Abstract
Breast tumors are heterogeneous with a complex etiology. The immune system plays a crucial role in the development of tumors and can facilitate tumor growth pleiotropically. Myeloid derived suppressor cells (MDSCs) generate reactive oxygen species (ROS) and cytokines to suppress T cells, dendritic cells and natural killer (NK) cells. Hence, the inhibition of MDSCs could be an important strategy for anticancer therapeutics. Phenethyl isothiocyanate (PEITC), a bioactive compound present in cruciferous vegetables, is known to have anticancer properties. However, the effects of PEITC administration on the immune system have not been previously reported. In the current study, we evaluated the effects of administering PEITC to immunocompromised NOD-SCID IL2Rγ−/− (SCID/NSG) host mice bearing MDA-MB-231 xenografts on MDSCs in the peripheral blood. Our results reveal that oral administration of 12 μmol PEITC attenuated tumor growth by 76%. This was marked tumor-inhibitory phenotype was associated with a significant reduction in the levels of MDSCs bearing the surface markers CD33, CD34 and CD11b in PEITC treated mice, indicating that overall tumor growth suppression by PEITC correlates with inhibition of MDSCs. To the best of our knowledge, this is the first study showing effects of PEITC on MDSCs.
Keywords: breast cancer, PBMC, PEITC, myeloid-derived tumor suppressor cells, T lymphocytes
Abbreviations: i.p., intraperitoneal; MDSC, myeloid derived suppressor cell; PBMC. peripheral blood mononuclear cell; PEITC, Phenethyl isothiocyanate; PSN, penicillin streptomycin neomycin; ROS, reactive oxygen species; SCID/NSG, NOD-SCID IL2Rγ−/−
Introduction
Breast tumors are complex tissues consisting of a variety of factors that promote tumor growth. Secretion of cytokines, chemokines and growth factors by surrounding tumor cells, promotes tumor progression by multiple mechanisms. Some of these factors are known to suppress the immune response, thereby affecting tumor growth. One major mechanism by which pro-inflammatory or tumor secreted factors suppress antitumor immunity is the accumulation of myeloid derived suppressor cells (MDSCs).1 This association between inflammation and immune suppression is one of the major protumorigenic mechanisms of promoting breast tumor.2
MDSCs are a diverse population of immature myeloid cells derived from the bone marrow. MDSCs are known to suppress immune function by inhibiting T-cell activity.3-6 In addition, a few studies also indicate MDSCs suppress the immunologic functions of natural killer (NK) and dendritic cells, while concurrently stimulating regulatory T cells and tumor-associated macrophages.7 MDSCs consist of cells at different stages in their maturation, such as, monocytes, granulocytes, macrophages, dendritic cells and neutrophils.8 MDSCs can be classified as monocytic or polymorphic based on distinguishing surface markers for each class of MDSC.9 Monocytic MDSCs are known to be key mediators of immune suppression in tumors.9 MDSCs migrate to the tumor stroma and differentiate into tumor-associated macrophages, while the polymorphonuclear (PMN) cells arise from peripheral differentiation of MDSCs.3-6,9 The process of MDSC regulation and expansion has been well characterized.1,10 Cancer progression and metastasis is known to be associated with an increase in MDSCs.7,11,12 MDSCs presence and quantitation is also used clinically as a predictor of patient prognosis.7,13
Epidemiological evidence suggests a strong association between consumption of cruciferous vegetables, such as water cress and broccoli, and reduced risk of breast cancer.14,15 Phenethyl isothiocyanate (PEITC) is formed by enzymatic hydrolysis of glucosinolates present in cruciferous vegetables. A plethora of pre-clinical studies suggest a strong anticancer activity of PEITC.15-24 Phase I and II clinical trials are also in progress to test PEITC against lung cancer and leukemia.25 Hence we evaluated the effects of PEITC on tumor-modulatory immune cells circulating in the blood.
The effect of PEITC on human MDSCs was evaluated in immunocompromised NOD-SCID IL2Rγ−/− (SCID/NSG) mice bearing breast tumor xenografts. We used CD33, CD34 and CD11b as distinguishing monocytic markers to study the effects of PEITC on MDSCs.1,26-30 Our results show that PEITC treatment in mice inhibited mammary xenograft tumor growth in association with reduced CD33+, CD34+ and CD11b+ monocytes. To the best of our knowledge, this is the first report on the immunomodulatory effects of PEITC in a breast cancer model.
