Abstract
Sorafenib is a multi-kinase inhibitor used alone or in combination with dacarbazine to treat metastasized melanoma. Our study investigated the relationship between metabolic response assessed by PET-CT and global transcriptome changes during sorafenib and dacarbazine therapy in patients with advanced melanoma. We conducted an open-label, investigator-initiated study that enrolled 13 sorafenib-naïve Stage IV melanoma patients, whose metastases were accessible for repeated biopsies. Treatment regimen included orally administered sorafenib and intravenous dacarbazine. Biopsies of skin or superficial lymph node metastases were taken before treatment (baseline), during sorafenib and after dacarbazine therapy and used for transcriptional profiling and validation experiments. Serum samples were evaluated for cytokine production. Metabolic response to therapy was observed in 45.5% of patients. The study drugs were well tolerated. We observed a clear upregulation of interferon (IFN)-stimulated immune response genes in profiled metastases. The IFNγ-induced gene signature seemed to be enhanced after addition of dacarbazine to sorafenib. Serum IFNγ also increased during therapy, particularly after addition of dacarbazine. Induction of IFNγ stimulated genes correlating with increased serum IFNγ was predictive of better clinical outcome and responders who had significantly higher serum IFNγ levels lived longer. Our data reveal in situ changes in melanoma metastases during treatment with sorafenib and dacarbazine and suggest an additional mechanism of action through immunomodulation.
Keywords: dacarbazine, gene expression profiling, interferon-γ, melanoma, sorafenib
Abbreviations: AE, adverse events CMR, complete metabolic response; ISG, IFN-stimulated gene; PMD, progressive metabolic disease; PMR, partial metabolic response; SAE, serious adverse events SMD; stable metabolic disease
Introduction
Over the last 4 decades, the rate of melanoma incidence has been constantly increasing.1 Despite regional variability, European countries have not yet reached the incidence rates of Australia and the United States (46.7 and 22.5 per 100,000 people, respectively).1-4 Treatment of metastatic melanoma remains challenging,1,3,4 even though prolonged survival has been achieved by the use of the recently FDA-approved molecules, such as the anti-CTLA-4 blocking antibody ipilimumab and the BRAF kinase inhibitors vemurafenib and dabrafenib.5 With registration of ipilimumab, which will surely be followed by other compounds intervening at immunological checkpoints, immunotherapy of melanoma has been implemented into standard therapeutic regimens worldwide.
Sorafenib is an orally available multi-kinase inhibitor that targets tumor cell proliferation and angiogenesis. It intervenes in the protein kinase RAF/MEK/ERK pathway by inhibiting several Raf kinase isoforms, including RAF1, wild-type BRAF and mutant BRAF (for a review see ref. 6) Furthermore, sorafenib acts against several other receptor tyrosine kinases including vascular endothelial growth factor receptor (VEGFR)2/3, platelet-derived growth factor receptor β PDGFRβ, fms-related tyrosine kinase 3 (FLT-3) and c-KIT. Sorafenib is currently registered for the treatment of metastasized renal cell carcinoma, hepatocellular carcinoma and differentiated thyroid cancer. In melanoma, sorafenib has been tested as monotherapy or in combination with different compounds, such as dacarbazine, temozolamide, carboplatin, paclitaxel and others.6 Dacarbazine is an alkylating agent, first introduced some 30 years ago and still considered as a reference single agent for the management of advanced melanoma.7 Phase I and II trials using sorafenib combined with dacarbazine in advanced melanoma demonstrated acceptable toxicity profiles and limited anti-tumor activity with disease stabilization in up to 20%.8-11 However, patients in Phase III trials of sorafenib added to other chemotherapeutics such as carboplatin and paclitaxel did not have better outcome compared to chemotherapy alone in the treatment of advanced melanoma.12,13 Despite the long history of dacarbazine use in melanoma therapy, little is known about mechanistic effects of the both sorafenib and dacarbazine, as well as their combination on antitumor responses in melanoma.
We initiated an open-label, investigator-initiated study for sorafenib-naïve patients with advanced melanoma to evaluate the in situ effects of dacarbazine and sorafenib on metastases that were accessible for repeated biopsies. Herein, we report that dacarbazine and sorafenib have additional immunomodulatory properties in addition to their cytotoxic and kinase inhibitory activities.
Results
Response and toxicity
Out of 14 enrolled patients with advanced melanoma, 13 patients (5 females, 9 males, mean age: 61.7 years ± 11.93) were actually treated with the study medication. Of these, 2 patients did not complete the study, with patient number 6 deceased on day 54 due to tumor progression and patient number 12 discontinued the medication at day 37 due to side effects (fatigue and loss of appetite). The remaining 11 patients were evaluable for the analysis at the end of the study (day 60). Metabolic response of target lesions evaluated using PERCIST 1.0 criteria was seen in 5 out of 11 patients (45.5%) (Table 1). At the end of the study (day 60), 5 responders (all complete metabolic response [CMR]) and 6 non-responders (2 stable metabolic disease [SMD] and 4 progressive metabolic disease [PMD]) could be defined. The median progression-free survival (PFS) was 63 days (range 24-307 days). Responders defined by PERCIST criteria showed a significantly longer median PFS (307 days) than non-responders (58.5 days; log rank test p = 0.001). Only one out of 5 responders had mutated BRAF (V600E mutation).
Table 1.
Patients' characteristics
| Patient No | Sex | Age | Stage at baseline | B-raf status | Previous therapies | PRECIST at day 60* | Status |
|---|---|---|---|---|---|---|---|
| 1 | M | 74 | IV | Wild-type | Surgery | CMR | Responder |
| 2 | M | 67 | IV | Wild-type | Surgery | SMD | Non-responder |
| 3 | M | 73 | IV | Wild-type | Surgery, chemotherapy | CMR | Responder |
| 4 | M | 63 | IV | Mutated (V600E) | Surgery, topical and experimental immunotherapy | CMR | Responder |
| 5 | F | 61 | IV | Mutated (V600E) | Surgery, interferon-α, experimental immunotherapy | PMD | Non-responder |
| 6 | M | 59 | IV | Wild-type | Surgery | n.e. | Non-responder |
| 7 | F | 48 | IV | Wild-type | Surgery, interferon-α | PMD | Non-responder |
| 9 | M | 60 | IV | Wild-type | Surgery | SMD | Non-responder |
| 10 | M | 75 | IV | Wild-type | Surgery | CMR | Responder |
| 11 | M | 42 | IV | Wild-type | Surgery | PMD | Non-responder |
| 12 | F | 76 | IV | Wild-type | Surgery | n.e. | Non-responder |
| 13 | F | 63 | IV | Wild-type | None | CMR | Responder |
| 14 | F | 41 | IV | Mutated (V600E) | Surgery, topical immunotherapy, interferon-α, radiotherapy | PMD | Non-responder |
*CMR (complete metabolic response), PMR (partial metabolic response), SMD (stable metabolic disease), PMD (progressive metabolic disease) according to PERCIST 1.0 criteria (see Material and Methods).
The treatment was well tolerated. Most of the adverse events (AEs) were mild or moderate and included constitutional symptoms, skin changes, impairment of bone marrow function and gastrointestinal side effects. A summary of AEs is shown in Table S1. Likewise, most of the serious adverse events (SAEs) were due to the impairment of bone marrow function, constitutional symptoms or toxicity to the gastrointestinal tract and liver. The death events on study were due to underlying disease (Table S1).
