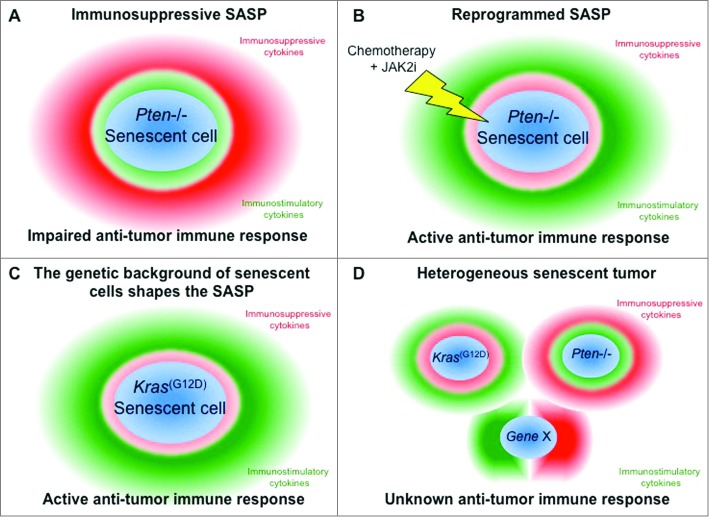
Senescent cells promote tumor clearance by evoking an antitumor immune response. However in Pten-null prostate tumors treated with chemotherapy, the antitumor immune response is blocked by activation of an immune suppressive senescence-associated secretory phenotype (SASP). Inhibition of the JAK2/STAT3 pathway reprograms the SASP of Pten-null tumors inducing a strong antitumor immune response and enhancing chemotherapy efficacy. Therefore, senescence surveillance induced by oncogenic stress or chemotherapy can be suppressed in certain genetic backgrounds but also reactivated by pharmacological treatments
Cellular senescence, a stable cell growth arrest, can be triggered by different insults including activation of oncogenes (oncogene-induced senescence (OIS)) or loss of tumor suppressor genes both in vitro and in vivo.1,2 Importantly, several evidences have demonstrated that senescence opposes tumor initiation and progression in different mouse models.2,3 However, senescent tumor cells secrete a variety of immune modulators and inflammatory cytokines, referred to as the SASP that mediates opposing and contradictory effects. Indeed, if on one hand the SASP stimulates the innate and adaptive antitumor immune response (a process designated as “senescence surveillance”) leading to tumor clearance, on the other hand it also promotes tumorigenesis by sustaining the proliferation of neighboring tumor cells.4,5 Therefore, the contradictories effects of the SASP cast doubts over the possibility to use and develop pro-senescence compounds for cancer therapy.3,6 We have previously demonstrated that loss of the tumor suppressor gene Pten triggers a strong senescence response both in vitro and in vivo.1,7 We named this novel senescence response Pten loss induced-cellular senescence (PICS).7 Pten null prostate conditional mice (Ptenpc−/−) develop a prostatic intraepithelial neoplasia (PIN) characterized by a senescence response that constrains tumorigenesis. In our study, we have characterized both the cytokine profile and the tumor-associated immune response of Ptenpc−/− senescent prostate tumors at the onset of tumorigenesis and in respect with tumor progressions.8 High-throughput multi-analyte profiling of different cytokines revealed that several factors reported to play a negative role in cancer pathogenesis (e.g. CXCL1, CXCL2, GM-CSF, M-CSF, and IL-10) were upregulated in Ptenpc−/− senescent tumors. However, chemo-attractant cytokines that have been previously shown to play a positive role in the process of inflammation associated to senescence surveillance in OIS (e.g., CXCL10 and MCP-1)9 were also upregulated in these tumors. Of note, CXCL1 and CXCL2 are potent recruiters of myeloid cells. In line with the cytokine array profile, FACS analysis showed that Ptenpc−/− tumors were strongly infiltrated by CD11b+Gr-1+ myeloid cells. Functional characterization of CD11b+Gr-1+ cells sorted from Ptenpc−/− senescent tumors, revealed that these cells were myeloid derived suppressor cells (MDSCs). The presence of MDSCs in the tumor microenvironment of Ptenpc−/− tumors explained the almost complete absence of tumor infiltrating cytotoxic CD8+ T and NK cells and lack of tumor immunosurveillance in Pten null mice before and after docetaxel treatments. Importantly, we could also show that the immunosuppressive SASP of PICS, and the consequent recruitment of MDSCs at the tumor site, was orchestrated by activation of the Jak2/Stat3 pathway in epithelial tumor cells. Indeed, both genetic and pharmacological inhibition of the Jak2/Stat3 pathway reprogrammed the SASP of PICS by reducing the overall level of immunosuppressive cytokines while retaining unchanged, or even increased, levels of immunostimulatory cytokines. The reprogrammed SASP (R-SASP) blunted the recruitment of MDSCs and evoked a strong antitumor immune response characterized by the presence of tumor infiltrating cytotoxic CD8+ T and NK cells. This antitumor immune response led to decreased tumor size and invasiveness. Notably, we found that in prostate tumors of Ptenpc−/−; Stat3pc−/− double knock mice and Ptenpc−/− mice treated with a JAK2 inhibitor senescence was not blocked and the Nf-kB signaling was still activated. This observation is line with previous reports describing the essential role of Nf-kB in sustaining the SASP and execution of the senescence program in tumor cells. Therefore, Stat3 is not essential for the execution of PICS and the overall activation of the SASP. However Stat3 acts as an enhancer of the negative components of the SASP by increasing the level of the immunosuppressive cytokines. Of great relevance from a therapeutic point of view, we also tested the effect of Jak2/Stat3 inhibition in the context of chemotherapy. Ptenpc−/− mice have been shown to be irresponsiveness to the chemotherapeutic agent Docetaxel.10 In Ptenpc−/− mice treated with Docetaxel we found an enhanced senescence response and increased phosphorylation of Stat3 that resulted in activation of the negative SASP of Ptenpc−/− tumors. Therefore, when we combined Docetaxel treatment with a Jak2 inhibitor, we observed an increased senescence response accompanied by an active antitumor immune response and tumor clearance. In this work, we also showed that activation of the Jak2/Stat3 pathway was sustained by downregulation (both protein and gene expression) of one of the major negative regulator of this pathway, the tyrosine phosphatase (PTP) SHP2 (gene known as PTPN11). Surprisingly, the levels of SHP2 (mRNA and protein) were correlating with the levels of PTEN also in human tumors. Notably, when PTEN was downregulated by mean of an siRNA in both normal and prostate cancer human cell lines, PTPN11 was concomitantly downregulated. Moreover, the correlation between the mRNA levels of PTEN and PTPN11 was also observed in several types of tumors, unveiling a novel PTEN-PTPN11 protein phosphatases network that may play a similar immunosuppressive role in different cancers.