Results
PEITC treatment inhibits tumor growth
In order to determine the effect of PEITC on the growth of MDA-MB-231 tumors in vivo, 5 × 106 cells were implanted subcutaneously into each mouse, a day after the intraperitoneal injection of PBMCs. Control mice received vehicle, while the treatment group received 12 μmol (81 mg/kg; average weight ∼24 g) PEITC was administered by oral gavage every day once the tumor volume reached 80 mm3, with tumor size calculated based on measurements taken using vernier calipers. PEITC administration significantly retarded mammary tumor growth, such that the average tumor volume in treated group of mice at the end of experiment was 76% less as compared to the control group (Fig 1A). At the end of experiment mice were euthanized, tumors were dissected out and weighed. We observed a 66% reduction in tumor weights in PEITC treated mice as compared to control mice (Fig 1B). These results suggest strong inhibitory effects of PEITC on human breast tumor growth.
Figure 1.
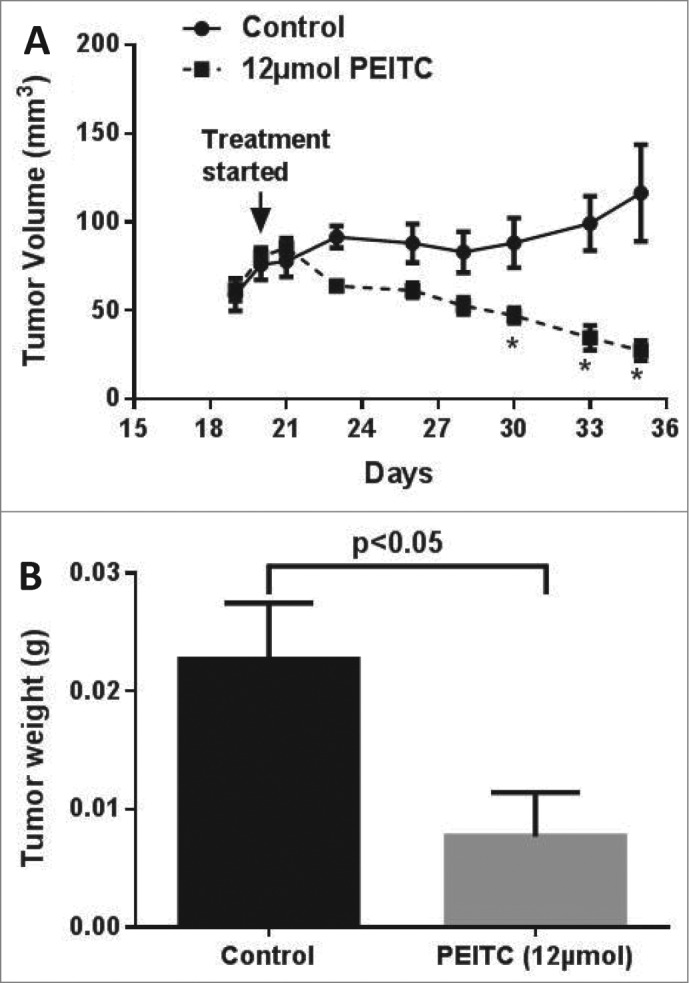
PEITC suppresses breast tumor growth. Human peripheral blood mononuclear cells (100×106) cells were injected i.p. in 100 μL PBS 1 d prior to s.c. transplantation of MDA-MB-231 in SCID/NSG mice. The treatment group received 12 μmol PEITC in PBS by oral gavage daily; control mice received vehicle alone; n = 8/group; statistical analysis was performed by Student's t test; asterisk indicates statistical significance (p < 0.05). (A) Tumor growth curves. (B) Endpoint tumor weights.