In vivo sequential transcriptome response to therapy
In-depth pathway analysis during therapy with sorafenib and its combination with dacarbazine
To evaluate transcriptional changes in melanoma metastases during therapy, we performed gene expression profiling using exon array analysis on melanoma metastases specimens obtained from 9 patients before treatment (baseline), during treatment with sorafenib (day 10) and during treatment with sorafenib and dacarbazine (day 16). Using our filtering criteria, we obtained 603 differentially expressed probes during sorafenib monotherapy (day 10) compared to baseline, accounting for 367 genes (169 upregulated, 198 downregulated). For the duration of sorafenib and dacarbazine therapy (day 16), we identified 786 differentially expressed probes compared to baseline, accounting for 455 genes (269 upregulated and 186 downregulated). A part of these genes, exactly 144, were shared between 2 time points (Fig. 1A). Genes from both treatment points were then used in pathway analysis. For this purpose ClueGO, a Cytoscape plug-in was employed using GO biological process descriptions, in order to assess the functional grouping of genes and enable visualization of interactions. Furthermore, a comparison analysis of genes differentially expressed during sorafenib monotherapy vs. sorafenib plus dacarbazine was also performed, in order to define biological processes specific for a particular treatment. The analysis criteria for ClueGO are described in Material & Methods and the results are shown in Figure 1. Function-based network of differentially expressed genes is shown in Figure S2.
Figure 1.
Melanoma metastases differentially express genes during therapy with sorafenib and/or dacarbazine. Exon array analysis on melanoma metastases specimens obtained from patients (n=9) before treatment (baseline), during treatment with sorafenib (day 10) and during treatment with sorafenib and dacarbazine (day 16). (A) Venn diagram of over/under differentially expressed genes in response to therapy and representing genes specific for or shared by different therapies. (B-C) Mining of functional gene associations was performed using Cytoscape, an open source application for visualization of gene interactions and pathway analysis (http://www.cytoscape.org). (B) Overview charts are specifying leading functional terms specific for or shared by sorafenib monotherapy or sorafenib plus dacarbazine. The size of the group indicates the number of the terms included in group. (C) Bar graphs representing gene ontology (GO) categories of genes specific for with sorafenib and/or dacarbazine combination, shared genes, their P-values and number of genes per GO category.
The majority of differentially expressed genes were allocated to functional groups implicated in the regulation of the cell cycle, cellular component organization and inflammatory / immune response (Fig. 1B, C). Control of cell cycle together with spindle apparatus / cytoskeleton organization appeared to be specific for the sorafenib monotherapy (Fig. 1B), with highest P-values for GO categories containing cell cycle and mitosis control (blue bars diagram, Fig. 1C). On the other hand, genes involved in inflammatory / immune response, cell and leukocyte migration were represented in the groups specific for sorafenib and dacarbazine combination (Fig. 1B), with highest P-values for GO categories comprising inflammatory response and cell migration (yellow bars diagram, Fig. 1C). Common to both treatment points were functional groups implicated in cytoskeleton and cellular compartment organization, the regulation of kinase activity and response to lipid (Fig. 1B, C). Functional signatures pertinent to sorafenib action on cell cycle processes could be therefore detected at both time points, whereas upregulation of genes involved in inflammatory response was observed particularly at day 16, i.e., following addition of dacarbazine to sorafenib therapy.
Delineation of genes predicting clinical response to therapy
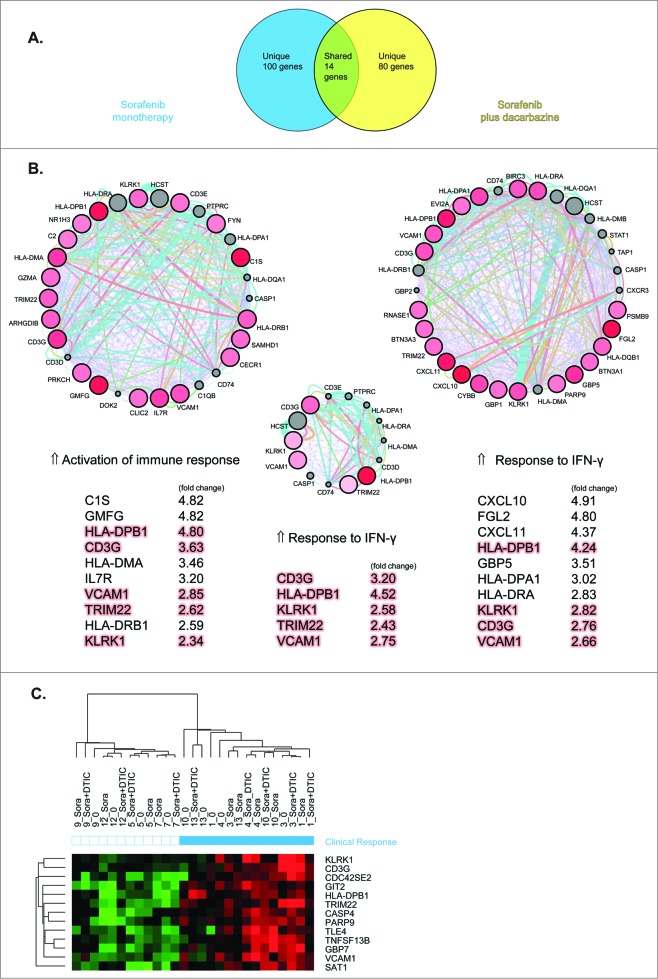
After having underlined the most important gene function categories expressed during sorafenib (day 10) and sorafenib plus dacarbazine therapy (day 16), we next sought to identify genes that could predict clinical response. For this purpose, we analyzed genes at these time points using SVM algorithm and their correlation to clinical outcome evaluated by PET-CT (see Methods) at the end of the study (day 60). In this way, we identified 114 genes for sorafenib and 94 genes for sorafenib plus dacarbazine therapy, which were predictive of clinical outcome with the lowest error rate. Fourteen genes were shared between the 2 treatment points (Fig. 2A). These predictor genes were then queried using Genemania and MCODE plug-ins in Cytoscape. Genemania data mining is performed using a databank of functional interaction data in combination with GO biological process descriptions, whereas MCODE determines the most densely connected regions (so-called modules). To our surprise, the majority of predictor genes assessed by Genemania plug-in represented genes involved in different aspects of immune response. The highest ranked functional categories during sorafenib monotherapy encompassed activation of immune response (P < 0.016 × 10−14) and its subcategories. At the point of sorafenib plus dacarbazine therapy, response to interferon (IFN) γ (P < 0.014 × 10−16), IFNγ signaling pathway and cellular response to IFNγ (P < 0.05 × 10−15) were the most significant functional categories, followed by antigen processing / presentation and their subcategories. Likewise, in the group of shared genes between 2 time points, response to IFNγ (P < 0.02 × 10−13) dominated over other functional themes. Using MCODE plug-in, we further dissected the most densely connected modules within the differentially expressed genes in the treatment groups and determined their overlapping genes. The highest ranked modules as circular networks and top 5-10 genes per module are shown in Figure 2B. In line with the above observation, we observed upregulation of IFNγ-stimulated genes, like CXCL10, CXCL11, HLA-DP/R, CD3G, VCAM1 and several encoding guanylate binding proteins (GBPs) to be more prominent during combined sorafenib and dacarbazine therapy. IFNα-stimulated genes, like OAS1, IFI44, IFIH1, several poly [ADP-ribose] polymerase (PARP) family members, and others, were upregulated at both time points but to a lower extent when compared to IFNγ-induced genes. Unsupervised 2-way clustering analysis using 14 shared predictor genes between treatment groups confirmed upregulated expression of these genes in responder patients (Fig. 2C). Although most of the treated patients clustered near each other in the responder arm, it was interesting to observe that some of the baseline samples were intermixed, suggesting the presence of an activated antitumor microenviroment prior to the initiation of therapy, which, in turn, may be permissive for positive responses in the course of the therapy.
