Abstract
Background
Dry eye may be caused or exacerbated by deficient lipid secretion. Recently, lipid-containing artificial tears have been developed to alleviate this deficiency. Our study compared the efficacy, safety, and acceptability of lipid-containing eye drops with that of aqueous eye drops.
Methods
A non-inferiority, randomized, parallel-group, investigator-masked multicenter trial was conducted. Subjects with signs and symptoms of dry eye were randomized to use one of two lipid-containing artificial tears, or one of two aqueous artificial tears. Subjects instilled assigned drops in each eye at least twice daily for 30 days. The primary efficacy analysis tested non-inferiority of a preservative-free lipid tear formulation (LT UD) to a preservative-free aqueous tear formulation (AqT UD) for change in Ocular Surface Disease Index (OSDI) score from baseline at day 30. Secondary measures included OSDI at day 7, tear break-up time (TBUT), corneal and conjunctival staining, Schirmer’s test, acceptability and usage questionnaires, and safety assessments.
Results
A total of 315 subjects were randomized and included in the analyses. Subjects reported instilling a median of three doses of study eye drops per day in all groups. At days 7 and 30, all groups showed statistically significant improvements from baseline in OSDI (P<0.001) and TBUT (P≤0.005). LT UD was non-inferior to AqT UD for mean change from baseline in OSDI score at day 30. No consistent or clinically relevant differences for the other efficacy variables were observed. Acceptability was generally similar across the groups and there was a low incidence of adverse events.
Conclusion
In this heterogeneous population of dry eye subjects, there were no clinically significant differences in safety, effectiveness, and acceptability between lipid-containing artificial tears and aqueous eye drops. The results suggest that lipid-containing artificial tears can be used to counteract lipid deficiency that is common in dry eye, without compromising overall acceptability.
Keywords: artificial tears, emulsion, dry eye, Ocular Surface Disease Index, tear break-up time, tear film lipid layer
Introduction
Dry eye is a multifactorial disease characterized by insufficient lubrication of the ocular surface.1 Lubricant eye drops, also known as artificial tears, are the mainstay of symptomatic treatment of dry eye. They may be used alone in mild to moderate disease, or in conjunction with other therapies (eg, pharmacological agents or surgical procedures) in moderate to severe disease.2
Artificial tear formulations aim to supplement the deficient tear film and lubricate the ocular surface, thereby providing symptomatic relief and reducing the potential for corneal damage.3–5 It is now recognized that excess evaporation of the tear film, often due to dysfunction of the meibomian glands and loss of the normal lipid layer of tears, is a key feature of the etiology of the disease in many individuals with dry eye.6 As a consequence, lipid-containing eye drops have been introduced as a means of replenishing both aqueous and lipid components and reducing the rate of evaporation of the tear film. In addition to the presence or absence of lipid, artificial tear formulations may be available in multidose bottles containing a preservative, or alternatively provided as preservative-free formulations in unit-dose packaging.
The purpose of the present study was to compare the efficacy, safety, and acceptability of novel lipid-containing eye drop formulations with and without a preservative, in subjects with dry eye disease, with that of otherwise similar aqueous eye drops that do not contain lipid.
Subjects and methods
Study design and participants
This 30-day, multicenter, randomized, subject-masked and investigator-masked, active-controlled, non-inferiority trial (ClinicalTrials.gov identifier NCT01459588) was conducted at 13 sites in the USA. The study was carried out in accordance with Good Clinical Practice guidelines. Institutional review board approval for the study was obtained at each center, and all subjects provided written informed consent. Adults with signs and symptoms of dry eye disease were enrolled into the study. The key inclusion and exclusion criteria are shown in Table 1.
Table 1.
Key inclusion and exclusion criteria assessed at baseline (day 1)
| Inclusion criteriaa |
| • ≥18 years of age and in good general health |
| • OSDI score 18–65 |
| • Use of artificial tears at least twice daily, on average, for ≥3 months prior to baseline |
| • Three consecutive measures of TBUT ≤10 seconds in at least one eye |
| • Mild to moderate corneal or conjunctival staining, as indicated by grade ≥1 (modified NEI grid) staining, related to dry eye in at least one eye |
| Exclusion criteria |
| • Schirmer’s test (with anesthesia) ≤2 mm/5 minutes in either eyeb |
| • Severe corneal or conjunctival staining, as indicated by grade 5 (modified NEI grid) staining, in either eyeb |
| • Wearing of contact lenses within 6 months prior to baseline |
| • Current use or use within 2 weeks of enrollment of topical ophthalmic medications such as corticosteroids, hypotensive agents, and generic cyclosporine (Restasis® [Allergan Inc., Irvine, CA, USA] was allowed if used ≥6 months prior to enrollment), or use of a systemic medication affecting dry eye |
| • Active ocular infection, inflammation, allergy, or blepharitis |
| • Abnormal corneal sensitivity, recent anterior segment surgery (eg, LASIK surgery or any surgery involving a limbal or corneal incision within 12 months of baseline visit) or trauma, anticipated or planned elective surgery during the study, or punctal occlusion |
Notes:
Subjects with Sjogren’s disease, rheumatoid arthritis, or thyroid disease were eligible for enrollment provided they met all inclusion criteria;
criteria used to identify subjects with severe dry eye.
