Abstract
A subset of CD1c-restricted T lymphocytes exhibits strong reactivity against leukemia cells. These T cells recognize methyl-lysophosphatidic acid (mLPA), a novel lipid antigen produced by acute leukemia cells. Considering that CD1c-restricted T cells display efficacious anti-leukemia activities in a mouse model, this lipid antigen thus represents a novel target in the immunotherapy of hematological malignancies.
Keywords: autoreactivity, CD1, immunesurveillance, immunotherapy, lipid antigens, leukemia
Improvements of poly-chemotherapy and allogeneic hematopoietic stem cell transplantation (HSCT) regimens have significantly ameliorated the prognosis of acute leukemia.1 Post-transplant recurrence of residual leukemia blasts surviving the conditioning regimen remains the major unmet clinical need for patients afflicted with acute leukemia,1 prompting the search of novel strategies to overcome this problem. The transfer of allogeneic donor-derived T cells into patients is currently utilized to maintain remission by inducing a beneficial graft vs. leukemia (GVL) effect.1 The grafted alloreactive T cells can also elicit detrimental graft vs. host disease (GVHD), resulting from the recognition of patient's non-hematopoietic tissues.1 This is due to transferred T cells that are specific for peptide antigens presented by the highly polymorphic MHC molecules, which generate histo-incompatible barriers between individuals.
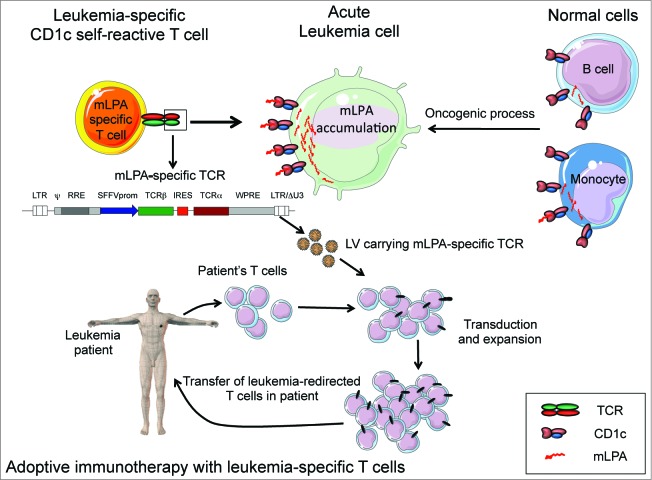
A promising therapeutic strategy to maximize GVL without inducing GVHD consists in the selective targeting of T-cell responses against the leukemia cells, while preserving both the normal donor-derived hematopoiesis and organ functions in patients. The adoptive transfer of T cells recognizing leukemia-associated peptide antigen is currently one of the most promising therapeutic approaches.1 Tumor-specific T lymphocytes can be directly isolated from patients or allogeneic donors and expanded in culture prior to infusion, or generated by engineering patients or donors T cells with T cell receptor (TCR) genes specific for leukemia-associated peptide antigens.2 One major caveat of this approach is represented by the polymorphism in MHC molecules, which requires the generation of T cells or TCRs that are restricted for the diverse MHC alleles displayed by the population of leukemia patients. Our study3 defines an original approach to exploit T lymphocytes against malignant cells bypassing the MHC-restricted histocompatibility. In recent years, it has becoming increasingly clear that T lymphocytes may also recognize lipid antigens presented by the non-polymorphic, MHC Class I-related CD1 molecules.4 There exists 5 CD1 molecules in humans, grouped by homology in CD1a, b, c (group 1), CD1d (group 2) and CD1e (group 3), respectively. CD1-restricted T cells recognize lipid antigens derived from infectious pathogens and may protect the host from infections.5 A large number of CD1-restricted T cells are also reactive against malignant cells expressing CD1, implying the recognition of endogenous self-lipids.6 By combining biochemical and cellular approaches, we identify a unique class of self-lipid antigens purified from leukemia cells and recognized by specific T cell clones when presented by CD1c molecules. This novel self-lipid antigens where characterized as methyl-lysophospatidic acid (mLPA), a molecule never described before, which we found accumulated in different types of primary leukemia cells and in established tumor cell lines of differing hematopoietic lineages.3 Hence, mLPA represents a novel class of leukemia-associated antigens. mLPA includes unusual structural characteristics, such as the presence of a methyl group on the phosphate moiety and an ether bonded alkyl chain, which is evidence of peroxisomal synthesis.7 Previous studies have reported the accumulation of methylated lysophosphatidic acids in cancer cells that promote tumor cell proliferation and migration,8 as well as an increase in peroxisome numbers and activities, which participate in tumor cell growth.9 Thus, mLPA-specific leukemia-reactive T cells recognize peroxisome-derived lipids as leukemia antigens, providing direct evidence of the capacity of the immune system to survey the lipidome of healthy cells and detect modifications occurring during oncogenesis (Fig. 1).
Figure 1.
Targeting leukemia by CD1c-restricted T cells specific for a novel lipid antigen. (A) CD1c self-reactive T cells recognize the self-lipid antigen methyl-lysophospatidic acid (mLPA), which is upregulated by oncogenic metabolic processes in malignant cells (acute myeloid or lymphocytic leukemias), compared with normal monocytes and B cells. (B) mLPA-specific T cell receptor (TCR) genes are cloned into lentivirus (LV) vectors that can be utilized to efficiently transfer the antigen-specificity into polyclonal T cells. LV vector structure: LTR/ΔU3, long terminal repeats, with deletions; ψ, packaging sequence; RRE, reverse response element; SFFV prom, Spleen Focus Forming Virus promoter; IRES, internal ribosome entry site sequence; WPRE, post-translational regulatory element of Woodchuck Hepatitis Virus. (C) T cells from acute leukemia patients are expanded and transduced ex vivo with the LV carrying mLPA-specific TCR genes, which redirects T cells against the leukemia target upon adoptive transfer into the patients.