Figure 2.
Gene predictors of clinical response of melanoma patients to therapy with sorafenib and/or dacarbazine. Differentially expressed genes from melanoma biopsy samples from patients (n = 9) treated with sorafenib (day 10) and sorafenib plus dacarbazine therapy (day 16) were analyzed for a correlation to clinical outcome. (A) Venn diagram representing gene predictors specific for or shared by sorafenib monotherapy or sorafenib plus dacarbazine therapy group. (B) Circle networks of most densely connected modules within treatment groups obtained by Genemania and MCODE plug-ins in Cytoscape. Shared genes by both groups are shown in the middle. The top upregulated genes are listed for each group and fold-change values to baseline are indicated. Orange highlights represent shared genes between the groups. Genes are identified by their gene symbols. (C) Two-way clustering analysis of the baseline, sorafenib (day 10) and sorafenib plus dacarbazine therapy (day 16) using 14 shared predictor genes. The similarity of gene expression profiles between examined samples is summarized in a dendrogram above the heatmap, in which the pattern and the length of the branches reflect the relatedness of the samples. The red color in the heatmap indicates a high relative level of expression, whereas the green color indicates a low relative level of expression. Clinical response is represented by blue squares, whereas non-responders are represented by white squares. Genes are represented by their gene symbols.
Validation of clinical response predictors by PCR and immunohistochemistry
We next focused on verifying the expression of predictor genes and their proteins in our samples by real-time PCR and immunohistochemistry. Samples from 10 patients (4 responders and 6 non-responders) were tested using real-time PCR. IFNγ (type II IFN) inducible transcripts (CD3G, CXCL11, VCAM1 and those encoding HLA-DR/HLA class II) and IFNα/β (type I IFN)-inducible MXA genes products were evaluated. The chemokine (C-X-C motif) ligand 11 (CXCL11, also known by the synonyms I-TAC and IP9) is a chemokine primarily induced by IFNγ with its best known role in leukocyte trafficking.14,15 CD3 and HLA-DR are cellular receptors present on T lymphocytes and antigen presenting cells, respectively.16 The VCAM1 gene encodes vascular cell adhesion molecule 1, a molecule involved in leukocyte migration to site of inflammation and is also IFNγ inducible.17 The mRNA expression of all tested IFNγ-inducible genes was significantly higher in responders (2-way ANOVA; CXCL11 P = 0.002, VCAM P = 0.026, CD3G P = 0.010 and HLA-DRA P < 0.0001, Fig. 3). Moreover, the expression levels of most of these genes at either of the 2 time points (i.e., day 10 or day 16) was able to discriminate between responders and non-responders in PET-CT as determined by receiver operating characteristic (ROC) curves (true predictors are shown in Fig. S3).
Figure 3.
Validation of gene predictor of clinical response to therapy. Expression of response predictor genes in biopsies (n = 10 patients), including 4 responders and 6 non-responders, were tested using real-time PCR. (A–D) Gene expression levels were assessed by quantitative PCR and tested for significance using 2-way ANOVA. The bars show mean expression of the respective gene normalized to RPL28 housekeeping genes at baseline, day 10 (sorafenib) and day 16 (sorafenib plus dacarbazine) as well as their grouping according to response, while whiskers represent standard error of the mean (SEM); *P < 0.05; **P < 0.01.
Although the MX1 gene was not present on the predictor genes list, we have chosen this gene and its product MX dynamin-like GTPase 1 (MX1, better known as MxA) protein as an additional molecular marker for type I IFN responses in the skin.18 The MX1 gene product (i.e., the MxA protein) is a large GTPase that is involved in cellular antiviral response.19,20 The expression of MX1 mRNA did not significantly differ between responders and non-responders (2-way ANOVA P = 0.352), implying that IFNγ-induced gene products are more relevant than those stimulated by IFNα in predicting clinical response. Nevertheless, MX1 gene expression in obtained biopsies could discriminate between responders and non-responders according to ROC analysis (Fig. S3).
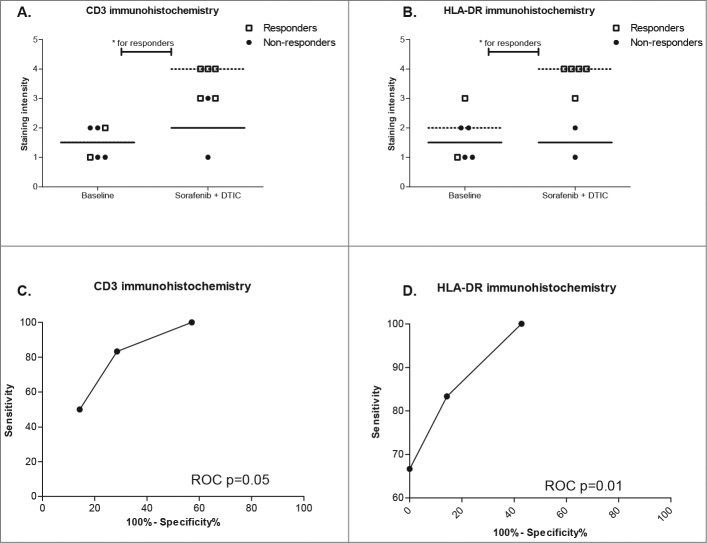
Similar results were obtained by immunohistochemistry, even though a reduced number and partially unpaired tissue samples were available. Total of 6 baseline and 7 samples from day 16 (sorafenib plus dacarbazine) were used for immunohistochemical stains for CD3 (T cells), HLA-DR (MHC class expressing inflammatory cells), MxA (IFNα activity) and VCAM-1 (adhesion molecule). CD3 expression revealing T-cell presence, albeit on the level of significance, was more prominent in the responder group (Mann-Whitney U-test P = 0.055, Fig. 4A). HLA-DR expression was also stronger in responder biopsies (Mann-Whitney U-test P = 0.008, Fig. 4B). MxA and VCAM-1 (data not shown) protein expression, on the other hand, did not show any statistical difference between responders and non-responders. ROC analysis of immunohistochemistry markers revealed analogous results, with only CD3 and HLA-DR discriminating response (Fig. 4C and D).
Figure 4.
Expression of response predictor gene products in melanoma biopsies. (A and B) CD3 and HLA-DR protein expression was evaluated by immunohistochemistry and difference between responders and non-responders tested using Mann-Whitney U-test. P values for each test are shown in the graph, P < 0.05 values are highlighted with one asterisk (*), P < 0.01 values with 2 asterisks (**). The scatter plots show individual staining intensity at baseline and day 16 (sorafenib plus dacarbazine), different labeling according to response, with horizontal line representing median value for each group. (C and D) ROC diagrams depict discrimination between responders and non-responders using CD3 and HLA-DR expression.