Abbreviations: LASIK, laser-assisted in situ keratomileusis; NEI, National Eye Institute; OSDI, Ocular Surface Disease Index; TBUT, tear film break-up time.
Randomization and study treatment
At the baseline study visit (day 1), subjects were randomized with a computer-generated randomization scheme in a 2:2:1:1 ratio to receive one of the four following artificial tear formulations: preservative-free unit-dose lipid tear formulation (LT UD), preservative-free unit-dose aqueous tear formulation (AqT UD), preserved multidose lipid tear formulation (LT MD), or preserved multidose aqueous tear formulation (AqT MD; Table 2). Randomization was stratified by baseline Ocular Surface Disease Index (OSDI) score (mild/moderate, 18–32; severe, 33–65).7 LT UD and AqT UD were supplied in identical 0.4 mL unit-dose vials, and LT MD and AqT MD were supplied in identical 15 mL multidose bottles. Subjects were instructed to instill one to two drops of the assigned study treatment in each eye as needed, but at least twice daily, for 30 days. The use of adjunctive treatments (such as warm compression or eye lid cleansing) was allowed to continue during the course of the study but any change in use (add or stop) was prohibited.
Table 2.
Artificial tear formulations used in the studya
| Formulation | LT UD (lipid-containing, emulsion eye drop) | AqT UD (aqueous eye drop) | LT MD (lipid-containing, emulsion eye drop) | AqT MD (aqueous eye drop) |
|---|---|---|---|---|
| Brand name (USA) | Refresh Optive® Advanced Sensitive | Refresh Optive® Sensitive | Refresh Optive® Advanced | Refresh Optive® |
| Dosing | Unit-dose | Unit-dose | Multidose | Multidose |
| Composition | CMC, glycerin, polysorbate 80, boric acid, Pemulen™, erythritol, levocarnitine, castor oil (0.25%), sodium hydroxide, purified water | CMC, glycerin, boric acid, sodium borate, sodium citrate, potassium chloride, erythritol, levocarnitine, calcium chloride, magnesium chloride, sodium hydroxide, purified water | CMC, glycerin, polysorbate 80, boric acid, Pemulen™, erythritol, levocarnitine, castor oil (0.25%), Purite®, sodium hydroxide, purified water | CMC, glycerin, boric acid, sodium borate, sodium citrate, potassium chloride, erythritol, levocarnitine, calcium chloride, magnesium chloride, Purite®, sodium hydroxide, purified water |
Notes:
All artificial tear formulations manufactured by Allergan, Inc., Irvine, CA, USA; all were isotonic with a neutral pH.
Abbreviations: LT UD, preservative-free unit-dose lipid tear formulation; AqT UD, preservative-free unit-dose aqueous tear formulation; LT MD, preserved multidose lipid tear formulation; AqT MD, preserved multidose aqueous tear formulation; AE, adverse event; CMC, carboxymethylcellulose sodium.
Outcome measures and efficacy endpoints
Study visits were scheduled for days 1 (baseline assessment, including eligibility), 7, and 30. The primary efficacy measure was the OSDI score, which was based on the frequency of symptoms over the previous week. Secondary efficacy measures (in order of conduct) included tear film break-up time (TBUT) assessed during the 2 minutes waiting period for corneal staining, corneal staining with fluorescein, conjunctival staining with lissamine green, and Schirmer’s test with anesthesia (performed last to avoid interfering with other ophthalmic tests). Questionnaires were used to assess study treatment usage and acceptability. Safety assessments included monitoring adverse events, biomicroscopy (slit lamp examination without pupillary dilation), and evaluation of distance visual acuity, with correction if necessary.
The primary efficacy endpoint was the change from baseline in the OSDI score at day 30. The treatment difference and 95% confidence interval (CI) in change from baseline in OSDI score at day 30 between LT UD and AqT UD were calculated based on the analysis of variance model. Non-inferiority was to be established if the upper limit of the 95% CI was less than the pre-specified margin of 7.3.8 Secondary efficacy endpoints were the change from baseline in the OSDI score at day 7, change from baseline in OSDI subscale scores at days 7 and 30, and TBUT, corneal and conjunctival staining, and Schirmer’s test values at days 7 and 30.
Sample size calculation and data analysis
A sample size of 96 subjects per treatment group was estimated to provide 90% power to establish non-inferiority of LT UD to AqT UD in mean change from baseline in OSDI score at day 30 based on a non-inferiority margin of 7.3. With the addition of the multidose treatment groups, total enrollment of approximately 300 subjects was planned. The intent-to-treat population consisted of all randomized subjects and was used for all efficacy analyses.
Two-way analysis of variance, accounting for treatment and baseline OSDI score stratification, was used to compare treatment differences for primary and secondary efficacy analyses. Within-treatment changes from baseline were analyzed using paired t-tests (alpha level 0.05). Additional analyses of OSDI subgroups and questionnaire data were also performed using analysis of variance. Safety data were summarized using descriptive statistics.