Finally, by comparing two different types of senescence responses in prostate, PICS (driven by loss of Pten) vs. OIS (driven by activation of the oncogene Kras(G12D)) we found that in Kras(G12D) driven senescent tumors, and in stark contrast to PICS, the levels of PTPN11 were not downregulated and the Jak2/Stat3 pathway was poorly activated. In OIS, absence of Stat3 activation and normal level of SHP2 were associated to a reduced intensity of the immunosuppressive SASP, when compared to PICS. Indeed, the SASP of OIS was characterized by lower levels of immune suppressive cytokines such as CXCL1, CXCL2, IL-10. This explained the absence of tumor infiltrating MDSCs and the presence of an active immunosurveillance in Kras(G12D) driven senescent tumors, confirming the results of other studies. In conclusion, our work suggests that activation of Jak2/Stat3 pathway is the key determinant underlying the difference between the SASPs of PICS and OIS, and demonstrates that the pro-tumorigenic features of the SASP depends on the genetic background of senescent tumor cells. Importantly, we showed that the SASP of senescent tumors can be pharmacologically reprogrammed. In future, it would be interesting to generate novel mouse models in which different genetic alterations are triggered at the same time in a specific tissue (Fig. 1). This will allow researchers to study how different genetic alterations in tumors influence the SASP and the associated antitumor immune response.
Figure 1.
Impact of the SASP on the tumor-associated immune response. (A) The SASP of Pten null senescent tumors is characterized by high levels of immunosuppressive cytokines that block the antitumor immune response. (B) The combinatorial treatment of Pten null tumors with chemotherapy (e.g. Docetaxel) in combination with a JAK2 inhibitor (JAK2i) enhances senescence and reprograms the SASP, leading to an active antitumor immune response. (C) The SASP of Kras(G12D) senescent tumors has reduced levels of immunosuppressive cytokines when compared to the SASP of Pten null tumors. Thus, the antitumor immune response is not impaired. (D) Unknown immune response in a heterogeneous senescent tumor.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005; 436:725-30; PMID:; http://dx.doi.org/ 10.1038/nature03918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collado M, Serrano M. Senescence in tumours: evidence from mice and humans. Nat Rev Cancer 2010; 10:51-7; PMID:; http://dx.doi.org/ 10.1038/nrc2772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nardella C, Clohessy JG, Alimonti A, Pandolfi PP. Pro-senescence therapy for cancer treatment. Nat Rev Cancer 2011; 11:503-11; PMID:; http://dx.doi.org/ 10.1038/nrc3057 [DOI] [PubMed] [Google Scholar]
- 4. Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011; 479:547-51; PMID:; http://dx.doi.org/ 10.1038/nature10599 [DOI] [PubMed] [Google Scholar]
- 5. Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol 2011; 192:547-56; PMID:; http://dx.doi.org/ 10.1083/jcb.201009094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bard-Chapeau EA, Li S, Ding J, Zhang SS, Zhu HH, Princen F, Fang DD, Han T, Bailly-Maitre B, Poli V, et al. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell 2011; 19:629-39; PMID:; http://dx.doi.org/ 10.1016/j.ccr.2011.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, Cheng K, Varmeh S, Kozma SC, Thomas G, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest 2010; 120:681-93; PMID:; http://dx.doi.org/ 10.1172/JCI40535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Toso A, Revandkar A, Di Mitri D, Guccini I, Proietti M, Sarti M, Pinton S, Zhang J, Kalathur M, Civenni G, et al. Enhancing chemotherapy efficacy in pten-deficient prostate tumors by activating the senescence-associated antitumor immunity. Cell Rep 2014; 9:75-89; PMID:; http://dx.doi.org/ 10.1016/j.celrep.2014.08.044 [DOI] [PubMed] [Google Scholar]
- 9. Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW, et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007; 445:656-60; PMID:; http://dx.doi.org/ 10.1038/nature05529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Di Mitri D, Toso A, Chen JJ, Sarti M, Pinton S, Jost TR, Krizhanovsky V, D'Antuono R, Montani E, Garcia-Escudero R, Guccini I, et al. Tumour-infiltrating Gr-1 myeloid cells antagonize senescence in cancer. Nature 2014; 515(7525):134-7; PMID: [DOI] [PubMed] [Google Scholar]