Systemic IFNγ induction during therapy with sorafenib and its combination with dacarbazine
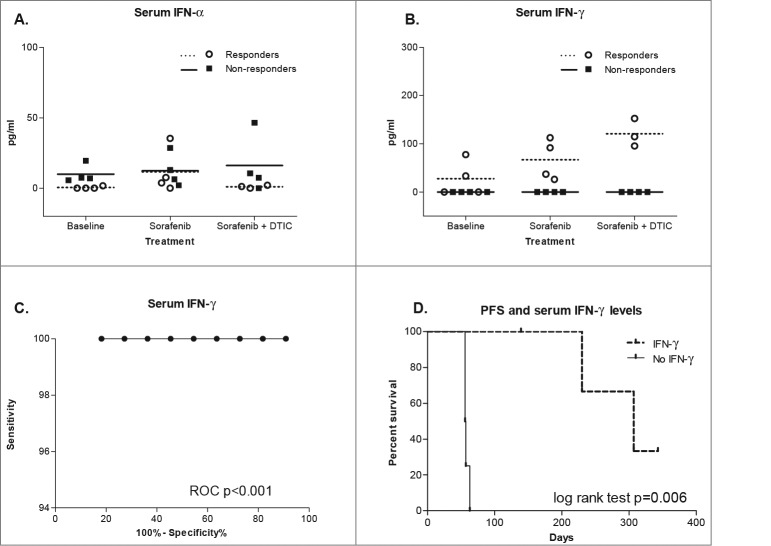
Since therapy with sorafenib, either monotherapy or in combination with dacarbazine, seems to induce expression of IFN-stimulated genes, we measured serum levels of IFNα and IFNγ in our patients. For this purpose, we analyzed sera of 8 patients (4 responders and 4 non-responders) at baseline, day 10 and day 16. Although detectable, serum IFNα levels were only marginally different between responders and non-responders (2-way ANOVA P = 0.055, Fig. 5A). In contrast and in conjunction with the PCR data presented above, it came as no surprise that serum IFNγ were only detectable in responders (P 7lt; 0.0001, Fig. 5B). Furthermore, serum IFNγ continuously increased during the treatment, showing the highest levels after addition of dacarbazine (0 to 66.89 pg/mL vs. 120.8 pg/mL; 2-way ANOVA P = 0.0095). Serum IFNγ levels were good discriminators between responders and non-responders in PET-CT using ROC analysis (P = 0.0009; Fig. 5C). Furthermore, patients who had detectable serum IFNγ levels had significantly longer progression-free survival than the ones without (307 days vs. 56.5 days; log-rank test P = 0.006; Fig. 5D). These data reveal, to our knowledge for the first time, detectable systemic IFNγ production in vivo during sorafenib and dacarbazine therapy and its positive relation to clinical response and survival in melanoma patients.
Figure 5.
Serum IFNα and IFNγ levels during therapy with sorafenib and/or dacarbazine. (A and B) Serum cytokine levels were measured by ELISA and tested by 2-way ANOVA. The scatter plots show individual interferon α (IFNα) and interferon γ (IFNγ) levels at baseline, day 10 (sorafenib) and day 16 (sorafenib plus dacarbazine) as well as their grouping according to response, the horizontal lines represent mean values of each response group. (B) ROC diagram depicts discrimination between responders and non-responders using IFNγ serum levels. (C) Kaplan-Meier curve depicts a difference in progression-free survival in study patients with detectable vs. undetectable serum IFNγ, as determined by log-rank test.
Discussion
Our pilot study revealed a crucial aspect of the response to kinase inhibitors and chemotherapy in melanoma, an immunologic effect that has so far been considered important primarily in immunotherapies. We evaluated the efficacy of sorafenib and dacarbazine combination in patients with advanced melanoma of Stage IV. Overall metabolic response rates of 45.5% observed in our study are better than the 12% reportedly observed by others9,11 but may be due to a low patient number and different response evaluation parameters (PERCIST) utilized in our study. Comparison studies of PERCIST and RECIST evaluations in solid tumors reveal higher sensitivity of PERCIST in detecting complete remission and stable disease,21,22 which may partly account for the high response rate observed in our patient population. The study drugs were well tolerated by our patients. Only one out of 5 responders had a mutated BRAF gene, suggesting that MAPK pathway inhibition by sorafenib was not the main mechanism governing the response in these patients. Similar data were published recently by Wilson et al., showing that BRAF mutation status was not predictive of response or survival in melanoma patients treated with sorafenib and chemotherapy.23
Subsequently, we focused on elucidating genome-wide transcriptional changes during sorafenib monotherapy followed by its combination with dacarbazine in accessible skin and lymph node metastases. Despite their potential influence on the Raf/MEK/ERK pathway,6 the molecular mechanisms by which sorafenib exerts its antitumor activity have not been fully elucidated. Taking into account that modulation of signaling through the Raf/MEK/ERK pathway can interfere with the cell cycle, cytoskeleton organization and cellular movement,24-26 our finding that these biological categories were highlighted in our analysis was anticipated. Similar results were obtained by others who performed gene expression profiling of tumors responding to sorafenib, including hepatocellular carcinoma27 and acute myeloid leukemia.28 We detected no significant changes related to inhibition of angiogenesis pathways by sorafenib. Dacarbazine, on the other hand, is classically considered to be a multimodal cytotoxic agent interfering with cell cycle and DNA organization, which could also be seen in our current study and by others.29
There is increasing evidence that both conventional and targeted therapies have broader and immunomodulatory activities.30,31 Sorafenib appears to enhance antitumor immunological responses by modulating macrophage and natural killer (NK) activity, thus rendering tumor cells susceptible to NK cell-mediated killing.32 Similarly, sorafenib selectively influences T-cell subsets, as it promotes activation of effector CD4+ T cells while reducing immunosuppressive regulatory T cells.33,34 Moreover, a recent study by Romero et al. showed that sorafenib promotes T helper (Th) type 1 polarization and accumulation of peripheral CD4+ T cells expressing NK group 2D (NKG2D) ligands in melanoma patients treated with sorafenib and temozolomide, an alkylating agent related to dacarbazine.35 The frequencies of NKG2D+ cell subtypes, however, failed to show an association with clinical response in this study. In our patient collective, the expression of NKG2D was one of the predictors of clinical response. Dacarbazine seems also to exert not only cytotoxic but also immunomodulatory effects. Hervieu at al. reported that dacarbazine upregulates the expression of NKG2D ligand on tumor cells leading to NK-cell activation.36 This, in turn, induces IFNγ secretion, which upregulates the expression of MHC class I molecules on the surface of tumor cells, making them susceptible to killing by cytotoxic CD8+ T cells in a mouse melanoma model. Interestingly, melanomas in T-cell deficient mice do not respond to dacarbazine, implying that the activation of NK cells is mandatory for the therapeutic in vivo effect of dacarbazine.36 This is in line with our data showing increased expression of NKG2D encoding transcripts (KLRK1) throughout the therapy with sorafenib and later with dacarbazine. More importantly, global gene expression profiling analysis of cutaneous melanoma metastases from patients treated with dacarbazine revealed gene expression signatures consistent with T-cell infiltration, immune activation and response to wounding.29 Therapy with dacarbazine induces expression of various chemokines, cytokines, lymphocytic markers and MHC class II genes, which we could also see in our analysis. Particular to our patient collective is the induction of multiple IFNγ stimulated genes, emphasizing the importance of adaptive antitumor immune response.
