Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population with the ability to suppress immune responses and are currently classified into three distinct MDSC subsets: monocytic, granulocytic and non-monocytic, and non-granulocytic MDSCs. Although NK cells provide an important first-line defense against newly transformed cancer cells, it is unknown whether NK cells can regulate MDSC populations in the context of cancer. In this study, we initially found that the frequency of MDSCs in non-Hodgkin lymphoma (NHL) patients was increased and inversely correlated with that of NK cells, but not that of T cells. To investigate the regulation of MDSC subsets by NK cells, we used an EL4 murine lymphoma model and found the non-monocytic and non-granulocytic MDSC subset, i.e., Gr1+CD11b+Ly6GmedLy6Cmed MDSC, is increased after NK cell depletion. The MDSC population that expresses MHC class II, CD80, CD124, and CCR2 is regulated mainly by CD27+CD11b+NK cells. In addition, this MDSC subset produces some immunosuppressive cytokines, including IL-10 but not nitric oxide (NO) or arginase. We also examined two subsets of MDSCs (CD14+HLA-DR− and CD14− HLA-DR− MDSC) in NHL patients and found that higher IL-10-producing CD14+HLA-DR−MDSC subset can be seen in lymphoma patients with reduced NK cell frequency in peripheral blood. Our analyses of MDSCs in this study may enable a better understanding of how MDSCs manipulate the tumor microenvironment and are regulated by NK cells in patients with lymphoma.
Keywords: IL-10, immunoregulation, malignant lymphoma, MDSC, NK cells
Abbreviations: DFS; disease-free survival; GM-CSF, granulocyte macrophage colony-stimulating factor; HLA, Human Leukocyte Antigen; IL, interleukin; LPS, Lipopolysaccharide; MDSC, myeloid-derived suppressor cell; NHL, non-Hodgkin lymphoma; NK, Natural killer cells; OS, overall survival; PBMC, peripheral blood mononucleated cell; TGFβ, transforming growth factor β; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor
Introduction
The host immune system plays a protective or suppressive role in the development and progression of cancer. It has been reported that a heterogeneous population of immature myeloid cells known as MDSCs can modulate NK1-3 and T cell function4-7 and may contribute to the immune escape of tumors.6,8 In fact, the elimination of MDSCs by chemotherapy in some models resulted in the augmentation of both the proportion and function of cytotoxic T cells.9-11 MDSCs have been intensively studied in spleen7,11,12 and within a tumor microenvironment.13,14 In mice, MDSCs are broadly characterized by the expression of CD11b and Gr-1. Using additional markers, these cells can be further divided into three subsets: Gr1hi or CD11b+Ly6GhiLy6Clow (granulocytic MDSCs) and Gr1low or CD11b+Ly6GlowLy6Chi (monocytic MDSCs) and Gr1int or CD11b+Ly6GintLy6Cint MDSCs (non-monocytic and non-granulocytic MDSCs).4,10,15-17 MDSC composition is dependent on tumor type as differences of MDSC subset frequency and/or function have been demonstrated between different tumor model systems.18
As a first defense, NK cells and other innate lymphocytes play an essential role in the inhibition of tumorigenesis, tumor growth, and tumor metastasis.19-21 We and other researchers previously demonstrated that dendritic cell (DC) therapy increases the activation of NK cells against tumor cells22-24 and that CD1d+ cells loaded with invariant NKT cell ligand generate NKT cell-mediated protection against tumor cells.25 Although there are reports in tumor-bearing mice of interactions between NK cells and various types of myeloid cells, i.e., DCs, macrophages, MDSCs, it is unclear whether NK cells influence the number or function of MDSCs.
IL-10 is known to be a major immune regulatory cytokine in lymphoma.26,27 In fact, increased serum levels of IL-10 in diffuse large B cell lymphoma was detected in 20% of NHL patients26,28 and was correlated with poor prognosis including significantly shortened overall survival (OS) and disease-free survival (DFS).26,29,30 It was shown that IL-10 is also produced by MDSCs.31-33 Therefore, we also focused on the importance of IL-10 production by MDSC subsets in this study.
In the current study, we initially evaluated a relation of NK cells to MDSCs in NHL patients. Next, we evaluated MDSC subset composition and function in murine lymphoma models and then translated these results to NHL patients. In murine lymphoma models, we studied about an effect of NK cells on MDSCs in the number and function. To confirm the clinical relevance of MDSC subsets, we evaluated peripheral blood from NHL patients for MDSC subsets in detail. These results imply that NK-MDSC interactions may be important in tumor growth and progression and could therefore be a target for novel immunotherapeutic approaches to alter the tumor-associated microenvironment.
Results
Inverse correlation in the number of MDSCs and NK cells in lymphoma patients
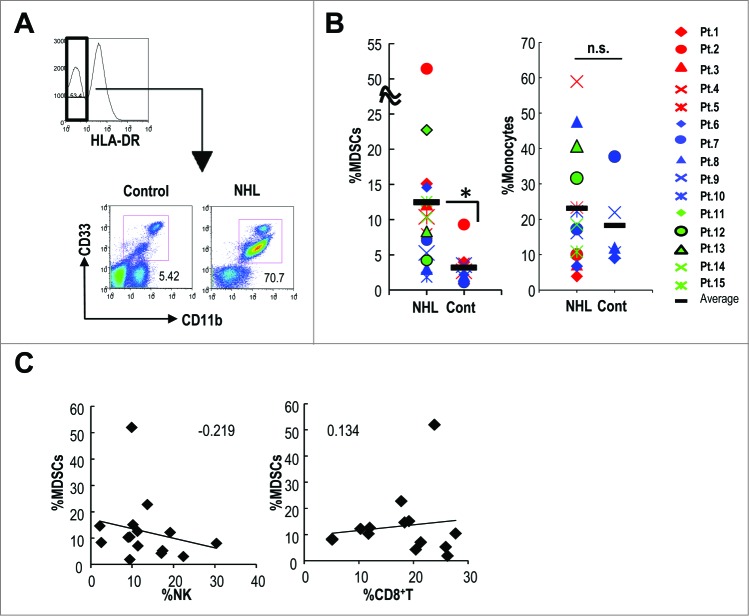
MDSCs are increased in many types of human cancers.34,35 However, only a few studies have attempted to identify MDSCs in patients with malignant lymphoma.6 We assessed the percentage of HLA-DR−CD11b+CD33+ MDSCs and CD14+HLA-DR+ monocytes in the peripheral blood of 15 patients with NHL (Table 1) and 12 healthy controls and found that HLA-DR−CD11b+CD33+ MDSCs, but not CD14+HLA-DR+ monocytes were significantly increased in NHL patients compared to healthy controls (Fig. 1A and 1B). In NHL patients, the frequency of MDSCs, but not of monocytes was increased compared to the healthy controls. We assessed the relationship between MDSCs and NK cells in the peripheral blood of 15 NHL patients and found an inverse correlation between numbers of MDSCs and NK cells, but not between MDSCs and CD8+ T cells (Fig. 1C).
Table 1.