Results
Subject disposition and baseline characteristics
A total of 315 subjects were enrolled and randomized (LT UD, n=105; AqT UD, n=103; LT MD, n=51; and AqT MD, n=56). Among the randomized subjects (intent- to-treat population), 98.4% (310/315) completed the study. Completion rates were similar across the treatment groups (96.1%–99.0%); of the five subjects who failed to complete the study, three discontinued as a result of adverse events (one LT UD, one LT MD, and one AqT MD) and two owing to protocol violation (one AqT UD and one LT MD). Ten subjects were excluded from the per-protocol population (n=305). Study eye drops were instilled a median of three times per day in each treatment group.
All randomized subjects had a history of dry eye disease and artificial tear use. Overall, only seven (2.2%) subjects had previously used lipid-containing eye drops for dry eye; four (3.9%) in the LT UD group, one (1.0%) in the AqT UD group, one (2.0%) in the LT MD group, and one (1.8%) in the AqT MD group. There were no statistically significant differences between the treatment groups in age, sex, or race. Similarly, disease characteristics and prior use of lubricating eye drops at baseline were comparable among the treatment groups (Table 3).
Table 3.
Baseline demographic and clinical characteristics of subjects
| Characteristic | LT UD (n=105) | AqT UD (n=103) | LT MD (n=51) | AqT MD (n=56) |
|---|---|---|---|---|
| Age, years | ||||
| Mean (SD) | 54.4 (14.8) | 55.8 (14.1) | 55.2 (14.5) | 53.5 (13.9) |
| Range | 22–85 | 24–84 | 23–81 | 22–83 |
| Female, n (%) | 83 (79.0) | 87 (84.5) | 44 (86.3) | 41 (73.2) |
| Race, n (%) | ||||
| Caucasian | 91 (86.7) | 83 (80.6) | 45 (88.2) | 47 (83.9) |
| Non-caucasian | 14 (13.3) | 20 (19.4) | 6 (11.8) | 9 (16.1) |
| OSDI score, mean (SD) | 41.5 (14.8) | 40.3 (13.5) | 38.3 (12.8) | 40.2 (13.4) |
| TBUT, seconds, mean (SD) | 4.9 (1.8) | 4.9 (1.8) | 4.6 (1.7) | 5.1 (1.7) |
| Schirmer’s test, mm/5 minutes, mean (SD) | 10.3 (7.4) | 10.4 (6.8) | 8.4 (7.3) | 10.1 (7.6) |
| Staining score, mean (SD) | ||||
| Corneal | 4.9 (3.8) | 5.0 (3.9) | 5.0 (3.8) | 4.7 (3.2) |
| Conjunctival | 6.7 (5.7) | 6.1 (4.4) | 6.3 (4.8) | 6.7 (5.4) |
| Meibomian gland dysfunction, n (%) | 7 (6.7) | 5 (4.9) | 3 (5.9) | 7 (12.5) |
| Use of cyclosporine ophthalmic emulsion, n (%)a | 8 (7.6) | 7 (6.8) | 7 (13.7) | 4 (7.1) |
| Use of Optive® lubricating eye drops, n (%) | 16 (15.2) | 10 (9.7) | 11 (21.6) | 8 (14.3) |
Notes:
Restasis® (Allergan, Inc., Irvine, CA, USA) use prior to enrollment and continuing during the study.
Abbreviations: LT UD, preservative-free unit-dose lipid tear formulation; AqT UD, preservative-free unit-dose aqueous tear formulation; LT MD, preserved multidose lipid tear formulation; AqT MD, preserved multidose aqueous tear formulation; OSDI, Ocular Surface Disease Index; SD, standard deviation; TBUT, tear break-up time.
Efficacy
For the primary efficacy measure, OSDI scores at day 30 were improved significantly compared with baseline in each treatment group (P<0.001; Figure 1). Improvements in the LT UD and AqT UD groups were similar, and met the non-inferiority criterion (the upper limit of the 95% CI for the mean change from baseline was 2.51, below the pre-specified margin of 7.3). OSDI scores were also significantly improved compared with baseline in each treatment group at day 7 (P<0.001; Figure 1). Additional comparisons of OSDI scores for mild/moderate versus severe subjects demonstrated some differences between LT UD and LT MD in the mild/moderate symptoms group at day 30 (P=0.004, in favor of LT UD; Figure 2). Examination of OSDI subscale scores for ocular symptoms, visual functions, and environmental triggers showed similar overall results among the four treatment groups.
Figure 1.