The importance of antitumor immunity in host control of tumor development is one of the cornerstones of the “cancer immunoediting” theory.37 Accordingly, the immune system is not only capable of destroying tumor cells through the activation of adaptive and innate immune IFN-dependent pathways, it can also facilitate the selection of tumor cells capable of escaping immune pressure, a 3-phase process composed of Elimination, Equilibrium and Escape.37,38 In the context of protective antitumor immunity and elimination, the role of intact lymphocyte compartment, type I (IFN-α/β) and type II (IFNγ) interferons is well recognized (reviewed in39). Antineoplastic agents such as conventional chemotherapeutics may thus stimulate immunosurveillance by increasing tumor antigenicity (e.g., via upregulation of MHC class I immunopeptidome), immunogenicity (via emitting danger signals) and susceptibility to immune effector cell killing (e.g., via upregulation of co-stimulatory molecules, death receptors, NK cell activating receptor ligands [mentioned above] or via downregulation of inhibitory molecules),31 most of which we could identify in our analysis (see Fig. 2). It has become increasingly appreciated that specific immune activation signatures can be associated with better prognosis and/or response to therapy in various types of human cancers including melanoma.39-42 The majority of these signatures falls in to functional categories encompassing Th1 activation (e.g., STAT1, IRF1/ IFNγ pathways), chemokines and their ligands (CXCR3/ CXCL9-11 and CCR5/ CCL3-5 pathways), cytotoxic factors (granzyme, perforin, granulysin/ TIA1/ caspase pathways) and adhesion molecules (VCAM1, ICAM1 and others).41 It is, therefore, not surprising that our response prediction analysis delivered some of the same genes as those previously described in other similar melanoma studies.43-47 This observation also refers to transcripts encoding CD3,43-46 HLA class II,43-46 CXCL1144,47 and VCAM1 43,45, markers which we used in our validation experiments. It is noteworthy that these immune activation signatures were typically described in patients receiving immunotherapy43,44,46,47 and rarely in chemotherapy.29,43. Our study clearly underlines the fact that tumor regression is, for the most part, immunologically mediated, not only in immune modulation-based but also in chemotherapy and targeted therapies.
The molecular pathways represented in signatures predictive of responsiveness to anticancer therapy largely involve coordinated expression of IFN-stimulated genes (ISGs). Both type I (IFNα/β) and type II (IFNγ) interferons induce a unique and partially overlapping set of ISGs, some of which were investigated here in the context of predicting responsiveness to therapy. Our results are consistent with downstream effects of IFNγ, which we could also detect in the serum of treated patients. Not only did we find higher IFNγ levels in patients who responded to sorafenib and dacarbazine therapy, patients who did not clinically respond to therapy presented no measurable serum IFNγ at all. Prior cytokine studies by others indicate that insufficient production of Th1 cytokines, including IFNγ, in patients with metastasized melanoma can be improved by immunotherapeutic interventions.48-50 In spite of the fact that our patients did not receive immunotherapy, we could still clearly detect an increase in IFNγ serum levels in patients after receiving dacarbazine in combination with sorafenib. This is to our knowledge the first such report showing differential modulation of IFNγ serum levels by tumor-specific therapies.
Lastly, responders presented with an activated antitumor microenviroment prior to the initiation of therapy (as shown by clustering analysis), which obviously favored positive response to therapy. Applied therapy probably resulted in the release of tumor antigens and an IFNγ-polarized immune response, which was detectable in the remaining tumors and serum. It is unlikely that serial biopsies might have accounted for the signatures that we observed, since wound healing after skin injury does not induce an IFNγ-dominated immune response.51-53
In conclusion, we show that combined therapy using sorafenib and dacarbazine can induce tumor regressions in patients with metastasized melanoma, which are associated with an unexpected immune effector mechanism. The limitation of our study is the small number of patients from which the results were drawn. Nevertheless, we demonstrated an induction of a proinflammatory environment dominated by IFNγ and its positive influence on clinical response. As IFNγ is considered to be one of the key players determining the clinical outcome to anticancer therapy,39-42 further investigations of other chemotherapeutics and targeted therapies are necessary to fully understand and exploit their immunomodulatory potential. There is evidence that sorafenib potentiates responses to regional chemotherapy with temozolomide,54 whereas its combination with alkylating agents like dacarbazine and temozolomide leads to more superior stimulation of antitumor immunity.32,35 Thus, unique combinations of different therapeutic agents may be more advantageous than monotherapy and such contexts should be taken into consideration.
Methods
Patients
The study protocol was approved by the institutional and regional ethical committee (reference number 799) and Swissmedic (notification number 2008DR1336). The trial was registered in www.clinicaltrial.gov (reference number NCT00794235). The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and good clinical practice.
Patients with advanced melanoma in Stage IV55 with histologically-confirmed skin or lymph node metastases, larger than 1 cm in diameter and measurable by PET/CT-scan were eligible for the study. Patients had to be sorafenib-naïve, not received any systemic chemotherapy for at least 3 months prior to study inclusion, show an elevated level of serum LDH (>1.1 ULN) and adequate bone marrow, liver and renal function. Patients with ocular melanoma were excluded as were individuals with significant co-morbidities (e.g., severe cardiovascular, renal, hepatic or psychiatric conditions), severe infections, metastatic neoplasm other than melanoma or symptomatic metastatic brain or meningeal tumors. All patients gave written informed consent prior to study inclusion. Patients' characteristics are shown in Table 1.
Study design and response evaluation
The study was an investigator-initiated, open-label, pilot study performed at the Department of Dermatology, University Hospital Zurich, Switzerland performed between January and September 2009. Treatment regimen included orally given sorafenib (Nexavar®, Bayer, Switzerland) 400 mg bid from day 1 to 56 and intravenous dacarbazine (DTIC, Dacin®, Lipomed, Switzerland) 1000 mg/m2 on days 14 and 42 (Fig. S1). In patients showing disease stabilization (i.e., tumor regression or stable disease) by the end of the study, the study medication was continued after the end of the study. In the event of disease progression, participation on the study was terminated. Adverse events were documented according to the National Cancer Institute Common Terminology for Criteria Adverse Events (NCI CTCAE) version 3.0 (www.cancer.gov). PET-CT scans with 2-deoxy-2-[fluorine-18]fluoro- D-glucose (FDG) were performed at baseline and day 60. Response to therapy was assessed by PET-CT using PERCIST 1.0 criteria as described by Wahl et al.22. Complete (CMR) and partial metabolic response (PMR) were considered as metabolic response; stable disease (SMD) and progressive metabolic disease (PMD) were evaluated as metabolic non-response.
Sequential response profiling
Biopsies of lymph node or superficial skin metastases were performed before treatment (baseline), during treatment with sorafenib (day 10) and after treatment with dacarbazine (day 16). The biopsies within one patient were taken from the same tumor and were divided and either preserved in RNAlater (Ambion, Life Technologies Corp., Carlsbad, CA, USA) for transcriptional profiling or in formalin for immunohistochemistry.
Transcriptional response profiling and data analysis
Total RNA was extracted from tissue biopsies using Qiagen Mini-Tissue kit (Qiagen, Venlo, Netherlands). RNA quality and integrity was assessed using Bioanalyzer 2100 (Agilent Technologies Inc.., Santa Clara, CA, USA). SPIA whole-genome cDNA amplification and labeling was performed using Applause kit (NuGen, San Carlos, CA, USA). The amplified, fragmented and labeled cDNA was purified from the reaction mix with Qiagen's MinElute® Reaction Cleanup Kit. Five μg of labeled cDNA were used for hybridization onto Human GeneChip® Exon 1.0 ST microarray (Affymetrix, Santa Clara, CA, USA) at the Functional Genomics Center Zurich.
