyses of isolated blastomeres. The possible significance of this asymmetric distribution of these two mRNAs and proteins in the establishment of the animal/vegetal axis is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. V., Jürgens G., Nüsslein-Volhard C. Establishment of dorsal-ventral polarity in the Drosophila embryo: genetic studies on the role of the Toll gene product. Cell. 1985 Oct;42(3):779–789. doi: 10.1016/0092-8674(85)90274-0. [DOI] [PubMed] [Google Scholar]
- Benson S., Sucov H., Stephens L., Davidson E., Wilt F. A lineage-specific gene encoding a major matrix protein of the sea urchin embryo spicule. I. Authentication of the cloned gene and its developmental expression. Dev Biol. 1987 Apr;120(2):499–506. doi: 10.1016/0012-1606(87)90253-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Davidson E. H. How embryos work: a comparative view of diverse modes of cell fate specification. Development. 1990 Mar;108(3):365–389. doi: 10.1242/dev.108.3.365. [DOI] [PubMed] [Google Scholar]
- Di Carlo M., Montana G., Bonura A. Analysis of the sequence and expression during sea urchin development of two members of a multigenic family, coding for butanol-extractable proteins. Mol Reprod Dev. 1990 Jan;25(1):28–36. doi: 10.1002/mrd.1080250106. [DOI] [PubMed] [Google Scholar]
- Driever W., Nüsslein-Volhard C. The bicoid protein is a positive regulator of hunchback transcription in the early Drosophila embryo. Nature. 1989 Jan 12;337(6203):138–143. doi: 10.1038/337138a0. [DOI] [PubMed] [Google Scholar]
- Franks R. R., Hough-Evans B. R., Britten R. J., Davidson E. H. Spatially deranged though temporally correct expression of Strongylocentrotus purpuratus actin gene fusion in transgenic embryos of a different sea urchin family. Genes Dev. 1988 Jan;2(1):1–12. doi: 10.1101/gad.2.1.1. [DOI] [PubMed] [Google Scholar]
- Giudice G., Sconzo G., Bono A., Albanese I. Studies on sea urchin oocytes. I. Purification and cell fractionation. Exp Cell Res. 1972 May;72(1):90–94. doi: 10.1016/0014-4827(72)90570-8. [DOI] [PubMed] [Google Scholar]
- Hardin P. E., Angerer L. M., Hardin S. H., Angerer R. C., Klein W. H. Spec2 genes of Strongylocentrotus purpuratus. Structure and differential expression in embryonic aboral ectoderm cells. J Mol Biol. 1988 Aug 5;202(3):417–431. doi: 10.1016/0022-2836(88)90275-6. [DOI] [PubMed] [Google Scholar]
- Hashimoto C., Hudson K. L., Anderson K. V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell. 1988 Jan 29;52(2):269–279. doi: 10.1016/0092-8674(88)90516-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lepage T., Ghiglione C., Gache C. Spatial and temporal expression pattern during sea urchin embryogenesis of a gene coding for a protease homologous to the human protein BMP-1 and to the product of the Drosophila dorsal-ventral patterning gene tolloid. Development. 1992 Jan;114(1):147–163. doi: 10.1242/dev.114.1.147. [DOI] [PubMed] [Google Scholar]
- Lepage T., Sardet C., Gache C. Spatial expression of the hatching enzyme gene in the sea urchin embryo. Dev Biol. 1992 Mar;150(1):23–32. doi: 10.1016/0012-1606(92)90004-z. [DOI] [PubMed] [Google Scholar]
- Levi G., Crossin K. L., Edelman G. M. Expression sequences and distribution of two primary cell adhesion molecules during embryonic development of Xenopus laevis. J Cell Biol. 1987 Nov;105(5):2359–2372. doi: 10.1083/jcb.105.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A. Pattern formation during animal development. Science. 1991 Apr 12;252(5003):234–241. doi: 10.1126/science.1672778. [DOI] [PubMed] [Google Scholar]
- Melton D. A. Translocation of a localized maternal mRNA to the vegetal pole of Xenopus oocytes. Nature. 1987 Jul 2;328(6125):80–82. doi: 10.1038/328080a0. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C., Frohnhöfer H. G., Lehmann R. Determination of anteroposterior polarity in Drosophila. Science. 1987 Dec 18;238(4834):1675–1681. doi: 10.1126/science.3686007. [DOI] [PubMed] [Google Scholar]
- Ransick A., Davidson E. H. A complete second gut induced by transplanted micromeres in the sea urchin embryo. Science. 1993 Feb 19;259(5098):1134–1138. doi: 10.1126/science.8438164. [DOI] [PubMed] [Google Scholar]
- Reynolds S. D., Angerer L. M., Palis J., Nasir A., Angerer R. C. Early mRNAs, spatially restricted along the animal-vegetal axis of sea urchin embryos, include one encoding a protein related to tolloid and BMP-1. Development. 1992 Mar;114(3):769–786. doi: 10.1242/dev.114.3.769. [DOI] [PubMed] [Google Scholar]
- Romancino D. P., Ghersi G., Montana G., Bonura A., Perriera S., Di Carlo M. Characterization of bep1 and bep4 antigens involved in cell interactions during Paracentrotus lividus development. Differentiation. 1992 Jun;50(2):67–74. doi: 10.1111/j.1432-0436.1992.tb00487.x. [DOI] [PubMed] [Google Scholar]
- Siu C. H. Cell-cell adhesion molecules in Dictyostelium. Bioessays. 1990 Aug;12(8):357–362. doi: 10.1002/bies.950120802. [DOI] [PubMed] [Google Scholar]
- Sprenger F., Stevens L. M., Nüsslein-Volhard C. The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature. 1989 Apr 6;338(6215):478–483. doi: 10.1038/338478a0. [DOI] [PubMed] [Google Scholar]
- St Johnston D., Nüsslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992 Jan 24;68(2):201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- Wessel G. M., McClay D. R. Sequential expression of germ-layer specific molecules in the sea urchin embryo. Dev Biol. 1985 Oct;111(2):451–463. doi: 10.1016/0012-1606(85)90497-x. [DOI] [PubMed] [Google Scholar]
- Yisraeli J. K., Sokol S., Melton D. A. A two-step model for the localization of maternal mRNA in Xenopus oocytes: involvement of microtubules and microfilaments in the translocation and anchoring of Vg1 mRNA. Development. 1990 Feb;108(2):289–298. doi: 10.1242/dev.108.2.289. [DOI] [PubMed] [Google Scholar]
- Yisraeli J. K., Sokol S., Melton D. A. The process of localizing a maternal messenger RNA in Xenopus oocytes. Development. 1989;107 (Suppl):31–36. doi: 10.1242/dev.107.Supplement.31. [DOI] [PubMed] [Google Scholar]