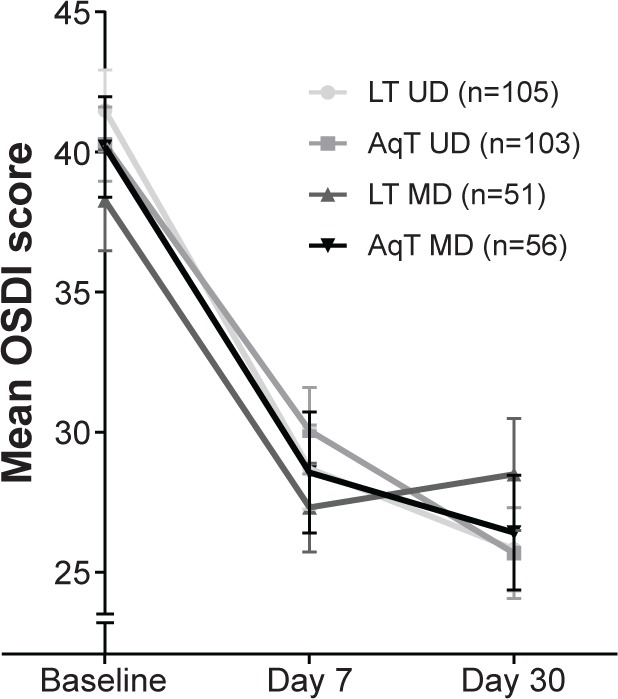
Mean OSDI scores at baseline and days 7 and 30 of study treatment.
Notes: OSDI scores were assessed on a scale of 0–100, where a higher score represents a more severe disease status. P<0.001 for LT UD, AqT UD, LT MD, and AqT MD at day 7 and day 30 compared with baseline; error bars represent SEM.
Abbreviations: LT UD, preservative-free unit-dose lipid tear formulation; AqT UD, preservative-free unit-dose aqueous tear formulation; LT MD, preserved multidose lipid tear formulation; AqT MD, preserved multidose aqueous tear formulation; OSDI, Ocular Surface Disease Index; SEM, standard error mean.
Figure 2.
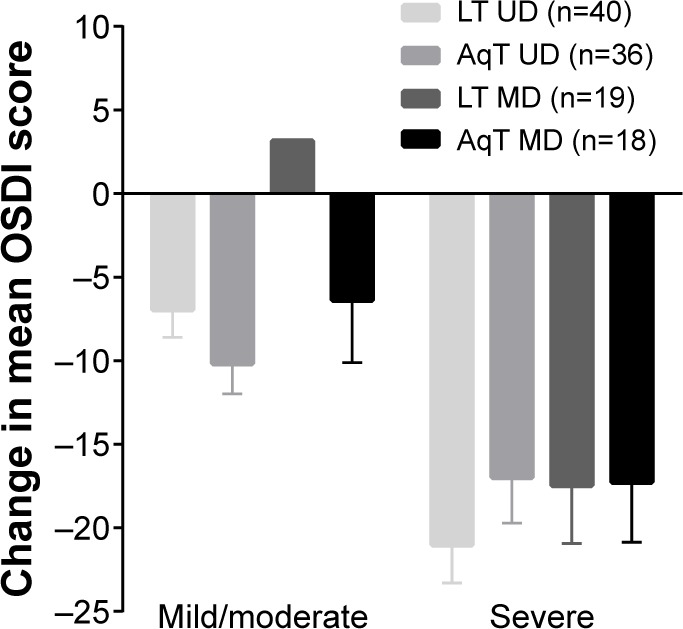
Mean change in OSDI scores at day 30 by baseline OSDI score.
Notes: OSDI scores ≥18–32 were grouped as mild/moderate; scores >32–65 were grouped as severe. P=0.004 for LT UD compared with LT MD in the mild/moderate group; error bars represent SEM.
Abbreviations: LT UD, preservative-free unit-dose lipid tear formulation; AqT UD, preservative-free unit-dose aqueous tear formulation; LT MD, preserved multidose lipid tear formulation; AqT MD, preserved multidose aqueous tear formulation; OSDI, Ocular Surface Disease Index; SEM, standard error mean.
For secondary efficacy measures, statistically significant improvements from baseline in TBUT were observed for each treatment group at days 7 and 30 (P≤0.005; Figure 3). In the LT UD group, TBUT improved from a mean (standard deviation) of 4.92 (1.79) seconds at baseline to 6.28 (3.29) seconds at day 30; in the LT MD group, TBUT improved from 4.61 (1.74) seconds at baseline to 5.82 (2.81) seconds at day 30. Similar increases in TBUT were observed with the aqueous eye drop formulations (Figure 3). No statistically significant differences were observed in any of the between-group comparisons. Similar to TBUT, at day 30, within-group mean changes from baseline were statistically significant for all groups in Schirmer’s test scores (P<0.05), but there were no significant differences in mean changes among the treatment groups (Figure 4).
Figure 3.