Microarray data were analyzed using Partek software as described by Mojica and Hawthorn.56 Baseline, sorafenib (day 10) and sorafenib plus dacarbazine (day 16) treated samples were used for analysis. Briefly, after RMA summarization and normalization using core annotations, principle component analysis and analysis of variance is performed followed by the development of mixed ANOVA model, Benjamini-Hochberg multiple testing correction (P-value cut-off 7lt;0.005) and fold change filtering (cut-off ≥ ±2.5). Analysis on exon level was performed as described previously.57,58 Subsequent response prediction analysis was done using support vector machine (SVM) module from Partek software.
Mining of functional gene associations was performed using Cytoscape, an open source application for visualization of gene interactions and pathway analysis (http://www.cytoscape.org).59 For this purpose, ClueGO,60 GeneMANIA61 and MCODE62 Cytoscape plug-ins were employed. Clustering analysis was performed with the Cluster program, and the display of the rearranged data was generated with the Tree View program (both programs are available through http://rana.lbl.gov).
Real-time quantitative PCR
Total RNA was isolated from biopsies using TRIzol reagent according to manufacturer's instructions (Invitrogen/Life technologies, Carlsbad, USA). One μg aliquots of RNA were reverse transcribed with Reverse Transcription System (Promega, Madison, USA) according to the manufacturer's instructions. Data collection and analysis were performed by ABI Viia7 Fast Real-Time PCR Systems (Applied Biosystems/ Life technologies, Carlsbad, USA). The primers were purchased from Qiagen (Venlo, Netherlands): OAS1 (Hs_OAS1_1_SG), MX1 (Hs_MX1_1_SG), HLA-DRA (Hs_HLA-DRA_1_SG), CXCL11 (Hs_CXCL11_2_SG), CD3g (Hs_CD3G_1_SG), and VCAM1 (Hs_VCAM_1_SG). Gene expression values of averaged triplicate reactions were normalized to RPL28 expression levels. The sequence of our RPL28 primers was: 5′-GCAATTGGTTCCGCTACAAC-3′ and 5′-TGTTCTTGCGGATCATGTGT-3′.
Immunohistochemistry
Paraffin-embedded blocks from biopsies were collected and used for validation. Immunohistochemical staining using alkaline-phosphatase-anti-alkaline-phosphatase technique (APAAP) was performed as previously described.18 The following antibodies were used: anti-CD3 (Dako, Baar, Switzerland), anti-HLA II (Novocastra, Leica Microsystems GmbH, Wetzlar, Germany), anti-VCAM1 (Abcam, Cambridge, United Kingdom), anti-MxA (clone CL143, kind gift of Dr. Jovan Pavlovic, Institute of Medical Virology, University of Zurich, Zurich, Switzerland). Staining intensity was graded from 0 (no staining) to 4 (maximal staining).
Measurement of serum IFNα and IFNγ levels
Serum levels of IFNα and IFNγ in patient sera were analyzed by human IFNα and IFNγ ELISA sets (eBioscience, San Diego, USA) according to manufacturer's protocol.
Statistical analysis
Statistical analysis of the data other than exon arrays was performed using GraphPad Prism software version 5.0 (GraphPad Software, San Diego California USA). Statistical analysis of PCR and ELISA data was done using 2-way ANOVA, for immunohistochemistry data using Mann-Whitney U Test. Comparison of Kaplan-Meier survival curves was performed using log-rank test. P values of less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Nikita Kobert and Ines Kleiber-Schaaf for technical assistance in validation experiments.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The study was supported in part by the University of Zurich, Gottfried und Julia Bangerter-Rhyner-Foundation for medical research and Bayer (Switzerland) AG.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Statement of Significance
To our knowledge this is the first report to show differential modulation of systemic interferon production by tumor-specific and non-immunomodulatory therapies, such as sorafenib and dacarbazine.
ClinicalTrials.gov Identifier: NCT00794235
References
- 1. Little EG, Eide MJ. Update on the current atate of melanoma incidence. Dermatol Clin 2012; 30:355-61; PMID:; http://dx.doi.org/ 10.1016/j.det.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 2. de Vries E, Bray FI, Coebergh JWW, Parkin DM, European Network of Cancer R. Changing epidemiology of malignant cutaneous melanoma in Europe 1953–1997: rising trends in incidence and mortality but recent stabilizations in Western Europe and decreases in Scandinavia. Int J Cancer 2003; 107:119-26; http://dx.doi.org/ 10.1002/ijc.11360 [DOI] [PubMed] [Google Scholar]
- 3. MacKie RM, Hauschild A, Eggermont AMM. Epidemiology of invasive cutaneous melanoma. Annals of Oncology 2009; 20:vi1-vi7; PMID:; http://dx.doi.org/ 10.1093/annonc/mdp252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arnold M, Holterhues C, Hollestein LM, Coebergh JWW, Nijsten T, Pukkala E, Holleczek B, Tryggvadóttir L, Comber H, Bento MJ, et al. Trends in incidence and predictions of cutaneous melanoma across Europe up to 2015. J Eur Acad Dermatol Venereol 2013; 8:1170-8; http://dx.doi.org/ 10.1111/jdv.12236 [DOI] [PubMed] [Google Scholar]
- 5. Coit DG, Olszanski AJ. Progress in the management of melanoma in 2013. J Natl Compr Canc Netw 2013; 11:645-8; PMID: [DOI] [PubMed] [Google Scholar]
- 6. Mangana J, Levesque MP, Karpova MB, Dummer R. Sorafenib in melanoma. Expert Opin Investig Drugs 2012; 21:557-68; PMID:; http://dx.doi.org/ 10.1517/13543784.2012.665872 [DOI] [PubMed] [Google Scholar]
- 7. Eggermont AM, Kirkwood JM. Re-evaluating the role of dacarbazine in metastatic melanoma: what have we learned in 30 years? Eur J Cancer 2004; 40:1825-36; PMID:; http://dx.doi.org/ 10.1016/j.ejca.2004.04.030 [DOI] [PubMed] [Google Scholar]
- 8. Eisen T, Ahmad T, Flaherty KT, Gore M, Kaye S, Marais R, Gibbens I, Hackett S, James M, Schuchter LM, et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br J Cancer 2006; 95:581-6; PMID:; http://dx.doi.org/ 10.1038/sj.bjc.6603291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eisen T, Marais R, Affolter A, Lorigan P, Robert C, Corrie P, Ottensmeier C, Chevreau C, Chao D, Nathan PD, et al. Sorafenib and dacarbazine as first-line therapy for advanced melanoma: phase I and open-label phase II studies. Br J Cancer 2011; 105:353-9; PMID:; http://dx.doi.org/ 10.1038/bjc.2011.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ott PA, Hamilton A, Min C, Safarzadeh-Amiri S, Goldberg L, Yoon J, Yee H, Buckley M, Christos PJ, Wright JJ, et al. A phase II trial of sorafenib in metastatic melanoma with tissue correlates. PLoS One 2010; 5:e15588; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0015588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McDermott DF, Sosman JA, Gonzalez R, Hodi FS, Linette GP, Richards J, Jakub JW, Beeram M, Tarantolo S, Agarwala S, et al. Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol 2008; 26:2178-85; PMID:; http://dx.doi.org/ 10.1200/JCO.2007.14.8288 [DOI] [PubMed] [Google Scholar]