The effect of PEITC on circulating leukocytes
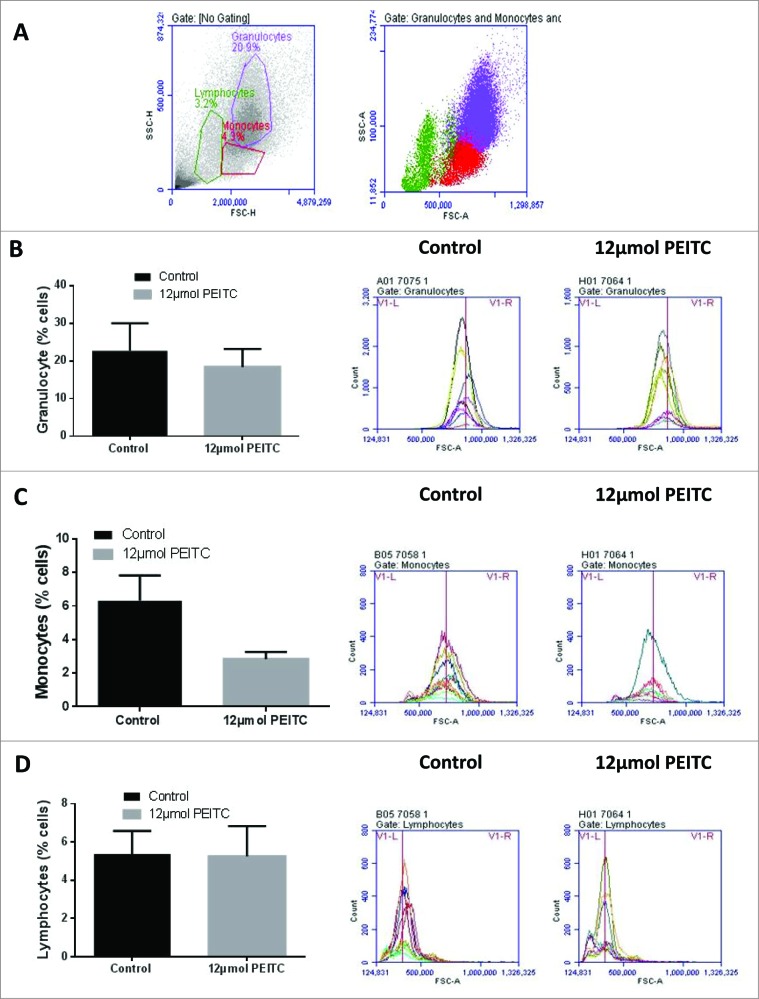
We observed significant inhibition of tumor growth by PEITC treatment in mice. To elucidate the mechanisms of antitumor effects, we evaluated the effects of PEITC treatment on blood leukocytes (Fig 2A). Peripheral blood mononuclear cells (PBMCs) were collected from the blood of treated versus control mice and the relative percentages of various populations of monocytes, granulocytes and lymphocytes were determined using flow cytometry. The events were plotted on dot plot and different populations were gated based on their light scatter profiles.31 Our results showed that PEITC treatment modestly reduced granulocyte and monocyte populations (Fig 2B & C). Surprisingly, PEITC treatment had no effect on the lymphocyte population (Fig 2D). These results indicated that there was no substantial effect of PEITC treatment on overall leukocyte counts.
Figure 2.
Effect of PEITC on different population of leukocytes. Peripheral blood leukocytes in PEITC treated versus control treated MDA-MB-231 tumor-bearing SCID/NSG mice (n = 3) were analyzed by immunostaining and fluorescence cytometry. (A) Representative dot plots for monocyte, lymphocyte and granulocyte populations in peripheral blood mononuclear cell samples collected from mice (B–D) Histograms showing the effects of PEITC treatment by flow cytometry on granulocytes (B), monocytes (C) and lymphocytes (D). The bar chart on the left shows the mean ± SEM percentage of the indicated leukocyte population; representative histograms for the control versus treatment groups are on the right, as indicated.
PEITC treatment reduces myeloid derived tumor suppressor cells
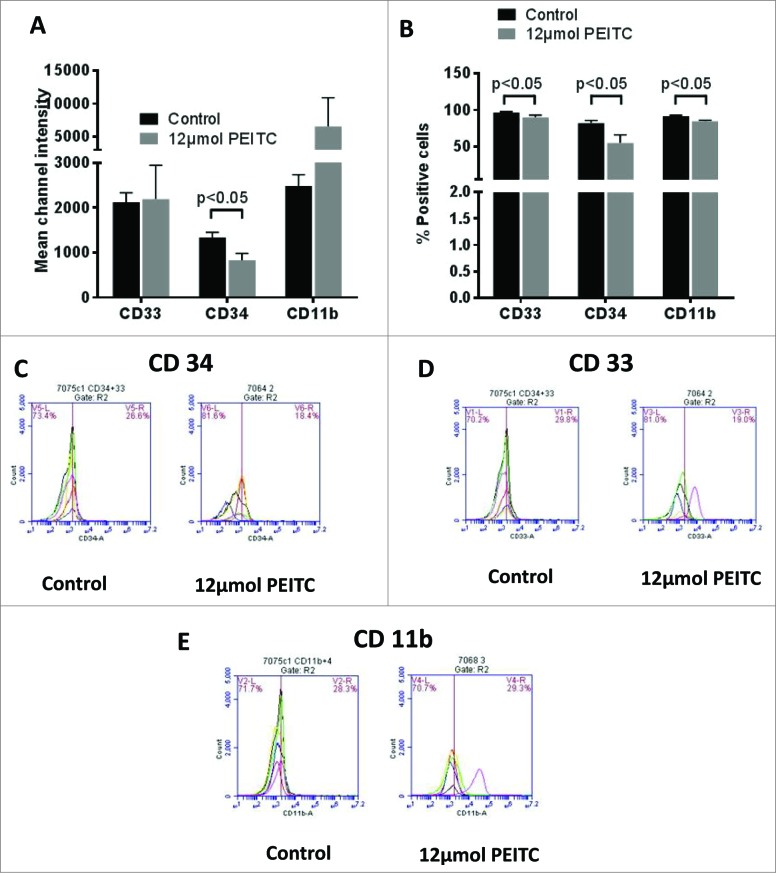