Clinical disease characteristics of patients
| Patients | Gender | Age | Diagnosis | Stage | Treatment status | WBC (K/mL) | Hb (g/dL) | Plts (K/mL) | LDH (IU/L) | sIL2R (U/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 73 | DLBCL | Relapsed | Post R-CHOP | 5 | 14.4 | 166 | 121 | 432 |
| 2 | Female | 75 | FL | Relapsed | Post R-CHOP | 5.3 | 11.9 | 179 | 172 | 837 |
| 3 | Male | 78 | DLBCL | Relapsed | Post R-CHOP | 6.9 | 15.7 | 168 | 304 | 299 |
| 4 | Male | 71 | PTCL | Newly diagnosed | — | 5.3 | 11.3 | 151 | 608 | 6200 |
| 5 | Female | 62 | DLBCL | Relapsed | Post R-CHOP | 7.5 | 10.6 | 140 | 384 | 1480 |
| 6 | Female | 59 | DLBCL | Newly diagnosed | — | 2.4 | 9.3 | 156 | 876 | 638 |
| 7 | Female | 82 | DLBCL | Newly diagnosed | — | 2.9 | 9.8 | 368 | 205 | 454 |
| 8 | Male | 50 | DLBCL | Newly diagnosed | — | 4.3 | 12.1 | 389 | 613 | 1780 |
| 9 | Male | 74 | FL | Newly diagnosed | — | 4.3 | 9.1 | 134 | 240 | 7350 |
| 10 | Male | 34 | DLBCL | Relapsed | Post R-CHOP | 11.4 | 15.2 | 218 | 151 | 219 |
| 11 | Male | 80 | AILT | Relapsed | Post CHOP | 4.4 | 12.5 | 163 | 236 | 10000 |
| 12 | Female | 58 | MALT | Newly diagnosed | — | 5.2 | 12.4 | 239 | 177 | 416 |
| 13 | Female | 82 | DLBCL | Newly diagnosed | — | 3.5 | 9.4 | 27 | 616 | 23900 |
| 14 | Male | 61 | FL | Relapsed | Post CHOP | 4.9 | 11.4 | 99 | 155 | 4550 |
| 15 | Female | 73 | FL | Newly diagnosed | — | 7.0 | 13 | 273 | 218 | 348 |
AILT, Angioimmunoblastic T-cell lymphoma; DLBCL, Diffuse large B-cell lymphoma; FL, Follicular lymphoma; MALT, Mucosa-associated lymphoid tissue; PTCL, Peripheral T-cell lymphoma.
Figure 1.
Increased MDSCs were inversely correlated with NK cells in lymphoma patients. (A) The MDSC subsets by gating HLA-DR−CD11b+CD33+ cells were evaluated in the peripheral blood of 15 non-Hodgkin lymphoma patients (NHL) and 12 healthy volunteers (control: cont) using anti-CD11b-Pacific Blue, anti-CD33-PE and anti-HLA-DR-PerCP antibodies. The representative flow cytometry data (A) and the frequencies (B) of HLA-DR−CD11b+CD33+ cells and CD14−HLA-DR+ monocytes were enumerated. The characterization of the patients is shown in Table 1. (*p < 0.01 for cont. versus NHL) (C) The correlation between the frequency of HLA-DR−CD11b+CD33+ cells and the frequency of NK or CD8+ T cells in blood of each patient was assessed. The number indicated the correlation coefficient.
NK cell depletion in mice increases CD11b+Gr1+ MDSCs capable of IL-10 production
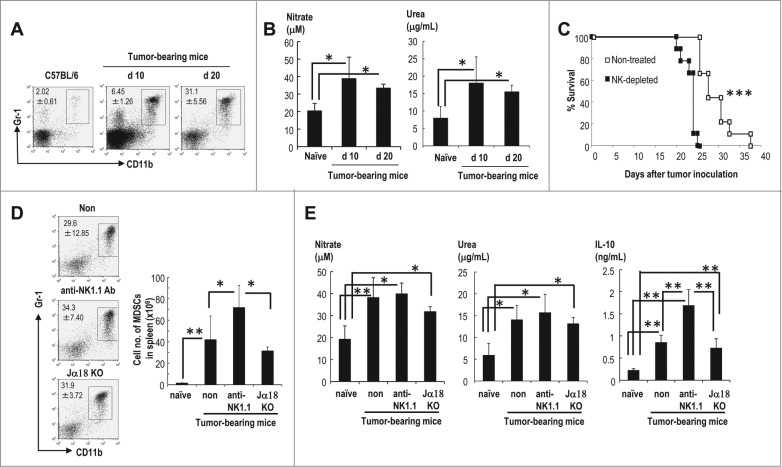
We assessed the regulation of MDSCs by NK cells using the EL4 murine lymphoma model. MDSCs, which are defined as CD11b+Gr1+ cells, were not detected on day 5 (data not shown) but were detected on day 10, with an increase on day 20 after an inoculation of EL4 lymphoma (Fig. 2A). These MDSCs demonstrated enhanced arginase activity and NO production at both time points, which induced an impairment of T cells (Fig. 2B). To evaluate the effect of NK cells on MDSCs, we analyzed MDSCs in tumor-bearing wild-type mice and tumor-bearing NK cell-depleted mice. Although EL4 lymphoma cells are generally known to be NK cell resistant in vitro,36 NK cell-depleted, tumor-bearing mice exhibited a significantly decreased survival time (Fig. 2C). We focused on immune cells in this study, although a variety of factors are suspected to be involved in decreased survival in NK cell-depleted mice, including the defect of IFNγ-promoted inhibition of angiogenesis at tumor sites.37 We could not find any difference in numbers of CD4+ T cells, regulatory T cells and CD8+ T cells in EL4 bearing mice and NK cell-depleted EL4 bearing mice (Fig. S1). CD11b+Gr1+MDSCs were increased in the NK cell-depleted group and could be one of the factors that contributed to the decreased survival seen in the NK cell-depleted mice (Fig 2C).
Figure 2.
MDSCs capable of producing IL-10 are increased in NK cell-depleted EL4 lymphoma-bearing mice. C57BL/6 (WT) mice were injected subcutaneously (s.c.) with EL4 lymphoma cells. (A) The percentages of CD11b+Gr1+ MDSCs in spleen on d 10 and 20 were analyzed using Gr1-APC and CD11b-FITC (n = 4–6, mean ± SEM). (B) The arginase activity (right) and NO production (left) of the sorted CD11b+Gr1+ splenic MDSCs were analyzed on day 10 and 20 after EL4 inoculation (n = 4–7, mean ± SEM; *p < 0.05). (C) The survival of EL4-bearing WT or NK cell-depleted mice was assessed. The survival curves were plotted using Kaplan–Meier estimates and compared through long-rank analysis (n = 9 per group; ***p < 0.001). (D) The frequencies of CD11b+Gr1+ MDSCs in tumor-bearing WT, anti-NK1.1 Ab-treated mice, and Jα18-KO mice on day 20 were analyzed by FACS (left). The total numbers of CD11b+Gr1+ splenic MDSCs in these animals were assessed 20 d after tumor inoculation (right; n = 4–6, mean ± SEM; **p < 0.01, *p < 0.05). (E) The activities of NO (left) and arginase (middle) and the production of IL-10 (right) by splenic MDSCs were assessed in these animals on day 20 (n = 4–6, mean ± SEM; **p < 0.01, *p < 0.05).
To rule out the possibility that NKT cells are also depleted with anti-NK1.1 Ab, we used NKT cell-lacking Jα18−/− mice and found the similar results as those in EL4 tumor-bearing WT mice (Fig. 2D). Interestingly, there was no difference in arginase activity and NO production in the MDSC subsets of tumor-bearing WT, anti-NK1.1-Ab treated WT, and Jα18−/− mice (Fig. 2E). However, the MDSCs from anti-NK1.1 Ab-treated mice produced more IL-10 than those from tumor-bearing WT or Jα18−/− mice (Fig. 2E). Thus, the depletion of NK cells either enhances the quantity of IL-10 produced by individual MDSCs or increases the number of the MDSC subset capable of producing IL-10. These results indicate that NK cells may work as a first defense against tumor and MDSCs.