- 12. Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, Eggermont A, Grabbe S, Gonzalez R, Gille J, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 2009; 27:2823-30; PMID:; http://dx.doi.org/ 10.1200/JCO.2007.15.7636 [DOI] [PubMed] [Google Scholar]
- 13. Flaherty KT, Lee SJ, Zhao F, Schuchter LM, Flaherty L, Kefford R, Atkins MB, Leming P, Kirkwood JM. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol 2013; 31:373-9; PMID:; http://dx.doi.org/ 10.1200/JCO.2012.42.1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol 2011; 89:207-15; PMID:; http://dx.doi.org/ 10.1038/icb.2010.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Singh AK, Arya RK, Trivedi AK, Sanyal S, Baral R, Dormond O, Briscoe DM, Datta D. Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev 2013; 24:41-9; PMID:; http://dx.doi.org/ 10.1016/j.cytogfr.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murphy K, Travers P, Walport M, Janeway C. Janeway's Immunobiology. New York: Garland Science, 2012. [Google Scholar]
- 17. Saha B, Jyothi Prasanna S, Chandrasekar B, Nandi D. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine 2010; 50:1-14; PMID:; http://dx.doi.org/ 10.1016/j.cyto.2009.11.021 [DOI] [PubMed] [Google Scholar]
- 18. Urosevic M, Fujii K, Calmels B, Laine E, Kobert N, Acres B, Dummer R. Type I IFN innate immune response to adenovirus-mediated IFN-gamma gene transfer contributes to the regression of cutaneous lymphomas. J Clin Invest 2007; 117:2834-46; PMID:; http://dx.doi.org/ 10.1172/JCI32077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schoggins JW, Rice CM. Interferon-stimulated genes and their antiviral effector functions. Curr Opin Virol 2011; 1:519-25; PMID:; http://dx.doi.org/ 10.1016/j.coviro.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haller O, Kochs G. Human MxA protein: an interferon-induced dynamin-like GTPase with broad antiviral activity. J Interferon Cytokine Res 2011; 31:79-87; PMID:; http://dx.doi.org/ 10.1089/jir.2010.0076 [DOI] [PubMed] [Google Scholar]
- 21. Ding Q, Cheng X, Yang L, Zhang Q, Chen J, Li T, Shi H. PET/CT evaluation of response to chemotherapy in non-small cell lung cancer: PET response criteria in solid tumors (PERCIST) versus response evaluation criteria in solid tumors (RECIST). J Thorac Dis 2014; 6:677-83; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med 2009; 50 Suppl 1:122S-50S; PMID:; http://dx.doi.org/ 10.2967/jnumed.108.057307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wilson MA, Zhao F, Letrero R, D'Andrea K, Rimm DL, Kirkwood JM, Kluger HM, Lee SJ, Schuchter LM, Flaherty KT, et al. Correlation of somatic mutations and clinical outcome in melanoma patients treated with Carboplatin, Paclitaxel, and sorafenib. Clin Cancer Res 2014; 20:3328-37; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fecher LA, Amaravadi RK, Flaherty KT. The MAPK pathway in melanoma. Curr Opin Oncol 2008; 20:183-9; PMID:; http://dx.doi.org/ 10.1097/CCO.0b013e3282f5271c [DOI] [PubMed] [Google Scholar]
- 25. Osborne JK, Zaganjor E, Cobb MH. Signal control through Raf: in sickness and in health. Cell Res 2012; 22:14-22; PMID:; http://dx.doi.org/ 10.1038/cr.2011.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pullikuth AK, Catling AD. Scaffold mediated regulation of MAPK signaling and cytoskeletal dynamics: a perspective. Cell Signal 2007; 19:1621-32; PMID:; http://dx.doi.org/ 10.1016/j.cellsig.2007.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cervello M, Bachvarov D, Lampiasi N, Cusimano A, Azzolina A, McCubrey JA, Montalto G. Molecular mechanisms of sorafenib action in liver cancer cells. Cell Cycle 2012; 11:2843-55; PMID:; http://dx.doi.org/ 10.4161/cc.21193 [DOI] [PubMed] [Google Scholar]
- 28. Man CH, Fung TK, Ho C, Han HHC, Chow HCH, Ma ACH, Choi WWL, Lok S, Cheung AMS, Eaves C, et al. Sorafenib treatment of FLT3-ITD(+) acute myeloid leukemia: favorable initial outcome and mechanisms of subsequent nonresponsiveness associated with the emergence of a D835 mutation. Blood 2012; 119:5133-43; PMID:; http://dx.doi.org/ 10.1182/blood-2011-06-363960 [DOI] [PubMed] [Google Scholar]
- 29. Nardin A, Wong W-C, Tow C, Molina TJ, Tissier F, Audebourg A, Garcette M, Caignard A, Avril M-F, Abastado J-P, et al. Dacarbazine promotes stromal remodeling and lymphocyte infiltration in cutaneous melanoma lesions. J Invest Dermatol 2011; 131:1896-905; PMID:; http://dx.doi.org/ 10.1038/jid.2011.128 [DOI] [PubMed] [Google Scholar]
- 30. Galluzzi L, Senovilla L, Zitvogel L, Kroemer G. The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 2012; 11:215-33; PMID:; http://dx.doi.org/ 10.1038/nrd3626 [DOI] [PubMed] [Google Scholar]
- 31. Zitvogel L, Galluzzi L, Smyth Mark J, Kroemer G. Mechanism of Action of Conventional and Targeted Anticancer Therapies: Reinstating Immunosurveillance. Immunity 2013; 39:74-88; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 32. Sprinzl MF, Reisinger F, Puschnik A, Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A, Galle PR, Schuchmann M, et al. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology 2013; 57:2358-68; PMID:; http://dx.doi.org/ 10.1002/hep.26328 [DOI] [PubMed] [Google Scholar]
- 33. Cabrera R, Ararat M, Xu Y, Brusko T, Wasserfall C, Atkinson MA, Chang LJ, Liu C, Nelson DR. Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother 2013; 62:737-46; PMID:; http://dx.doi.org/ 10.1007/s00262-012-1380-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chen M-L, Yan B-S, Lu W-C, Chen M-H, Yu S-L, Yang P-C, Cheng A-L. Sorafenib relieves cell-intrinsic and cell-extrinsic inhibitions of effector T cells in tumor microenvironment to augment antitumor immunity. Int J Cancer 2014; 134:319-31; PMID:; http://dx.doi.org/ 10.1002/ijc.28362 [DOI] [PubMed] [Google Scholar]
- 35. Romero AI, Chaput N, Poirier-Colame V, Rusakiewicz S, Jacquelot N, Chaba K, Mortier E, Jacques Y, Caillat-Zucman S, Flament C, et al. Regulation of CD4(+)NKG2D(+) Th1 cells in patients with metastatic melanoma treated with sorafenib: role of IL-15Rα and NKG2D triggering. Cancer Res 2014; 74:68-80; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1186 [DOI] [PubMed] [Google Scholar]
- 36. Hervieu A, Rebe C, Vegran F, Chalmin F, Bruchard M, Vabres P, Apetoh L, Ghiringhelli F, Mignot G. Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth. J Invest Dermatol 2013; 133:499-508; PMID:; http://dx.doi.org/ 10.1038/jid.2012.273 [DOI] [PubMed] [Google Scholar]