Since PEITC treatment did not have any effect on the number of leukocytes, we evaluated its effects on immunosuppressive MDSCs using specific markers. MDSCs are well known for their tumor promoting effects. We used CD33, CD34 and CD11b markers to determine the effects of PEITC on MDSCs. Monocytic MDSCs were analyzed by gating monocytes on the basis of on light scattering profiles of the cells. Our results showed that PEITC significantly suppressed CD33+CD34+CD11b+ monocytes. We observed a 38% reduction in overall intensity for CD34 staining in PBMCs collected from PEITC treated mice (Fig 3A). Our results also showed a 33% reduction in the CD34+ monocytes (Fig 3B & C). In addition, we observed about 8-9% reduction in the number of CD33+ and CD11b+ monocytes (Fig 3B, D & E). However, the intensity of CD33 staining remained unchanged (Fig 3A). The PEITC treated group showed an increase in CD11b staining, but this difference was not observed to be statistically significant (Fig 3A). Taken together, these observations indicate that PEITC suppresses the monocytic population of MDSCs.
Figure 3.
Effect of PEITC on MDSCs. Peripheral blood mononuclear cells (PBMCs) were collected from the blood obtained from control and PEITC treated MDA-MB-231 tumor-bearing SCID/NSG mice (n = 3). Modulation of CD33, CD34 and Cd11b was analyzed by immunostaining and fluorescence cytometry to determine the effects of PEITC on myeloid derived suppressor cells (MDSCs); statistical analysis was performed by Student's t test. (A) Bar charts showing mean channel intensities for CD33, CD34 or CD11b staining on monocytes. (B) Histograms for monocytes positive for CD33, CD34 or CD11b. (C–E) Flow cytometry showing monocytes positive for CD34 (C), CD33 (D) or CD11b (E) respectively in control and PEITC treated samples.
PEITC reduces CD4+ T lymphocytes
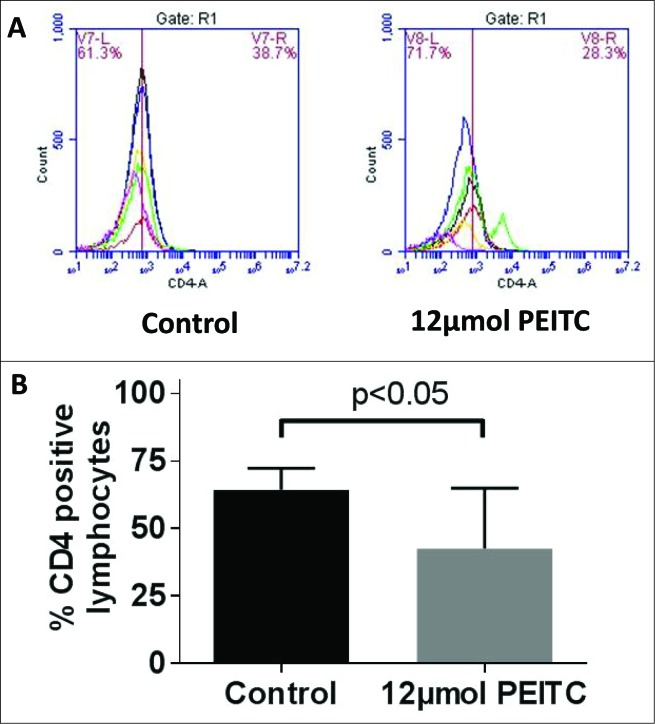
MDSCs can cause the expansion of regulatory CD4+ T cell population. Hence, it was important to evaluate the effect of PEITC on CD4+ T cells. Our results revealed that PEITC treatment caused a small reduction in CD4+ lymphocytes. The percentage of lymphocytes positive for CD4 was 34% less in PEITC treated PBMC samples as compared to control PBMC samples (Fig 4A & B). These observations suggested that PEITC treatment reduced the levels of the CD4+ T cell subset.
Figure 4.