CD11b+Gr1+Ly6GmedLy6Cmed and CD11b+Gr1+Ly6GhiLy6Cmed MDSC subsets are increased in NK-depleted tumor-bearing mice
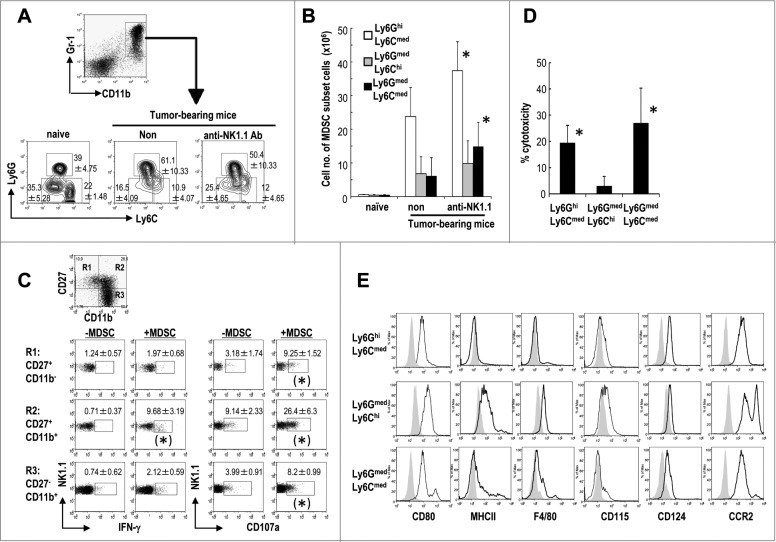
The CD11b+Gr1+ MDSCs can be separated into three subsets.10,15-17 Differential expression of Ly6C and Ly6G divides CD11b+Gr1+ cells into three distinct MDSC subsets: Ly6GhiLy6Cmed (granulocytic), Ly6GmedLy6Chi (monocytic), and Ly6GmedLy6Cmed (non-monocytic and non-granulocytic). Initially, we compared the frequency of the three subsets in EL4 tumor bearing mice and found Ly6GhiLy6Cmed MDSCs were the most prevalent and the other two subsets were equivalent in number. In NK cell-depleted tumor-bearing mice, the frequency and absolute numbers of the CD11b+Gr1+Ly6Gmed Ly6Cmed MDSCs were increased (Figs. 3A and B). The absolute number of CD11b+Gr1+Ly6GhiLy6Cmed MDSCs was also increased in NK cell-depleted tumor-bearing mice (Fig. 3B). To assess which subset of NK cells primarily responded to CD11b+Gr1+MDSCs, we cultured NK cells with CD11b+Gr1+ MDSCs from tumor bearing mice and verified three subsets of NK cells, i.e., CD27+CD11b−, CD27+CD11b+, CD27−CD11b+ after gating CD3−NK1.1+ cells. We analyzed each NK cell subset for IFNγ production and CD107a expression. As shown in Fig. 3C, CD27+CD11b+NK cells produced IFNγ and expressed CD107a in response to co-culture with MDSCs. Further analysis showed both Ly49D+ and Ly49D− NK cells, or both Ly49H+ and Ly49H− NK cells expressed CD107a in response to co-culture with MDSCs (data not shown). NK cells are highly cytotoxic against Ly6GmedLy6Cmed and Ly6GhiLy6Cmed MDSCs but not against Ly6GmedLy6Chi MDSCs (Fig. 3D). These findings suggested that CD27+CD11b+ mature NK cells may mainly have the cytotoxic capacity against two subsets of MDSCs.
Figure 3.
Characterization of three subsets of CD11b+Gr1+ MDSCs in NK-depleted tumor-bearing mice. C57BL/6 mice were inoculated with EL4 s.c. and assessed 20 d later. In some of the experiments, the mice were treated with anti-NK1.1 Ab to deplete NK cells. Splenocytes from EL4 tumor-bearing mice were analyzed by flow cytometry. (A) The cells were gated to identify the CD11b+Gr1+ MDSC subsets. EL4-tumor bearing mice were either treated with anti-NK1.1Ab or not, and after gating on CD11b+Gr1+ cells, each CD11b+Gr1+ MDSC fraction was analyzed to determine its expression levels of Ly6C and Ly6G using anti-CD11b-PerCP/Cy5.5, anti-Gr1-APC, anti-Ly6C-FITC, and anti-Ly6G-Pacific Blue antibodies (n = 4–6, mean ± SEM). (B) The absolute numbers of three MDSC subsets in the spleen from mice groups described in (A) were quantified after gating based on the expression levels of Gr1 and CD11b (n = 4–6, mean ± SEM; *p < 0.05 for non- vs. anti-NK1.1). (C) NK cells were directly isolated from spleen of Rag1−/− mice using anti-DX5 Ab-conjugated bead were cocultured with CD11b+Gr1+MDSCs at a 1:1 ratio for 6 h. CD107 expression was analyzed using Alexa488-CD107a and IFNγ production by intracellular staining as previously described53. (n = 4, mean ± SEM; IFNγ; *p < 0.05 for -MDSC vs. +MDSC in R2, CD107a; *p < 0.05 –MDSC vs. +MDSC in R1, R2, and R3) (D) NK cell cytotoxicity against each MDSC subset was determined as described in Methods (n = 4, mean ± SEM; *p < 0.05 for Ly6GhiLy6Cmed vs. Ly6GmedLy6Chi, and Ly6GhiLy6Cmed vs. Ly6GmedLy6Cmed). (E) CD11b+Gr1+ MDSC subsets from EL4 tumor-bearing mice were stained with PE-labeled anti-CD80, MHC II, F4/80, CD115, CD124, and CCR2, and the expression levels of these markers were analyzed. The data are representative of four experiments (n ≥ 4 per group).
Characterization of CD11b+Gr1+ Ly6GmedLy6Cmed MDSCs
We were interested in further evaluating the different subsets of MDSCs, particularly CD11b+Gr1+Ly6GmedLy6Cmed MDSCs, which have not been well characterized. Therefore, we compared phenotypic markers on these MDSCs with those present on granulocytic MDSCs and monocytic MDSCs. All three MDSC subsets expressed CD80, CD124, and CCR2 (Fig. 3E), but not CD11c (data not shown). They did not express B220, CD36, CD40, CD86, CD103, TIM1, and TIM4 (data not shown). Ly6GmedLy6Chi MDSCs preferentially expressed CD115 (M-CSF receptor) (Fig. 3E), and both Ly6GmedLy6Chi and Ly6GmedLy6Cmed MDSC subsets expressed MHC class II (I-Ab) (Fig. 3E).