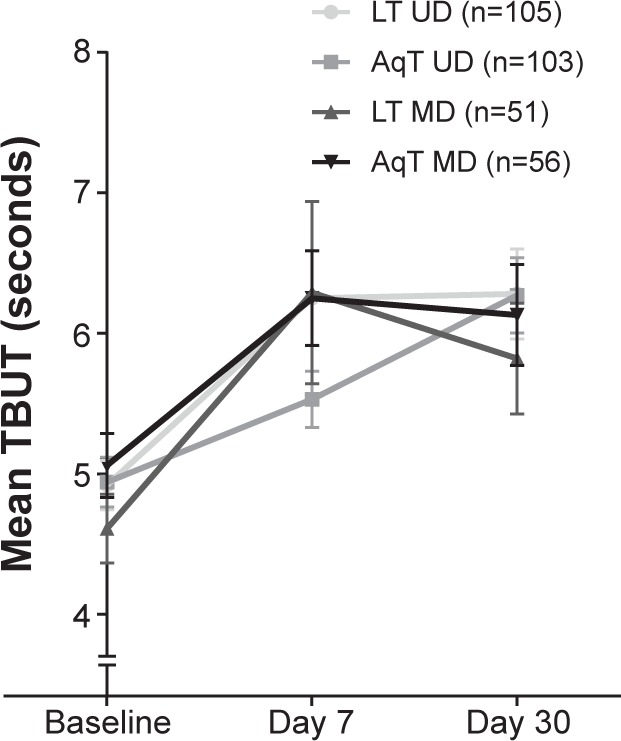
Mean TBUT at baseline and days 7 and 30 of study treatment.
Notes: The eye with the shorter average TBUT at baseline for each subject was used in the analysis. P≤0.005 for LT UD, AqT UD, LT MD, and AqT MD at day 7 and day 30 compared with baseline; error bars represent the SEM.
Abbreviations: LT UD, preservative-free unit-dose lipid tear formulation; AqT UD, preservative-free unit-dose aqueous tear formulation; LT MD, preserved multidose lipid tear formulation; AqT MD, preserved multidose aqueous tear formulation; TBUT, tear break-up time; SEM, standard error mean.
Figure 4.
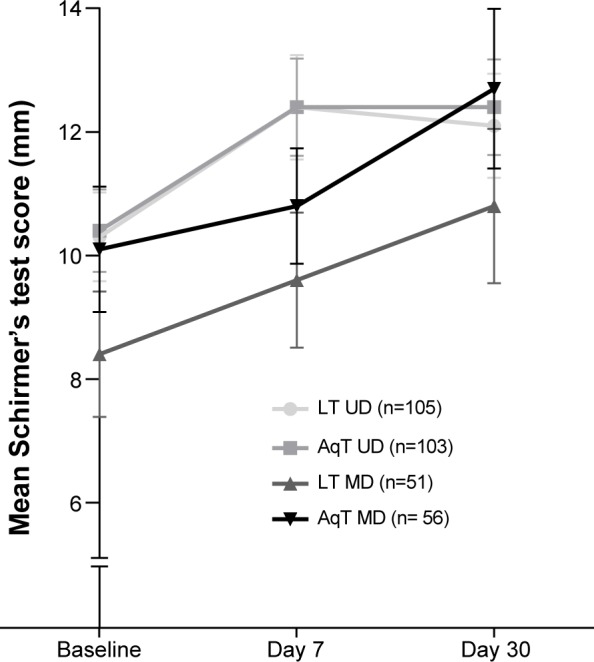
Mean Schirmer’s score at baseline and days 7 and 30 of study treatment.
Notes: Schirmer’s score was measured for 5 minutes (with anesthesia). P≤0.01 for LT UD at day 7 and day 30 compared with baseline; P<0.04 for LT MD at day 7 compared with baseline; P<0.01 for LT MD at day 30 compared with baseline; P≤0.001 for AqT UD at day 7 and day 30 compared with baseline; P=0.004 for AqT MD at day 30 compared with baseline; error bars represent SEM.
Abbreviations: LT UD, preservative-free unit-dose lipid tear formulation; AqT UD, preservative-free unit-dose aqueous tear formulation; LT MD, preserved multidose lipid tear formulation; AqT MD, preserved multidose aqueous tear formulation; SEM, standard error mean.
Statistically significant reductions from baseline in corneal staining were observed at days 7 and 30 for the LT UD, AqT UD, and AqT MD groups (P<0.05), but not for the LT MD group (Table 4). Although the reduction in corneal staining from baseline to day 30 was significantly greater for the AqT UD group than the LT UD group (mean between-group difference 0.78; P=0.045), the 95% CI for the between-group comparison of these two groups was 0.02–1.54 at day 30. Since the upper limit (1.54) was less than the pre-specified clinical margin of 3.0, the data do not support a conclusion of clinically relevant differences between AqT UD and LT UD. Statistically significant reductions from baseline in conjunctival staining were observed at days 7 and 30 for the AqT UD group (P<0.001) and at day 7 for the AqT MD group (P<0.05), but not for the LT UD or LT MD groups (Table 4). However, the upper limits of the 95% CI for the between-group analyses were less than the pre-specified clinical margin of 3.0, indicating that there were no clinically relevant differences in between-group comparisons.
Table 4.