Effect of PEITC on T cells levels. Peripheral blood mononuclear cells (PBMCs) were collected from blood obtained from control and PEITC treated MDA-MB-231 tumor-bearing SCID/NSG mice (n = 3). Modulation of CD4 was monitored by immunostaining and fluorescence cytometry to determine the effects of PEITC specifically on CD4+ T cells; statistical analysis was performed by Student's t test. (A) Representative histograms showing reduced CD4+ lymphocytes (B) Bar charts showing mean percentage of lymphocytes positive for CD4 in control and PEITC treated samples.
Discussion
The etiology of breast cancer is highly variable and involves numerous factors. Recent advancements have shown that the immune system can foster cancer progression in multiple ways.2 MDSCs are known to be an important cancer-promoting component of the immune system hijacked by the malignant microenvironment.10,32 MDSCs are immature cells with myeloid origin that are known to suppress antitumor immunity by inhibiting the activity of NK cells, dendritic cells, and cytotoxic T lymphocytes.7 In addition, MDSCs cause expansion of CD4+ regulatory T cells, which further promote tumor growth. Previous studies from our lab and others have shown strong anticancer activity of PEITC against mammary carcinoma.17,18,20 Our present study demonstrates that the antitumor effect of PEITC is associated with the inhibition of MDSCs in breast cancer.
Halasi et al. previously evaluated the antitumor effects of PEITC on MDA-MB-231 xenografts.33 About 2×106 MDA-MB-231 cells were implanted subcutaneously and 25 mg/kg PEITC was administered by intraperitoneal route, 4-5 times a week for 4 weeks.33 However, no effect of PEITC on tumor growth was observed when administered alone. However, when PEITC was administered along with bortezomib, a proteasome inhibitor, a 75% reduction in tumor volume was observed.33 We administered 12 μmol (81 mg/kg) PEITC by oral route every day to the mice implanted with 5 × 106 MDA-MB-231 cells. We observed a 76% suppression of tumor growth after 5 weeks of PEITC treatment. The difference in the efficacy of PEITC could be attributed to the relatively higher dose of PEITC used in our study.
PEITC acts as a strong antitumor agent by inhibiting survival pathways, such as, nuclear factor kB (NFkB), signal transducer and activator of transcription 3 (STAT3) and hypoxia inducible factor 1α (HIF1α).16 Previous studies have shown that inhibition of these pathways suppresses MDSC activity and inhibits cancer progression.34-36 Tsou et al. used 80 mg/kg PEITC for 4 weeks to study its effects on macrophages, T cells, NK cells and B cells in a mouse model of leukemia.37 This study showed that PEITC treatment induced activity of NK cells, as determined by the count of target cells.37 In contrast, we did not see any effect of PEITC on NK cells as evaluated by surface marker CD56 (data not shown). Differences in the mode of evaluation could be responsible for this discrepancy. Nonetheless, the effect of PEITC on NK cells needs to be evaluated in other tumor models.
Inhibition of MDSCs can suppress tumor growth and metastasis, as well as increase efficacy of antitumor vaccines in murine models.32 However, tumor suppressive effects or accumulation rate of MDSC vary significantly with the model used.38 Hence we decided to demonstrate antitumor effects of PEITC on breast tumor xenografts using transplanted human PBMCs. To mimic human conditions, we injected human PBMCs before implantation of tumors to allow better interaction of PBMCs with the tumor cells. In addition, this also allowed maturation of PBMC cells into MDSCs with a human phenotype. Human MDSCs can be characterized by the presence of surface markers CD11b, CD34 and CD33.7,32 Although Tsou et al. did not determine the effects of PEITC on MDSCS, CD11b was assessed for effects of PEITC on macrophages. The study showed that PEITC treatment increased CD11b level in white blood cells in control BALB/c mice, while in WEHI-3 leukemia mice, PEITC has no effect. Our study showed reduced expression of CD11b+ monocytes in response to PEITC treatment. We used monocytes to evaluate CD11b expression as compared to the method evaluating total white blood cells used by Tsou et al. In addition, we examined the effects of PEITC treatment on MDSCs using cell surface markers CD11b, CD33 and CD34. Our results showed that CD33+ and CD34+ cells were significantly reduced by PEITC treatment. Hence, our results suggest PEITC treatment-induced inhibition of MDSCs.
MDSCs induce regulatory CD4+ T-cell activity to suppress immune activity against breast tumor cells.7 Tsou et al. have reported increased T-cell (CD3+) population by PEITC, while PEITC treatment inhibited the differentiation of T-cell precursor cells.37 However, in contrast, our results showed a reduced population of CD4+ T cells in PEITC treated samples. Nonetheless, mode of action of PEITC on T cells, as well the specific T-cell population affected by PEITC, remains to be further studied in detail.