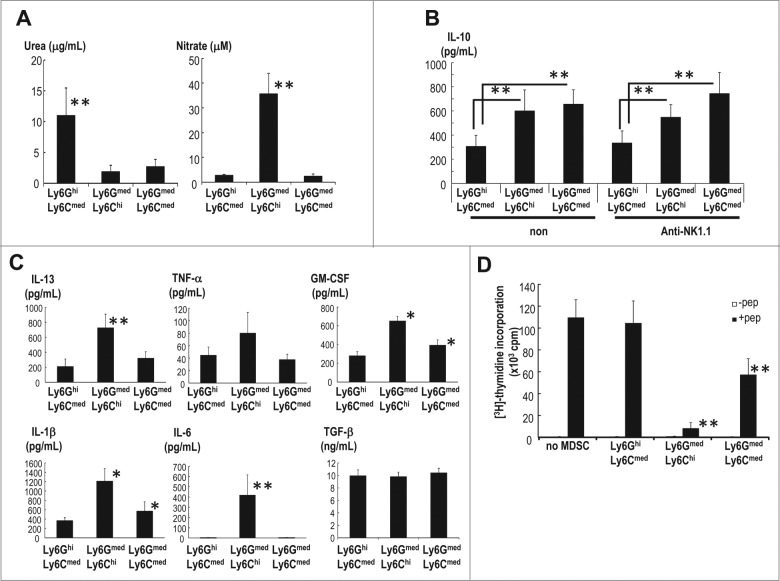
We then assessed the function of the three MDSC subsets. Ly6GhiLy6Cmed MDSCs showed increased levels of arginase activity, whereas Ly6GmedLy6Cmed MDSCs did not (Fig. 4A, left). In contrast, Ly6GmedLy6Chi MDSCs produced NO while Ly6GmedLy6Cmed MDSCs did not (Fig. 4A, right). IL-10 was preferentially produced by Ly6GmedLy6Cmed and Ly6GmedLy6Chi subsets (Fig. 4B). The number of Ly6GmedLy6Cmed MDSCs but not Ly6GmedLy6Chi MDSCs increased after depletion of NK cells (Fig. 3B), however levels of IL-10 production did not change (Fig. 4B). NK cell depletion did not enhance the quantity of IL-10 produced per cell (Fig. 4B). The number of MDSCs capable of producing IL-10 is inversely regulated by NK cells. Some immunosuppressive and inflammatory cytokines, such as IL-13, GM-CSF, TNF-α and IL-1β were also assessed. Ly6GmedLy6Chi and Ly6GmedLy6Cmed MDSC subsets produced more of these cytokines than Ly6GhiLy6Cmed MDSCs (Fig. 4C). TGF-β was released in similar amounts by all three groups, whereas IL-6 was secreted only by Ly6GmedLy6Chi MDSCs.
Figure 4.
Functional characterization of MDSC subsets. (A) The three subsets of MDSCs in spleen were sorted 20 d after EL4 inoculation s.c. The arginase activity and NO production were evaluated (n = 4–6, mean ± SEM; **p < 0.01 for the comparison of urea between Ly6GhiLy6Cmed and others and for the comparison of nitrate between Ly6GmedLy6Chi and others). (B) The IL-10 production by each splenic MDSC subset in untreated (non) and anti-NK1.1 Ab-treated tumor-bearing mice was measured by ELISA (n = 4–6, mean ±SEM; **p < 0.01 Ly6GhiLy6Cmed vs. Ly6GmedLy6Chi, and Ly6GhiLy6Cmed vs. Ly6GmedLy6Cmed). (C) The cytokine production in the mice described in (B) was assessed by Luminex after 24 h of culture (n = 4–6, mean ± SEM; **p < 0.01 for the comparison of IL-6 and IL-13 between Ly6Chi and others; *p < 0.05 for the comparison of GM-CSF and IL-1β between the Ly6GmedLy6Chi and Ly6GmedLy6Cmed cells and the Ly6GhiLy6Cmed cells). (D) Individual three MDSC subsets (6 × 104 cells/well) were isolated from EL4-bearing mice and co-cultured with spleen cells from OT-II TCR Tg mice (2 × 105 cells/well) in the presence or absence of 10 μM OVA323-339 peptide (ISQAVHAAHAEINEAGR) for 64 h. For the last 16 h of culture, (3H)-thymidine was added, and the cell proliferation was measured (n = 6, mean ± SEM; **p < 0.01 for Ly6GmedLy6Chi and Ly6GmedLy6Cmed cells vs. the group without MDSCs and the Ly6GhiLy6Cmed group).
Next, we tested the capacity of the three MDSC subsets to inhibit antigen-specific proliferation of CD4+ T cells. Individual MDSC subsets were isolated from EL4 bearing mice and co-cultured with spleen cells of OT-II transgenic mice in the presence of OVA peptide. Interestingly, CD11b+Gr1+Ly6GmedLy6Chi monocytic MDSCs significantly inhibited CD4+ OT-II T cells, but CD11b+Gr1+Ly6GhiLy6Cmed granulocytic MDSCs did not (Fig. 4D). In addition to Ly6GmedLy6Chi MDSCs, CD11b+Gr1+Ly6GmedLy6Cmed MDSCs exhibited the suppressive activity on antigen-specific CD4+ T cells (Fig. 4D).
Characterization of MDSCs in Eμ-myc spontaneous B cell lymphoma mouse models
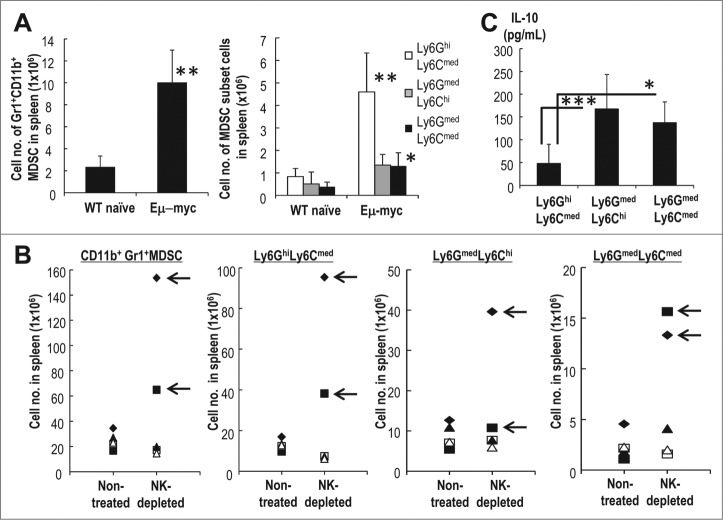
We then used Eμ-myc transgenic mice to assess the phenotypes of three MDSC subsets and their capacity for IL-10 production. The Eμ-myc transgenic mouse, in which the Myc proto-oncogene is under the control of the IgH enhancer, is a valuable model for the study of spontaneously occurring Myc-driven B cell lymphomas. The onset of lymphoma in Eμ-myc mice occurs at approximately 4 mo of age and is heralded by lymph node swelling. To assess the role of the MDSC subsets in the context of spontaneous lymphoma, we analyzed the MDSCs from 4 mo old Eμ-myc mice with lymphadenopathy. The total number of CD11b+Gr1+ MDSC cells were increased in the Eμ-myc transgenic mice as compared to C57BL/6 wild type mice and Eμ-myc mice without lymphadenopathy and the distribution and phenotypes of the three subsets were similar to those found in mice injected with EL4 (Fig. 5A). Next, we focused on the development of lymphoma of Eμ-myc mice. We treated the 4 mo-old Eμ-myc mice without lymphadenopathy with anti-NK1.1Ab for 1 mo, and compared the development of lymphoma and frequency of MDSC subsets as to the control Eμ-myc mice without lymphadenopathy that were not treated with anti-NK1.1Ab. Two out of five mice treated with anti-NK1.1 Ab developed lymphoma (Fig. 5B). They demonstrated an increased frequency of at least two subsets of MDSCs (arrow in Fig. 5B), i.e., Ly6GhiLy6Cmed and Ly6GmedLy6Cmed. On the other hand, the increase in number of MDSCs apparently did not occur in non-treated mice group or anti-NK1.1Ab-treated, Eμ-myc mice without lymphadenopathy (Fig. 5B). Progression to Eμ-myc lymphoma mice was correlated with an increased frequency of MDSCs. Compared to CD11b+Gr1+Ly6GhiLy6Cmed MDSCs, both CD11b+Gr1+Ly6GmedLy6Chi and CD11b+Gr1+Ly6GmedLy6Cmed MDSCs preferentially produced IL-10 (Fig. 5C). Therefore, the phenotype of three MDSC subsets and their capacity for IL-10 production were similar in both the EL4 lymphoma model and the Eμ-myc spontaneous lymphoma model and may suggest how lymphoma progresses in humans.