Severity rating of corneal and conjunctival staining
| LT UD | AqT UD | LT MD | AqT MD | Between-group comparisons (P-value)
|
|||
|---|---|---|---|---|---|---|---|
| LT UD versus AqT UD | LT UD versus LT MD | LT MD versus AqT MD | |||||
| Corneal, mean (SD) | |||||||
| Baseline | 4.9 (3.8) | 5.0 (3.9) | 5.0 (3.8) | 4.7 (3.2) | 0.813 | 0.777 | 0.603 |
| Change to day 7 | −0.7 (2.6)** | −1.3 (2.2)*** | −0.3 (2.6) | −1.1 (2.5)** | 0.074 | 0.291 | 0.076 |
| Change to day 30 | −0.7 (2.9)* | −1.5 (2.4)*** | 0.1 (3.2) | −1.4 (2.9)*** | 0.045 | 0.079 | 0.004 |
| Conjunctival, mean (SD) | |||||||
| Baseline | 6.7 (5.7) | 6.1 (4.4) | 6.3 (4.8) | 6.7 (5.4) | 0.368 | 0.590 | 0.655 |
| Change to day 7 | −0.5 (3.9) | −1.2 (2.9)*** | 0.2 (4.0) | −1.1 (2.7)** | 0.185 | 0.248 | 0.069 |
| Change to day 30 | −0.6 (4.2) | −1.2 (3.7)*** | 0.5 (3.8) | −0.8 (3.7) | 0.227 | 0.088 | 0.076 |
Notes: P-values for within-group analysis of changes from baseline using paired t-test:
P<0.05;
P<0.01; and
P<0.001. Staining was rated on the modified NEI rating scale of 0–5. For each subject, the eye with the higher score at baseline was used in the analysis.
Abbreviations: LT UD, preservative-free unit-dose lipid tear formulation; AqT UD, preservative-free unit-dose aqueous tear formulation; LT MD, preserved multidose lipid tear formulation; AqT MD, preserved multidose aqueous tear formulation; NEI, National Eye Institute; SD, standard deviation.
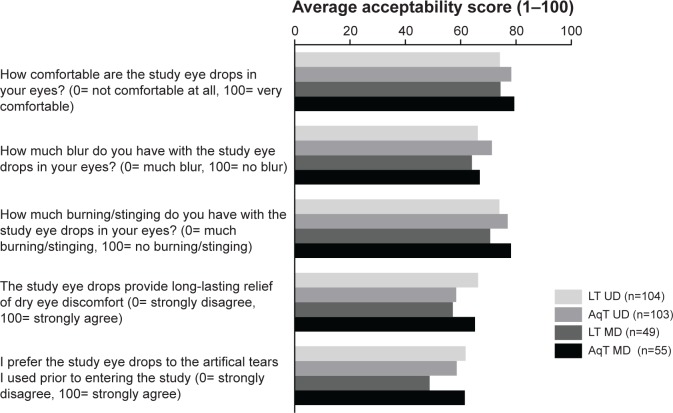
Product usage and acceptability questionnaires showed that the lipid eye drop formulations were equally utilized and acceptable as the aqueous eye drop formulations. Overall, acceptability scores were similar across the treatment groups at each visit, except for responses to question 5 (“I prefer the study eye drops to the artificial tears I used prior to entering the study”) in the LT MD versus AqT MD (P=0.023 at day 7, in favor of AqT MD) and LT UD versus LT MD (P=0.021 at day 30, in favor of LT UD) comparisons. Acceptability data at day 30 are shown in Figure 5. There were no between-group differences in the number of times the study treatments were used during the week preceding the day 7 and day 30 visits, and subjects instilled study treatments at a median of 3.0 (range 2–20) times a day prior to the visits for each group.
Figure 5.
Acceptability of study treatment at day 30 of study treatment.
Notes: Acceptability was assessed with a 5-item questionnaire, scored on a visual analog scale of 0–100, with 0 representing minimal acceptability and 100 representing maximum acceptability.
Abbreviations: LT UD, preservative-free unit-dose lipid tear formulation; AqT UD, preservative-free unit-dose aqueous tear formulation; LT MD, preserved multidose lipid tear formulation; AqT MD, preserved multidose aqueous tear formulation.
Safety
Treatment-emergent adverse events were reported in 11.4%, 15.5%, 13.7%, and 10.7% of subjects in the LT UD, AqT UD, LT MD, and AqT MD groups, respectively (Table 5). A total of 21 subjects reported treatment-related adverse events; the most frequent were instillation site pain and blurred vision. There were no statistically significant differences between the treatment groups in the incidence of clinically relevant biomicroscopy findings or changes in distance visual acuity at days 7 or 30.
Table 5.
Summary of adverse events
| Incidence, n (%) | LT UD (n=105) | AqT UD (n=103) | LT MD (n=51) | AqT MD (n=56) |
|---|---|---|---|---|
| Treatment-emergent AEs | 12 (11.4) | 16 (15.5) | 7 (13.7) | 6 (10.7) |
| Treatment-related AEs | 5 (4.8) | 9 (8.7) | 4 (7.8) | 3 (5.4) |
| Serious AEsa | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (1.8) |
| Treatment-emergent AEs reported in ≥2% of subjects in any treatment group | ||||
| Instillation site pain | 4 (3.8) | 4 (3.9) | 2 (3.9) | 2 (3.6) |
| Vision blurred | 3 (2.9) | 4 (3.9) | 2 (3.9) | 2 (3.6) |
| Foreign body sensation in eyes | 1 (1.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Visual acuity reduced | 1 (1.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Ocular discomfort | 0 (0.0) | 3 (2.9) | 1 (2.0) | 0 (0.0) |
| Eyelid pruritus | 0 (0.0) | 1 (1.0) | 1 (2.0) | 0 (0.0) |
| Conjunctival hemorrhage | 0 (0.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Contusion | 0 (0.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
| Madarosis | 0 (0.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) |
Notes:
Bile duct stone (LT UD, not treatment-related) and ankle fracture (AqT MD, not treatment-related).