Taken together, our study reports a novel antitumor mechanism of PEITC. Our results indicate that PEITC inhibits the CD4+ T-cell population, which is regulated by MDSCs. These immunologic effects of PEITC were observed in conjunction with reduced tumor growth in mice. Nonetheless, previous studies from our lab and others have shown multivariate effects of PEITC on cancer, suggesting possible broader effects of PEITC on the immune system.16 Hence, it is possible that PEITC works to suppress CD4+ T cells as well as tumor growth partly via mechanisms distinct from MDSC inhibition. This is of interest because MDSCs function along with other cells of the immune system to form an integral aspect of the complex tumor microenvironment. Our study lays the foundation for further explorations into the immunotherapeutic effects of PEITC on the tumor microenvironment and the myriad of immune components regulating tumor growth.
Materials and Methods
Ethics Statement. Experiments in mice were conducted in accordance with the ethical standards and approved protocol by our Institutional Animal Care and Use Committee (IACUC).
Cell culture. Human breast carcinoma cell lines MDA-MB-231 were purchased from ATCC (# HTB-26). ATCC uses DNA fingerprinting (microsatellite analysis) for cell line authentication. The cells used in this study were within twenty passages after receipt or resuscitation.
Human PBMCs isolation from buffy coat. The healthy human buffy coat was obtained from an anonymous donor by the Coffee Memorial Blood Bank, Amarillo, TX. To isolate PBMCs, the buffy coat was diluted 2 fold with sterile PBS and 25 mL of this was gently layered over 10 mL of Ficoll-Paque reagent (# 17-1440-02, GE Lifesciences). The separation was performed per manufacturer's protocol. Briefly, the prepared sample was centrifuged at 400g at 20°C for 40 min (with no brakes applied) and the PBMC layer was collected from the interface of Ficoll-Paque and plasma. The collected PBMCs were washed with PBS and incubated at 37°C with red blood cell (RBC) lysis buffer. The cells were then centrifuged, washed with PBS and collected.
Injection of PBMCs in mice. Female SCID/NSG mice (4-6 weeks old) were obtained from the animal facility (Texas Tech University Health Sciences Center, Lubbock) and maintained under specific pathogen-free conditions. Mice were kept on antioxidant-free AIN-76A special diet (# CA170481, Harlan Laboratories) 1 week before starting the experiment. The PBMCs were counted and 100 × 106 cells in 100 μL PBS were intraperitoneal (IP) injected in each mouse.
Tumor therapy model. The day following PBMCs injection, MDA-MB-231 breast cancer xenografts were implanted into each mouse. MDA-MB-231 cells were harvested during exponential phase of growth using trypsin and washed with PBS. The cells were resuspended in 1:1 PBS/matrigel at a density of 50 × 106 cells per mL. A 100 μL cell suspension volume comprising 5 × 106 cells was injected subcutaneously into the left flank of recipient mice. Vernier calipers were used to measure tumor volumes 3 times a week, as described previously.39,40 Once the tumor volume reached 80 mm3, mice were randomly segregated into 2 groups with 8 mice in each group. The treatment group of mice received 12 μmol PEITC (# 253731, Sigma-Aldrich) in PBS by oral gavage every day till day 35. The control mice received vehicle alone. The experiment was terminated at day 35 and mice were sacrificed by CO2 overdose in accordance with IACUC guidelines. The tumors were dissected out and weighed.
PBMC collection from mouse blood and fluorescence cytometric analysis. To analyze the effects of PEITC treatment, PBMCs were collected from the blood of experimental and control mice. The blood from each mouse was diluted 10-fold with the RBC lysis buffer. The diluted blood was incubated as described above. The cells were washed with PBS followed by a second wash in FACS buffer [2% heat inactivated fetal bovine serum and 2 mM ethylenediaminetetraacetic acid (EDTA)] in PBS. The cells collected from each mouse were equally divided into 3 sets. Each set of cells was re-suspended in equal volume of FACS buffer. Fluorophore-conjugated human specific antibodies CD13-R-Phycoerythrin (R-PE) (# MHCD1304), CD33-fluorescein isothiocyanate (FITC) (#MHCD3301), CD34-R-PE (#CD34-581-04), CD11b-FITC (#CD11b01) and CD4-Allophycocyanin (APC) (#MHCD0405) were purchased from Invitrogen (Life Technologies). The samples to be analyzed were first incubated with human FcR blocking reagent at 4°C in dark for 15 min (#130-059-90, Miltenyi Biotech). The cells were then washed and appropriate antibodies were added to each set of cells and the samples were incubated on ice for 30 min in dark. The samples were then washed and re-suspended in 300 μL of FACS buffer. The cell phenotype was determined using cytofluorimetric analysis on an Accuri C6 flow cytometer (BD Biosciences). To identify monocytic and lymphocytic cell subsets, cells were gated based on their light scatter profiles on dot plots.31 The monocytic cell population was used to evaluate effects on MDSCs and the lymphocytic population was used to determine T-cell modulations. Accuri C6 flow cytometer software was used to compare CD11b, CD33, CD34 or CD4 expressing cell populations as well as surface densities for these receptors in control and PEITC treated groups. The parameter used to obtain positive cell populations was overall peak shifts for each staining antibody, while for surface receptor densities, mean channel intensities were used for the comparison. For each staining antibody, mean channel intensities were used for comparison.