Figure 5.
Characterization of MDSCs in spontaneous lymphoma mice. (A) The absolute numbers of CD11b+Gr1+ MDSCs (a, left) or each subset of MDSCs (a, right) from naïve or Eμ-myc mice that had been developed with lymphadenopathy were measured (n = 5, mean ± SEM; **p < 0.01 and *p < 0.05 for WT naïve vs. Eμ-myc). (B) Ten Eμ-myc mice that had been 4 mo after a birth, but still did not develop the lymphadenopathy were assessed. In some mice, NK cells were depleted with anti-NK1.1Ab for one month (n = 5). One month later, the MDSCs in spleen were evaluated. Arrows in the figure indicate the development of lymphoma. (C) As shown in (A), splenic MDSCs from Eμ-myc mice with lymphadenopathy were stimulated with LPS, and the levels of IL-10 in the supernatants of the cell cultures were assessed by ELISA (n = 5, mean ± SEM; ***p < 0.001 Ly6GmedLy6Chi vs. Ly6GhiLy6Cmed, *p < 0.05 Ly6GmedLy6Cmed vs. Ly6GhiLy6Cmed).
The relationship of MDSC subsets and NK cell number in lymphoma patients
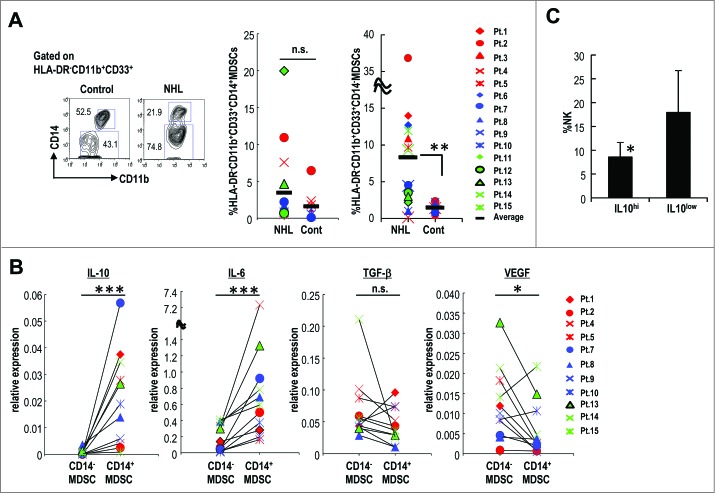
As shown in Fig. 1, the percentage of HLA-DR−CD11b+CD33+ MDSCs was significantly increased in the peripheral blood of NHL patients. MDSCs in humans can be further separated into CD33+CD11b+CD14+ and CD33+CD11b+CD14− after gating on HLA-DR (Fig. 6A left).6 Using this gating strategy, we assessed the percentage of CD14+HLA-DR− MDSCs and CD14−HLA-DR− MDSCs in the peripheral blood of 15 patients with NHL (Table 1) and 12 healthy controls. CD14−HLA-DR− MDSCs were significantly increased in the peripheral blood of NHL patients (Fig. 6A right). CD14+HLA-DR− MDSCs were not significantly increased, but there was a trend toward higher frequency of these MDSCs in NHL patients (Fig. 6A middle).
Figure 6.
Characterization of MDSC subsets in lymphoma patients. (A) The MDSC subsets were evaluated as CD14+ or CD14− by gating HLA-DR−CD11b+CD33+ cells in the peripheral blood of 15 NHL and 12 healthy volunteers (control: cont) using anti-CD11b-Pacific Blue, anti-CD14-Alexa647, anti-CD33-PE, and anti-HLA-DR-PerCP antibodies. (**p< 0.01 for control vs. NHL) (B) The cytokine (IL-10, IL-6, TGF-β, and VEGF) gene expression levels in CD14+HLA-DR− MDSCs and CD14−HLA-DR− MDSCs from lymphoma patients was measured by real-time PCR after stimulation with LPS. (*p < 0.05 and ***p < 0.001 for CD14−MDSC vs. CD14+MDSC) (C) The frequency of NK in blood of each patient was assessed between IL-10 high (relative intensity > 0.01) or IL-10 low (relative intensity < 0.01) in CD14+HLA-DR− MDSCs. (*p < 0.05 for IL10hi vs. IL10low).
We evaluated the production of immunosuppressive cytokines, including IL-10, by each MDSC subset found in the peripheral blood of NHL patients. Compared to CD14−HLA-DR− MDSCs, CD14+HLA-DR− MDSCs produced higher levels of IL-10 and IL-6, but not TGF-β and VEGF production (Fig. 6B).
Elevated serum IL-10 is a known poor prognostic indicator for patients with malignant lymphoma.26,27 In addition, it was reported that increased numbers of CD14+HLA-DR− cells in NHL patients inversely correlated with survival.38 We then evaluated the correlation between IL-10 production by CD14+HLA-DR− MDSC and NK cell frequency. CD14+HLA-DR− MDSCs producing high levels of IL-10 which indicates the high relative intensity (>0.01) of IL-10 expression, were found in patients with low frequency of NK cells in their peripheral blood, while CD14+HLA-DR− MDSCs producing low intensity IL-10 expression (<0.01) were found in patients with preserved NK cells in their peripheral blood. Thus, CD14+HLA-DR− MDSC can be still separated into two populations. These findings suggested that the high IL-10-producing CD14+HLA-DR− MDSC subset in NHL patients appears to be equivalent to the mouse Ly6GmedLy6Chi and Ly6GmedLy6Cmed subsets, which also preferentially produce IL-10 in the EL4 and Eμ-myc mouse lymphoma models.
Discussion
MDSCs are a heterogeneous population of immune suppressor cells that are known to inhibit NK and T cell function, resulting in tumor metastasis.1-3,39-44 The current study demonstrates for the first time that certain MDSC subsets are susceptible to NK cells, and NK cells control the MDSC subsets. We evaluated the relationship between MDSC subsets and NK cells in two murine lymphoma models as well as NHL patients. In the lymphoma mouse models, we characterized three distinct subsets of MDSC: CD11b+Gr1+Ly6GmedLy6ChiMDSCs, CD11b+Gr1+Ly6GhiLy6CmedMDSCs, and CD11b+Gr1+Ly6GmedLy6Cmed MDSCs. Conventional Ly6C MDSCs (also denoted as CD11b+Gr1+Ly6GmedLy6ChiMDSCs) are the most immunosuppressive of the MDSC subsets (Fig. 4D); however these cells are not targets of NK cell killing (Fig. 3D). Conversely, the other conventional MDSC subset, Ly6G, (also denoted as CD11b+Gr1+Ly6GhiLy6CmedMDSCs), though the most abundant and sensitive to NK cell cytotoxicity (Fig. 3B and D), are not the most immune suppressive (Fig. 4D). We also describe a third non-monocytic, non-granulocytic MDSC subset, CD11b+Gr1+Ly6GmedLy6Cmed MDSC, which produces IL-10, is immune suppressive, and sensitive to NK cell cytotoxicity (Figs. 3D and 4B).