We reported that mLPA-specific T cells kill CD1c+ leukemia cell lines and primary blasts and display therapeutic efficacy in a mouse xenograft model of human T-cell acute lymphoblastic leukemia (T-ALL) and acute myeloid leukemia (AML), significantly restraining the progression of the transplanted malignant cells compared to mLPA-non specific control T cells.3 Notably, mLPA-specific T cells discriminate between normal and malignant cells, as normal primary circulating B cells and monocytes represent poor targets for these T lymphocytes, compatible with the detection of trace amounts of mLPA in normal cells.3 An exception is represented by the recognition of in vitro differentiated monocyte-derived dendritic cells (Mo-DCs) in which we measured high levels of mLPA.3 Consistently, MoDCs efficiently stimulated mLPA-specific T cells.3 As Mo-DCs are generated in vitro, the physiological relevance of their recognition by mLPA-specific T cells warrants further investigations.
All together, these findings indicated that the expression pattern of mLPA resembles that of other tumor-associated antigens of a protein nature, such that mLPA accumulates in tumor cells but is also present in some healthy cells.10
Our study provides proof-of-concept evidence that it is possible to generate large numbers of CD1c-restricted anti-leukemia T cells that target leukemia cells in vivo. This is achieved by lentivirus transfer of mLPA-specific TCR genes into polyclonal T cell lines, which recognize and kill CD1c-expressing malignant cells.3 Importantly the non-polymorphic CD1c molecules are broadly expressed by primary blasts representative of AML and precursor B-ALL disease occurring in both adult and pediatric patients, whereas they are not expressed in hematopoietic precursors and parenchymatous organs.3 These characteristics will probably minimize GVHD in transduced patients. The weak recognition of healthy monocytes and DCs by mLPA-specific T cells might be clinically tolerated in the course of adoptive cell therapy in the peritransplant period considering the benefit of prevention/control of post-transplant leukemia relapses.
Our results have twofold implications. First, the identification of mLPA as new lipid antigen, probably the product of altered lipid metabolism associated with the oncogenic process. Second, the definition of a new strategy to target leukemia that may efficiently complement the current cell-based immunotherapies relying on antitumor responses directed against protein antigens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood 2008; 112:4371-83; PMID:; http://dx.doi.org/ 10.1182/blood-2008-03-077974 [DOI] [PubMed] [Google Scholar]
- 2. Maus MV, Fraietta JA, Levine BL, Kalos M, Zhao Y, June CH. Adoptive immunotherapy for cancer or viruses. Ann Rev Immunol 2014; 32:189-225; PMID:; http://dx.doi.org/ 10.1146/annurev-immunol-032713-120136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lepore M, de Lalla C, Gundimeda SR, Gsellinger H, Consonni M, Garavaglia C, et al. A novel self-lipid antigen targets human T cells against CD1c(+) leukemias. J Exp Med 2014; 211:1363-77; PMID:; http://dx.doi.org/ 10.1084/jem.20140410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Porcelli SA, Modlin RL. The CD1 system: antigen-presenting molecules for T cell recognition of lipids and glycolipids. Annu Rev Immunol 1999; 17:297-329; PMID:; http://dx.doi.org/ 10.1146/annurev.immunol.17.1.297 [DOI] [PubMed] [Google Scholar]
- 5. De Libero G, Mori L. Recognition of lipid antigens by T cells. Nat Rev Immunol 2005; 5:485-96; PMID:; http://dx.doi.org/ 10.1038/nri1631 [DOI] [PubMed] [Google Scholar]
- 6. de Lalla C, Lepore M, Piccolo FM, Rinaldi A, Scelfo A, Garavaglia C, Mori L, De Libero G, Dellabona P, Casorati G. High-frequency and adaptive-like dynamics of human CD1 self-reactive T cells. Eur J Immunol 2011; 41:602-10; PMID:; http://dx.doi.org/ 10.1002/eji.201041211 [DOI] [PubMed] [Google Scholar]
- 7. Wanders RJ, Ferdinandusse S, Brites P, Kemp S. Peroxisomes, lipid metabolism and lipotoxicity. Biochim Biophys Acta 2010; 1801:272-80; PMID:; http://dx.doi.org/ 10.1016/j.bbalip.2010.01.001 [DOI] [PubMed] [Google Scholar]
- 8. Endo T, Kano K, Motoki R, Hama K, Okudaira S, Ishida M, Ogiso H, Tanaka M, Matsuki N, Taguchi R, et al. Lysophosphatidylmethanol is a pan lysophosphatidic acid receptor agonist and is produced by autotaxin in blood. J Biochem 2009; 146:283-93; PMID:; http://dx.doi.org/ 10.1093/jb/mvp068 [DOI] [PubMed] [Google Scholar]
- 9. Schrader M, Fahimi HD. Peroxisomes and oxidative stress. Biochim Biophys Acta 2006; 1763:1755-66; PMID:; http://dx.doi.org/ 10.1016/j.bbamcr.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 10. Gilboa E. The makings of a tumor rejection antigen. Immunity 1999; 11:263-70; PMID:; http://dx.doi.org/ 10.1016/S1074-7613(00)80101-6 [DOI] [PubMed] [Google Scholar]