Statistical Analysis: Statistical analysis was performed using Prism 6.0 (GraphPad software Inc.). Results were represented as means ± SEM with minimum value of n = 3. Data was analyzed by Student's t-test. Differences were considered statistically significant at p < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by R01 grant CA129038 (to Sanjay K. Srivastava) awarded by the National Cancer Institute, NIH. Dr. Sanjay K. Srivastava is an International Scholar at Kyunghee University, Seoul, South Korea.
Authors’ Contributions
Parul Gupta was responsible for designing the study, performing the experiments and writing the first draft of the manuscript. Stephen E Wright and Sanjay K Srivastava were responsible for designing the study, analyzing the data and writing the manuscript.
References
- 1.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of Immunology 2009; 182:4499-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646-74. [DOI] [PubMed] [Google Scholar]
- 3.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews. Immunology 2009; 9:162-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Seminars in Cancer Biology 2006; 16:53-65. [DOI] [PubMed] [Google Scholar]
- 5.Poggi A, Musso A, Dapino I, Zocchi MR. Mechanisms of tumor escape from immune system: Role of mesenchymal stromal cells. Immunology Letters 2014; 159:55-72. [DOI] [PubMed] [Google Scholar]
- 6.Solito S, Bronte V, Mandruzzato S. Antigen specificity of immune suppression by myeloid-derived suppressor cells. Journal of Leukocyte Biology 2011; 90:31-6. [DOI] [PubMed] [Google Scholar]
- 7.Markowitz J, Wesolowski R, Papenfuss T, Brooks TR, Carson WE., 3rd. Myeloid-derived suppressor cells in breast cancer. Breast Cancer Research and Treatment 2013; 140:13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunological Reviews 2008; 222:162-79. [DOI] [PubMed] [Google Scholar]
- 9.Nagaraj S, Youn JI, Gabrilovich DI. Reciprocal relationship between myeloid-derived suppressor cells and T cells. Journal of Immunology 2013; 191:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nature Reviews. Immunology 2012; 12:253-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Cruz-Merino L, Barco-Sanchez A, Henao Carrasco F, Nogales Fernandez E, Vallejo Benitez A, Brugal Molina J, Martinez Peinado A, Grueso Lopez A, Ruiz Borrego M, Codes Manuel de Villena M, et al. New insights into the role of the immune microenvironment in breast carcinoma. Clinical & Developmental Immunology 2013; 2013:785317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Ye TH, Han YP, Song H, Zhang YK, Xia Y, Wang NY, Xiong Y, Song XJ, Zhu YX, et al. Reductions in Myeloid-Derived Suppressor Cells and Lung Metastases using AZD4547 Treatment of a Metastatic Murine Breast Tumor Model. Cellular Physiology and Biochemistry 2014; 33:633-45. [DOI] [PubMed] [Google Scholar]
- 13.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clinical Cancer Research 2000; 6:1755-66. [PubMed] [Google Scholar]
- 14.Boggs DA, Palmer JR, Wise LA, Spiegelman D, Stampfer MJ, Adams-Campbell LL, Rosenberg L. Fruit and vegetable intake in relation to risk of breast cancer in the Black Women's Health Study. American Journal of Epidemiology 2010; 172:1268-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. The Journal of Nutrition 2004; 134:1134-8. [DOI] [PubMed] [Google Scholar]
- 16.Gupta P, Wright S, Kim S, Srivastava SK. Phenethyl Isothiocyanate: A comprehensive review of anti-cancer mechanisms. BBA Reviews on Cancer 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta P, Kim B, Kim SH, Srivastava SK. Molecular targets of isothiocyanates in cancer: Recent advances. Molecular Nutrition & Food Research 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta P, Adkins C, Lockman P, Srivastava SK. Metastasis of breast tumor cells to brain is suppressed by phenethyl isothiocyanate in a novel in vivo metastasis model. PLoS One 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loganathan S, Kandala PK, Gupta P, Srivastava SK. Inhibition of EGFR-AKT axis results in the suppression of ovarian tumors in vitro and in preclinical mouse model. PLoS One 2012; 7:e43577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta P, Srivastava SK. Antitumor activity of phenethyl isothiocyanate in HER2-positive breast cancer models. BMC Medicine 2012; 10:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wattenberg LW. Inhibition of carcinogenesis by minor anutrient constituents of the diet. Proceedings of the Nutrition Society 1990; 49:173-83. [DOI] [PubMed] [Google Scholar]