NK cells have the natural capacity directly to kill virally infected or malignantly transformed cells, but the interaction between NK cells and infected or malignant cells is altered in the presence of myeloid cells. In the bidirectional cross-talk between NK cells and DCs, NK cells can induce DC maturation through the secretion of pro-inflammatory cytokines. NK cells can also eliminate immature DCs via the NK cell activating molecules, NKp30 and DNAM-1, and the absence of inhibitory KIRs specific for self-HLA class I alleles on immature DCs.45-47 NK cells can also directly regulate the adaptive immune response. It has been reported that NK cell-derived IFNγ induces direct upregulation of the major Th1 transcription factor T-bet and inhibition of the Th2 transcription factor GATA3.48 It has also recently been reported in infection models that CD4+ T cells are eliminated by NK cells via TRAIL,49 whereas the elimination of CD8+ T cells is 2B4, NKG2D and perforin mediated.50,51 In the current study, to investigate how NK cells recognize CD11b+Gr1+Ly6GmedLy6Cmed MDSCs, we assessed NK cell cytotoxicity against MDSCs by using anti-NKG2D or anti-NKp46 blocking antibodies. Anti-NKG2D Ab partially blocked NK cytotoxicity, but anti-NKp46 Ab did not (data not shown). Further studies are needed to completely understand the mechanism of NK cell-mediated MDSC cytotoxicity.
In this study, we demonstrated an inverse correlation between NK cells and a novel MDSC subset capable of producing IL-10. This correlation could offer insight into the pathogenesis of lymphoma and could potentially be used as a prognostic indicator for patients with lymphoma. The identification of the role MDSCs play in cancer progression uncovers potential new treatment modalities, including targeting NK-sensitive MDSCs with NK cell therapy for the treatment of recalcitrant tumors.
Materials and Methods
Mice and cell lines
Pathogen-free 6- to 8-wk-old C57BL/6 (WT) and Rag1−/− mice were purchased from CLEA Japan. B6 Jα18−/− mice and TCR-transgenic mice harboring ovalbumin (OVA)-specific CD4+ T cells (OT-II) were bred in the IMS animal facility. Eμ-Myc mice were obtained from Jackson Laboratory. All of the mice were maintained under specific pathogen-free conditions and studied in compliance with institutional guidelines. EL4 thymoma were purchased from the American Type Culture Collection.
Human samples
Peripheral blood samples from 12 healthy volunteers and 15 non-Hodgkin type malignant lymphoma patients who were newly diagnosed or had recurrent disease were obtained from National Hospital Organization Kumamoto Medical Center (Kumamoto, Japan). PBMCs were separated by Ficoll-Hypaque (Amersham Pharmacia Biotech) density centrifugation, washed three times with PBS, and stored in liquid nitrogen until use. Written informed consent was obtained from all of the patients according to the Declaration of Helsinki. All of the studies were approved by the National Hospital Organization Kumamoto Medical Center review board and the RIKEN institutional review board. The clinical characteristics of patients are demonstrated in Table 1.
Reagents
The following monoclonal antibodies (mAbs) were purchased from BD Bioscience, BioLegend, or e-Bioscience: anti-mouse CD11b(M1/70), CD27(LG.3A10), CD80(16-10A1), CD107a(1D4B), CD115(AFS98), CD124(mIL4R-M1), CCR2(475301), F4/80(BM8), Gr-1(RB6-8C5), I-Ab(AF6-120.1), Ly6C(AL-21), Ly6G(1A8), NK1.1(PK136), Ly49D((4E5), Ly49H(3D10), CD3(145-2C11), TCRβ(H57-597), IFN-γ (XMG1.2), anti-human CD11b(ICRF44), CD14 (M5E2), CD15(HI98), CD33(WM53), HLA-DR(L243), CD3(HIT3a) and CD8+(RPA-T8). LIVE/DEAD Fixable Aqua Dead Cell stain kit (Invitrogen) was used to eliminate dead cells. For analysis, a FACSCaliburTM or Canto II instrument and the CELLQuestTM or FACSDiva (BD Biosciences) or FlowJo software packages were used. For depletion in vivo, anti-NK1.1 Ab was prepared in our laboratory from a hybridoma (PK136, ATCC), and anti-asialoGM1 Ab was purchased from Wako Pure Chemical Industries, Ltd.
Cell preparation
Splenocytes were obtained by crushing the spleen through a 70-μm cell strainer, lysing erythrocytes with ACK lysing buffer (Invitrogen), and two washes in RPMI. In some of the experiments, spleens were digested with collagenase D (Roche). MDSCs were isolated from spleens of control or tumor-bearing mice (on day 10 or 20). CD11b+Gr-1+ MDSCs were isolated using anti-Gr1-PE and PE magnetic beads (MACS), and the purity of the resulting population was higher than 90%. Each subset of MDSCs was sorted from pooled spleens using a BD FACSAria cell sorter.
In vivo tumor experiments
WT mice were inoculated with 1 × 106 EL4 lymphoma cells. In some of the experiments, mice were treated with anti-asialoGM1 Ab (50 μL/mouse) to study their survival or anti-NK1.1 Ab (200 μg/mouse) for MDSC analysis every 2 d starting on day 2 before EL4 inoculation.
Arginase activity
Arginase activity in cell lysates was measured as previously described.4,52 Briefly, 1 × 106 cells were lysed for 30 min with 100 μL of 0.1% TritonX-100. Subsequently, 100 μL of 25 mM Tris-HCl and 10 μL of 10 mM MnCl2 were added to the mixture, and the enzyme was activated by heating for 10 min at 56°C. Arginine hydrolysis was conducted by incubating the lysate with 100 μL of 0.5 M L-arginine (pH 9.7) at 37°C for 120 min. The reaction was stopped by the addition of 900 μL of H2SO4 (96%)/H3PO4 (85%)/H2O (1/3/7, v/v/v). The urea concentration was measured at 540 nm after the addition of 40 μL of α-isonitrosopropiophenone (dissolved in 100% ethanol) and subsequent heating at 95°C for 30 min.
Nitric oxide (NO) production
Equal volumes of culture (1 × 106 cells) supernatants (100 μL) were mixed with Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% N-1-naphthylethylenediamine dihydrochloride in double-distilled water). After a 10-min incubation at room temperature, the absorbance at 550 nm was measured using a microplate plate reader.
Inhibition assay of antigen-specific CD4+ T cell proliferation
Spleen cells from OT-II TCR Tg mice were cultured at a density of 2 × 105 cells/well with or without each subset of MDSCs (6 × 104 cells/well) isolated from EL4-bearing mice in the presence or absence of 10 μM OVA323-339 peptide (ISQAVHAAHAEINEAGR) for 64 h. During the last 16 h of culture, [3H]-thymidine was added to the culture, and thymidine incorporation was measured.
Cytokine secretion assays and intracellular staining
MDSCs were cultured at a density of 1 or 2 × 105 cells/well in the presence of 100 ng/mL LPS (SIGMA) for 24 h. The supernatants were collected, and the IL-10 production was measured using an ELISA kit (BD). In some of the experiments, the concentrations of IL-1β, IL-6, IL-13, GM-CSF, TGF-β, and TNF-α were measured by Luminex (Bio Rad).