Abbreviations: LT UD, preservative-free unit-dose lipid tear formulation; AqT UD, preservative-free unit-dose aqueous tear formulation; LT MD, preserved multidose lipid tear formulation; AqT MD, preserved multidose aqueous tear formulation; AE, adverse event.
Discussion
This study demonstrated that both unit-dose and multidose lipid-containing eye drops are similar to standard aqueous eye drops in terms of efficacy, safety, and acceptability. All four eye drop formulations resulted in statistically significant improvements in OSDI scores from baseline at days 7 and 30, and LT UD was shown to be non-inferior to AqT UD in reducing the severity of dry eye symptoms at day 30 (primary endpoint). There were no clinically significant differences in performance in TBUT, corneal staining, conjunctival staining, and Schirmer’s test among the artificial tear formulations. Mean conjunctival staining improved compared with baseline in all treatment groups, reaching statistical significance in the AqT UD group at day 30; however, the changes observed were small (less than 2 units) and did not lead to any clinically meaningful conclusions. All treatment groups had similar incidences of adverse events; biomicroscopy and distance visual acuity assessments did not reveal any safety concerns.
Eye drop utilization and acceptability scores were positive and similar across treatment groups, except for the question about subject preference of the study eye drops over their previous artificial tears. This could possibly be due to the higher percentage of subjects who previously used Optive® in the LT MD group (21.6%), compared with the AqT MD group (14.3%), which may have minimized the change observed at day 7 in the LT MD group. However, the higher percentage of subjects who previously used Optive® in the preserved LT MD group (21.6%) compared with the preservative-free LT UD group (15.2%), is unlikely to have favored LT UD at 30 days as there was no significant difference in tolerability between the two groups. Additionally, subjects used artificial tears a median of three times a day, thus there may have been a difference between the adverse effects observed with preservative versus non-preservative formulations in subjects who used the eye drops more frequently for treatment of more severe dry eye.
Previously published studies have reported that a single instillation of an emulsion eye drop containing castor oil induced restructuring of the lipid tear film in patients with dry eye disease.9,10 Lipid-based eye drops used for 2 weeks11 or 30 days12 have also been found to have a significantly greater effect than aqueous drops in reducing the rate of tear film evaporation. A more recent prospective, multicenter study of 1,209 patients conducted in a routine clinical setting in Germany confirmed the effectiveness of the same multidose artificial tear formulation containing castor oil (LT MD) evaluated in the current study in reducing the signs and symptoms of all types of dry eye, although it was recommended for lipid-deficient dry eye disease.13 In addition, eye drops containing a 2% concentration of castor oil have been shown to be effective in treating non-inflamed, obstructive meibomian gland dysfunction, without causing complications such as blurred vision.14 Other studies of emulsion eye drops with higher concentrations of castor oil and without carboxymethylcellulose or osmoprotectants have reported adverse event rates (including blurring of vision and burning/stinging) as high as 19.5% in subjects with severe dry eye.15 Therefore, the results from the current study suggest that reduction in the total amount of oil, both LT UD and LT MD contained 0.25% castor oil which is lower than the 1.25% to 5.5% concentrations reported in other eye drop products containing oils,12,14,16 and addition of carboxymethylcellulose and osmoprotectants, provide a substantial improvement in subject comfort and acceptability compared with earlier lipid-based eye drops.
A recent systematic review concluded that there is substantial evidence that lipid-containing eye drops are effective in improving signs and symptoms of dry eye, and recommended their use in clinical practice.17 Since this study demonstrates that efficacy, safety, and acceptability were similar for the lipid-containing formulations and advanced aqueous drops, the results support the use of these lipid-containing artificial tears in individuals with dry eye to improve tear film stability without compromising overall clinical performance.
A potential limitation of the study was the use of the OSDI score, which assesses the frequency of symptoms but does not account for the severity of each symptom episode. In addition, the absence of a washout period before initiation of study treatment could have influenced outcomes, especially at day 7. However, overall results of the study were consistent at days 7 and 30.
Conclusion
The lipid-containing artificial tears tested were as safe, effective, and acceptable as the aqueous eye drops. All four formulations produced statistically significant improvements in OSDI scores from baseline at days 7 and 30, and LT UD was non-inferior to AqT UD in reducing the severity of dry eye symptoms at day 30 (primary endpoint). Overall acceptability was also similar across the groups.