- 22.Palmer S. Diet, nutrition, and cancer. Progress in Food & Nutrition Science 1985; 9:283-341. [PubMed] [Google Scholar]
- 23.Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C, Cheng JR, Shu XO, Gao YT, Zheng W. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Research 2003; 63:3980-6. [PubMed] [Google Scholar]
- 24.Wang X, Di Pasqua AJ, Govind S, McCracken E, Hong C, Mi L, Mao Y, Wu JY, Tomita Y, Woodrick JC, et al. Selective Depletion of Mutant p53 by Cancer Chemopreventive Isothiocyanates and Their Structure-Activity Relationships. Journal of Medicinal Chemistry 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelloff GJ, Crowell JA, Hawk ET, Steele VE, Lubet RA, Boone CW, Covey JM, Doody LA, Omenn GS, Greenwald P, et al. Strategy and planning for chemopreventive drug development: clinical development plans II. Journal of Cellular Biochemistry. Supplement 1996; 26:54-71. [DOI] [PubMed] [Google Scholar]
- 26.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. Journal of Immunology 2010; 185:2273-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. Journal of Clinical Oncology 2007; 25:2546-53. [DOI] [PubMed] [Google Scholar]
- 28.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Research 2005; 65:3044-8. [DOI] [PubMed] [Google Scholar]
- 29.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Research 2006; 66:9299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients’ CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunology, Immunotherapy : CII 2008; 57:1493-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colovai AI, Giatzikis C, Ho EK, Farooqi M, Suciu-Foca N, Cattoretti G, Orazi A. Flow cytometric analysis of normal and reactive spleen. Modern Pathology 2004; 17:918-27. [DOI] [PubMed] [Google Scholar]
- 32.Wesolowski R, Markowitz J, Carson WE., 3rd. Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer. Journal for Immunotherapy of Cancer 2013; 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halasi M, Pandit B, Wang M, Nogueira V, Hay N, Gartel AL. Combination of oxidative stress and FOXM1 inhibitors induces apoptosis in cancer cells and inhibits xenograft tumor growth. The American Journal of Pathology 2013; 183:257-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J, Wang Y, Yan F, Zhang P, Li H, Zhao H, Yan C, Yan F, Ren X. Noncanonical NF-kappaB Activation Mediates STAT3-Stimulated IDO Upregulation in Myeloid-Derived Suppressor Cells in Breast Cancer. Journal of Immunology 2014; 193:2574-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mace TA, King SA, Ameen Z, Elnaggar O, Young G, Riedl KM, Schwartz SJ, Clinton SK, Knobloch TJ, Weghorst CM, et al. Bioactive compounds or metabolites from black raspberries modulate T lymphocyte proliferation, myeloid cell differentiation and Jak/STAT signaling. Cancer Immunology, Immunotherapy : CII 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. The Journal of Experimental Medicine 2010; 207:2439-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsou MF, Tien N, Lu CC, Chiang JH, Yang JS, Lin JP, Fan MJ, Lu JJ, Yeh SP, Chung JG. Phenethyl isothiocyanate promotes immune responses in normal BALB/c mice, inhibits murine leukemia WEHI-3 cells, and stimulates immunomodulations in vivo. Environmental Toxicology 2011. [DOI] [PubMed] [Google Scholar]
- 38.Kapanadze T, Gamrekelashvili J, Ma C, Chan C, Zhao F, Hewitt S, Zender L, Kapoor V, Felsher DW, Manns MP, et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. Journal of Hepatology 2013; 59:1007-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boreddy SR, Pramanik KC, Srivastava SK. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clinical Cancer Research 2011; 17:1784-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahu RP, Srivastava SK. The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. Journal of the National Cancer Institute 2009; 101:176-93. [DOI] [PMC free article] [PubMed] [Google Scholar]