Killing assay in vitro
NK cells were prepared from spleen and liver of Rag1−/− mice and stimulated with 10 ng/mL IL-12 and 50 ng/mL IL-18 for 24 h. PKH-26-labeled spleen cells of EL4-bearing mice were co-cultured with activated NK cells at an E:T ratio of 20. Four hours later, the spleen cells were stained with anti-Gr-1-PerCP/Cy5.5, CD11b-PE/Cy7, Ly6C-FITC, Ly6G-PB, and To-PRO3 immediately prior to their analysis to identify dead cells. The spontaneous target cell death (SD) was determined by the labeling of the cells that were cultured alone with PKH-26. As a positive control for total cytotoxicity (TD), the labeled target cells were permeabilized with BD Cytofix/Cytoperm reagent (BD PharMingen). The specific lysis was calculated using the following formula: (Sample-SD/TD-SD) × 100.
Quantitative PCR assay
To evaluate the gene expression profile of human MDSC, PBMCs were stimulated with 100 ng/mL LPS overnight. Each subset of MDSCs was then sorted by an Aria sorter. The FACS-sorted MDSCs were directly subjected to cDNA synthesis and pre-amplification without RNA purification using a CellsDirect One-Step qRT-PCR kit (Invitrogen) with a mixture of pooled gene-specific primers (0.2 μM each). After 18 cycles of pre-amplification (each cycle consisted of 95°C for 30 sec and 60°C for 4 min), an aliquot was used as the template for quantitative PCR using FastStart Universal Probe Master (Roche), a gene-specific forward and reverse primer pair as below, and the corresponding FAM-labeled hydrolysis probe (Universal Probe Library Set, Roche). Quantitative PCR was performed on a StepOne Plus instrument (Applied Biosystems). The gene expression was measured by the ΔΔCT method using the expression of GAPDH as the internal control. The primers used in these experiments were purchased from Invitrogen, and the sequences were the following: IL-6 forward, 5′-caggagcccagctatgaact-3′, and reverse, 5′-gaaggcagcaggcaacac-3′; IL-10 forward, 5′-tgccttcagcagagtgaaga-3′, and reverse, 5′-gcttggcaacccaggtaa-3′; TGF-β forward, 5′-actactacgccaaggaggtcac-3′, and reverse, 5′-tgcttgaacttgtcatagatttcg-3′; VEGF forward, 5′-gcagcttgagttaaacgaacg-3′, and reverse, 5′-ggttcccgaaaccctgag-3′; and GAPDH forward, 5′-agccacatcgctcagacac-3′, and reverse, 5′-gcccaatacgaccaaatcc-3′.
Statistical analysis
The survival curves of the treatment groups were plotted using the Kaplan–Meier estimates and compared through long-rank analysis. The Mann–Whitney U-test was used for the statistical analysis of the remaining data. p < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank H. Fujimoto and Y. Hachiman (RIKEN, IMS) for providing technical assistance and Dr. K. Bickham (Columbia University) for the critical review of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, Zhang HG. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood 2007; 109:4336-42; PMID:; http://dx.doi.org/ 10.1182/blood-2006-09-046201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-b1. J Immunol 2009; 182:240-9; PMID:; http://dx.doi.org/ 10.4049/jimmunol.182.1.240 [DOI] [PubMed] [Google Scholar]
- 3. Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50:799-807; PMID:; http://dx.doi.org/ 10.1002/hep.23054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181:5791-802; PMID:; http://dx.doi.org/ 10.4049/jimmunol.181.8.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162-74; PMID:; http://dx.doi.org/ 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253-68; PMID:; http://dx.doi.org/ 10.1038/nri3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Capietto AH, Kim S, Sanford DE, Linehan DC, Hikida M, Kumosaki T, Novack DV, Faccio R. Down-regulation of PLCg2-b-catenin pathway promotes activation and expansion of myeloid-derived suppressor cells in cancer. J Exp Med 2013; 210:2257-71; PMID:; http://dx.doi.org/ 10.1084/jem.20130281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol 2010; 22:238-44; PMID:; http://dx.doi.org/ 10.1016/j.coi.2010.01.021 [DOI] [PubMed] [Google Scholar]
- 9. Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005; 11:6713-21; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-05-0883 [DOI] [PubMed] [Google Scholar]
- 10. Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013; 38:729-41; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 11. Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res 2014; 74:104-18; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol 2013; 14:211-20; PMID:; http://dx.doi.org/ 10.1038/ni.2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu T, Ramakrishnan R, Altiok S, Youn JI, Cheng P, Celis E, Pisarev V, Sherman S, Sporn MB, Gabrilovich D. Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 2011; 121:4015-29; PMID:; http://dx.doi.org/ 10.1172/JCI45862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fan Q, Gu D, Liu H, Yang L, Zhang X, Yoder MC, Kaplan MH, Xie J. Defective TGF-beta signaling in bone marrow-derived cells prevents hedgehog-induced skin tumors. Cancer Res 2014; 74:471-83; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-2134-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol 2010; 40:22-35; PMID:; http://dx.doi.org/ 10.1002/eji.200939903 [DOI] [PubMed] [Google Scholar]
- 16. Elkabets M, Ribeiro VS, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN, Vosshenrich CA. IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol 2010; 40:3347-57; PMID:; http://dx.doi.org/ 10.1002/eji.201041037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sumida K, Wakita D, Narita Y, Masuko K, Terada S, Watanabe K, Satoh T, Kitamura H, Nishimura T. Anti-IL-6 receptor mAb eliminates myeloid-derived suppressor cells and inhibits tumor growth by enhancing T-cell responses. Eur J Immunol 2012; 42:2060-72; PMID:; http://dx.doi.org/ 10.1002/eji.201142335 [DOI] [PubMed] [Google Scholar]
- 18. Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 2010; 40:2969-75; PMID:; http://dx.doi.org/ 10.1002/eji.201040895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest 2007; 117:1137-46; PMID:; http://dx.doi.org/ 10.1172/JCI31405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44-9; PMID:; http://dx.doi.org/ 10.1126/science.1198687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29:235-71; PMID:; http://dx.doi.org/ 10.1146/annurev-immunol-031210-101324 [DOI] [PubMed] [Google Scholar]
- 22. Granucci F, Zanoni I, Pavelka N, Van Dommelen SL, Andoniou CE, Belardelli F, Degli Esposti MA, Ricciardi-Castagnoli P. A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J Exp Med 2004; 200:287-95; PMID:; http://dx.doi.org/ 10.1084/jem.20040370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol 2005; 174:727-34; PMID:; http://dx.doi.org/ 10.4049/jimmunol.174.2.727 [DOI] [PubMed] [Google Scholar]
- 24. Shimizu K, Asakura M, Fujii S. Prolonged antitumor NK cell reactivity elicited by CXCL10-expressing dendritic cells licensed by CD40L+ CD4+ memory T cells. J Immunol 2011; 186:5927-37; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1003351 [DOI] [PubMed] [Google Scholar]