Acknowledgments
The authors would like to thank Genming Shi and Ru Chen (former employees of Allergan, Inc.) for their contribution to the data analysis and development of the manuscript. The following principal investigators participated in this study: Jason Bacharach, Petaluma, CA; Gregg J Berdy, St Louis, MO; David T Douglass, Bangor, ME; Paul Hartman, Rochester, NY; Michael S Korenfeld, Washington, MO; Christopher W Lievens, Memphis, TN; Stephen Montaquila, Warwick, RI; Lee Rigel, East Lansing, MI; Kenneth Sall, Artesia, CA; Mary Jo Stiegemeier, Beachwood, OH; Anthony J Verachtert, Kansas City, MO; Eric M White, San Diego, CA; Kenneth A Young, Brentwood, TN.
Footnotes
Disclosure
This study was sponsored by Allergan, Inc., Irvine, CA, USA. The authors are employees of Allergan, Inc. The formulations used in the study are investigational or marketed products of Allergan, Inc. Writing and editorial assistance was provided to the authors by Sarah Whitfield, of Evidence Scientific Solutions, Philadelphia, PA, USA, and funded by Allergan, Inc. All authors met the International Committee of Medical Journal Editors authorship criteria. Neither honoraria nor payments were made for authorship.
References
- 1.The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):75–92. doi: 10.1016/s1542-0124(12)70081-2. No authors listed. [DOI] [PubMed] [Google Scholar]
- 2.Behrens A, Doyle JJ, Stern L, et al. Dysfunctional tear syndrome: a Delphi approach to treatment recommendations. Cornea. 2006;25(8):900–907. doi: 10.1097/01.ico.0000214802.40313.fa. [DOI] [PubMed] [Google Scholar]
- 3.Lee JH, Ahn HS, Kim EK, Kim TI. Efficacy of sodium hyaluronate and carboxymethylcellulose in treating mild to moderate dry eye disease. Cornea. 2011;30(2):175–179. doi: 10.1097/ICO.0b013e3181e9adcc. [DOI] [PubMed] [Google Scholar]
- 4.Aragona P, Papa V, Micali A, Santocono M, Milazzo G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol. 2002;86(2):181–184. doi: 10.1136/bjo.86.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doughty MJ, Glavin S. Efficacy of different dry eye treatments with artificial tears or ocular lubricants: a systematic review. Ophthalmic Physiol Opt. 2009;29(6):573–583. doi: 10.1111/j.1475-1313.2009.00683.x. [DOI] [PubMed] [Google Scholar]
- 6.Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol. 2000;118(5):615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 8.Miller KL, Walt JG, Mink DR, et al. Minimal clinically important difference for the ocular surface disease index. Arch Ophthalmol. 2010;128(1):94–101. doi: 10.1001/archophthalmol.2009.356. [DOI] [PubMed] [Google Scholar]
- 9.Di Pascuale MA, Goto E, Tseng SC. Sequential changes of lipid tear film after the instillation of a single drop of a new emulsion eye drop in dry eye patients. Ophthalmology. 2004;111(4):783–791. doi: 10.1016/j.ophtha.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Maïssa C, Guillon M, Simmons P, Vehige J. Effect of castor oil emulsion eyedrops on tear film composition and stability. Cont Lens Anterior Eye. 2010;33(2):76–82. doi: 10.1016/j.clae.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Tomlinson A, Madden LC, Simmons PA. Effectiveness of dry eye therapy under conditions of environmental stress. Curr Eye Res. 2013;38(2):229–236. doi: 10.3109/02713683.2012.757323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khanal S, Tomlinson A, Pearce EI, Simmons PA. Effect of an oil- in-water emulsion on the tear physiology of patients with mild to moderate dry eye. Cornea. 2007;26(2):175–181. doi: 10.1097/ICO.0b013e31802b492d. [DOI] [PubMed] [Google Scholar]
- 13.Kaercher T, Thelen U, Brief G, Morgan-Warren RJ, Leaback R. A prospective, multicenter, noninterventional study of Optive Plus® in the treatment of patients with dry eye: the prolipid study. Clin Ophthalmol. 2014;8:1147–1155. doi: 10.2147/OPTH.S58464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goto E, Shimazaki J, Monden Y, et al. Low-concentration homogenized castor oil eye drops for noninflamed obstructive meibomian gland dysfunction. Ophthalmology. 2002;109(11):2030–2035. doi: 10.1016/s0161-6420(02)01262-9. [DOI] [PubMed] [Google Scholar]
- 15.Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000;107(4):631–639. doi: 10.1016/s0161-6420(99)00176-1. [DOI] [PubMed] [Google Scholar]
- 16.Scaffidi RC, Korb DR. Comparison of the efficacy of two lipid emulsion eyedrops in increasing tear film lipid layer thickness. Eye Contact Lens. 2007;33(1):38–44. doi: 10.1097/01.icl.0000247638.50568.c0. [DOI] [PubMed] [Google Scholar]
- 17.Lee SY, Tong L. Lipid-containing lubricants for dry eye: a systematic review. Optom Vis Sci. 2012;89(11):1654–1661. doi: 10.1097/OPX.0b013e31826f32e0. [DOI] [PubMed] [Google Scholar]