- 37. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3:991-8; PMID:; http://dx.doi.org/ 10.1038/ni1102-991 [DOI] [PubMed] [Google Scholar]
- 38. Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006; 6:836-48; PMID:; http://dx.doi.org/ 10.1038/nri1961 [DOI] [PubMed] [Google Scholar]
- 39. Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases-elimination, equilibrium and escape. Curr Opin Immunol 2014; 27C:16-25; http://dx.doi.org/ 10.1016/j.coi.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 2012; 12:298-306; PMID:; http://dx.doi.org/ 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 41. Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity 2013; 39:11-26; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 42. Ascierto M, Giorgi VD, Liu Q, Bedognetti D, Spivey T, Murtas D, Uccellini L, Ayotte B, Stroncek D, Chouchane L, et al. An immunologic portrait of cancer. J Translat Med 2011; 9:146; PMID:; http://dx.doi.org/ 10.1186/1479-5876-9-146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bogunovic D, O'Neill DW, Belitskaya-Levy I, Vacic V, Yu Y-L, Adams S, Darvishian F, Berman R, Shapiro R, Pavlick AC, et al. Immune profile and mitotic index of metastatic melanoma lesions enhance clinical staging in predicting patient survival. Proc Natl Acad Sci U S A 2009; 106:20429-34; PMID:; http://dx.doi.org/ 10.1073/pnas.0905139106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ji R-R, Chasalow S, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure Kunkel M, Siemers N, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012; 61:1019-31; PMID:; http://dx.doi.org/ 10.1007/s00262-011-1172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mann G, Pupo G, Campain A, Carter C, Schramm S-J, Pianova S, Gerega S, De Silva C, Lai K, Wilmott J, et al. BRAF Mutation, NRAS Mutation, and the Absence of an Immune-Related Expressed Gene Profile Predict Poor Outcome in Patients with Stage III Melanoma. JInvest Dermatol 2013; 133:509-17; PMID:; http://dx.doi.org/ 10.1038/jid.2012.283 [DOI] [PubMed] [Google Scholar]
- 46. Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, Lehmann FF, Suciu S, Kruit WHJ, Eggermont AMM, Vansteenkiste J, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol 2013; 31:2388-95; PMID:; http://dx.doi.org/ 10.1200/JCO.2012.44.3762 [DOI] [PubMed] [Google Scholar]
- 47. Bedognetti D, Spivey TL, Zhao Y, Uccellini L, Tomei S, Dudley ME, Ascierto ML, De Giorgi V, Liu Q, Delogu LG, et al. CXCR3/CCR5 pathways in metastatic melanoma patients treated with adoptive therapy and interleukin-2. Br J Cancer 2013; 109:2412-23; PMID:; http://dx.doi.org/ 10.1038/bjc.2013.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Moretti S, Chiarugi A, Semplici F, Salvi A, De Giorgi V, Fabbri P, Mazzoli S. Serum imbalance of cytokines in melanoma patients. Melanoma Res 2001; 11:395-9; PMID:; http://dx.doi.org/ 10.1097/00008390-200108000-00010 [DOI] [PubMed] [Google Scholar]
- 49. Schiltz PM, Dillman RO. Serum cytokines in metastatic melanoma patients treated with an autologous tumor vaccine. Cancer Biother Radiopharm 2003; 18:879-86; PMID:; http://dx.doi.org/ 10.1089/108497803322702842 [DOI] [PubMed] [Google Scholar]
- 50. Grimm EA, Smid CM, Lee JJ, Tseng CH, Eton O, Buzaid AC. Unexpected cytokines in serum of malignant melanoma patients during sequential biochemotherapy. Clin Cancer Res 2000; 6:3895-903; PMID: [PubMed] [Google Scholar]
- 51. Osuka K, Suzuki Y, Saito K, Takayasu M, Shibuya M. Changes in serum cytokine concentrations after neurosurgical procedures. Acta Neurochir (Wien) 1996; 138:970-6; PMID:; http://dx.doi.org/ 10.1007/BF01411287 [DOI] [PubMed] [Google Scholar]
- 52. Tredget EE, Yang L, Delehanty M, Shankowsky H, Scott PG. Polarized Th2 cytokine production in patients with hypertrophic scar following thermal injury. J Interferon Cytokine Res 2006; 26:179-89; PMID:; http://dx.doi.org/ 10.1089/jir.2006.26.179 [DOI] [PubMed] [Google Scholar]
- 53. Xu H, Wan H, Sandor M, Qi S, Ervin F, Harper JR, Silverman RP, McQuillan DJ. Host response to human acellular dermal matrix transplantation in a primate model of abdominal wall repair. Tissue Eng Part A 2008; 14:2009-19; PMID:; http://dx.doi.org/ 10.1089/ten.tea.2007.0316 [DOI] [PubMed] [Google Scholar]
- 54. Augustine CK, Toshimitsu H, Jung S-H, Zipfel PA, Yoo JS, Yoshimoto Y, Selim MA, Burchette J, Beasley GM, McMahon N, et al. Sorafenib, a multikinase inhibitor, enhances the response of melanoma to regional chemotherapy. Mol Cancer Ther 2010; 9:2090-101; PMID:; http://dx.doi.org/ 10.1158/1535-7163.MCT-10-0073 [DOI] [PubMed] [Google Scholar]
- 55. Balch CM, Gershenwald JE, Soong S-J, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol 2009; 27:6199-206; PMID:; http://dx.doi.org/ 10.1200/JCO.2009.23.4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mojica W, Hawthorn L. Normal colon epithelium: a dataset for the analysis of gene expression and alternative splicing events in colon disease. BMC Genomics 2010; 11:5; PMID:; http://dx.doi.org/ 10.1186/1471-2164-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Okoniewski MJ, Miller CJ. Comprehensive analysis of affymetrix exon arrays using BioConductor. PLoS Comput Biol 2008; 4:e6; PMID:; http://dx.doi.org/ 10.1371/journal.pcbi.0040006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Okoniewski MJ, Yates T, Dibben S, Miller CJ. An annotation infrastructure for the analysis and interpretation of Affymetrix exon array data. Genome Biol 2007; 8:R79; PMID:; http://dx.doi.org/ 10.1186/gb-2007-8-5-r79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Saito R, Smoot ME, Ono K, Ruscheinski J, Wang P-L, Lotia S, Pico AR, Bader GD, Ideker T. A travel guide to Cytoscape plugins. Nat Methods 2012; 9:1069-76; PMID:; http://dx.doi.org/ 10.1038/nmeth.2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman W-H, Pagès F, Trajanoski Z, Galon J. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009; 25:1091-3; PMID:; http://dx.doi.org/ 10.1093/bioinformatics/btp101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Montojo J, Zuberi K, Rodriguez H, Kazi F, Wright G, Donaldson SL, Morris Q, Bader GD. GeneMANIA Cytoscape plugin: fast gene function predictions on the desktop. Bioinformatics 2010; 26:2927-8; PMID:; http://dx.doi.org/ 10.1093/bioinformatics/btq562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bader GD, Hogue CWV. An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinformatics 2003; 4:2; PMID:; http://dx.doi.org/ 10.1186/1471-2105-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.