- 25. Shimizu K, Mizuno T, Shinga J, Asakura M, Kakimi K, Ishii Y, Masuda K, Maeda T, Sugahara H, Sato Y, et al. Vaccination with antigen-transfected, NKT cell ligand-loaded, human cells elicits robust in situ immune responses by dendritic cells. Cancer Res 2013; 73:62-73; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-0759 [DOI] [PubMed] [Google Scholar]
- 26. Lossos IS, Morgensztern D. Prognostic biomarkers in diffuse large B-cell lymphoma. J Clin Oncol 2006; 24:995-1007; PMID:; http://dx.doi.org/ 10.1200/JCO.2005.02.4786 [DOI] [PubMed] [Google Scholar]
- 27. Cao HY, Zou P, Zhou H. Genetic association of interleukin-10 promoter polymorphisms and susceptibility to diffuse large B-cell lymphoma: a meta-analysis. Gene 2013; 519:288-94; PMID:; http://dx.doi.org/ 10.1016/j.gene.2013.01.066 [DOI] [PubMed] [Google Scholar]
- 28. Lech-Maranda E, Baseggio L, Bienvenu J, Charlot C, Berger F, Rigal D, Warzocha K, Coiffier B, Salles G. Interleukin-10 gene promoter polymorphisms influence the clinical outcome of diffuse large B-cell lymphoma. Blood 2004; 103:3529-34; PMID:; http://dx.doi.org/ 10.1182/blood-2003-06-1850 [DOI] [PubMed] [Google Scholar]
- 29. Blay JY, Burdin N, Rousset F, Lenoir G, Biron P, Philip T, Banchereau J, Favrot MC. Serum interleukin-10 in non-Hodgkin's lymphoma: a prognostic factor. Blood 1993; 82:2169-74; PMID: [PubMed] [Google Scholar]
- 30. Nacinovic-Duletic A, Stifter S, Dvornik S, Skunca Z, Jonjic N. Correlation of serum IL-6, IL-8 and IL-10 levels with clinicopathological features and prognosis in patients with diffuse large B-cell lymphoma. Int J Lab Hematol 2008; 30:230-9; PMID:; http://dx.doi.org/ 10.1111/j.1751-553X.2007.00951.x [DOI] [PubMed] [Google Scholar]
- 31. Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 2007; 179:977-83; PMID:; http://dx.doi.org/ 10.4049/jimmunol.179.2.977 [DOI] [PubMed] [Google Scholar]
- 32. De Wilde V, Van Rompaey N, Hill M, Lebrun JF, Lemaitre P, Lhomme F, Kubjak C, Vokaer B, Oldenhove G, Charbonnier LM. Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant 2009; 9:2034-47; PMID:; http://dx.doi.org/ 10.1111/j.1600-6143.2009.02757.x [DOI] [PubMed] [Google Scholar]
- 33. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, Bronte V, Chouaib S. PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med 2014; 211:781-90; PMID:; http://dx.doi.org/ 10.1084/jem.20131916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer 2013; 13:739-52; PMID:; http://dx.doi.org/ 10.1038/nrc3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci 2014; 1319:47-65; PMID:; http://dx.doi.org/ 10.1111/nyas.12469 [DOI] [PubMed] [Google Scholar]
- 36. Dennert G, Yogeeswaran G, Yamagata S. Cloned cell lines with natural killer activity. Specificity, function, and cell surface markers. J Exp Med 1981; 153:545-56; PMID:; http://dx.doi.org/ 10.1084/jem.153.3.545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, Saiki I. IFN-g-mediated inhibition of tumor angiogenesis by natural killer T- cell ligand, alpha-galactosylceramide. Blood 2002; 100:1728-33; PMID: [PubMed] [Google Scholar]
- 38. Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood 2011; 117:872-81; PMID:; http://dx.doi.org/ 10.1182/blood-2010-05-283820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 2006; 66:1123-31; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-05-1299 [DOI] [PubMed] [Google Scholar]
- 40. Nagaraj S, Nelson A, Youn JI, Cheng P, Quiceno D, Gabrilovich DI. Antigen-specific CD4(+) T cells regulate function of myeloid-derived suppressor cells in cancer via retrograde MHC class II signaling. Cancer Res 2012; 72:928-38; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-11-2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagaraj S, Youn JI, Gabrilovich DI. Reciprocal relationship between myeloid-derived suppressor cells and T cells. J Immunol 2013; 191:17-23; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1300654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schouppe E, Mommer C, Movahedi K, Laoui D, Morias Y, Gysemans C, Luyckx A, De Baetselier P, Van Ginderachter JA. Tumor-induced myeloid-derived suppressor cell subsets exert either inhibitory or stimulatory effects on distinct CD8(+) T-cell activation events. Eur J Immunol 2013; 43:2930-42; PMID:; http://dx.doi.org/ 10.1002/eji.201343349 [DOI] [PubMed] [Google Scholar]
- 43. Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182:4499-506; PMID:; http://dx.doi.org/ 10.4049/jimmunol.0802740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Smith HA, Kang Y. The metastasis-promoting roles of tumor-associated immune cells. J Mol Med (Berl) 2013; 91:411-29; PMID:; http://dx.doi.org/ 10.1007/s00109-013-1021-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pende D, Castriconi R, Romagnani P, Spaggiari GM, Marcenaro S, Dondero A, Lazzeri E, Lasagni L, Martini S, Rivera P. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood 2006; 107:2030-6; PMID:; http://dx.doi.org/ 10.1182/blood-2005-07-2696 [DOI] [PubMed] [Google Scholar]
- 46. Chijioke O, Munz C. Dendritic cell derived cytokines in human natural killer cell differentiation and activation. Front Immunol 2013; 4:365; PMID:; http://dx.doi.org/ 10.3389/fimmu.2013.00365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Van Elssen CH, Oth T, Germeraad WT, Bos GM, Vanderlocht J. Natural killer cells: the secret weapon in dendritic cell vaccination strategies. Clin Cancer Res 2014; 20:1095-103; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-2302 [DOI] [PubMed] [Google Scholar]
- 48. Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-g for TH1 priming. Nat Immunol 2004; 5:1260-5; PMID:; http://dx.doi.org/ 10.1038/ni1138 [DOI] [PubMed] [Google Scholar]
- 49. Schuster IS, Wikstrom ME, Brizard G, Coudert JD, Estcourt MJ, Manzur M, O'Reilly LA, Smyth MJ, Trapani JA, Hill GR, et al. . TRAIL+ NK Cells Control CD4+ T cell responses during chronic viral infection to limit autoimmunity. Immunity 2014; 41:646-56; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2014.09.013 [DOI] [PubMed] [Google Scholar]
- 50. Lang PA, Lang KS, Xu HC, Grusdat M, Parish IA, Recher M, Elford AR, Dhanji S, Shaabani N, Tran CW, et al. Natural killer cell activation enhances immune pathology and promotes chronic infection by limiting CD8+ T-cell immunity. Proc Natl Acad Sci U S A 2012; 109:1210-5; PMID:; http://dx.doi.org/ 10.1073/pnas.1118834109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Waggoner SN, Cornberg M, Selin LK, Welsh RM. Natural killer cells act as rheostats modulating antiviral T cells. Nature 2012; 481:394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Corraliza IM, Campo ML, Soler G, Modolell M. Determination of arginase activity in macrophages: a micromethod. J Immunol Methods 1994; 174:231-5; PMID:; http://dx.doi.org/ 10.1016/0022-1759(94)90027-2 [DOI] [PubMed] [Google Scholar]
- 53. Shimizu K, Asakura M, Shinga J, Sato Y, Kitahara S, Hoshino K, Kaisho T, Schoenberger SP, Ezaki T, Fujii S. Invariant NKT cells induce plasmacytoid dendritic cell (DC) cross-talk with conventional DCs for efficient memory CD8+ T cell induction. J Immunol 2013; 190:5609-19; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1300033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.