Abstract
In melanoma, both the induction of immunosuppression by tumor cells and the inflammatory antitumor response can induce an upregulation of counter-regulatory mechanisms such as indoleamine 2,3-dioxygenase (IDO), programmed death-ligand 1 (PD-L1) and CTLA-4+ regulatory T-cells (Tregs) in the tumor microenvironment. Even though these immunosuppressive mediators are targets for immunotherapy, research investigating their expression in the peripheral blood is lacking. We therefore, performed flow cytometry on PBMCs of stage I–IV melanoma patients. IDO expression was detected in plasmacytoid dendritic cells (pDC) and monocytic myeloid-derived suppressor cells (mMDSC), and increased in advanced disease stage (p = 0.027). Tryptophan breakdown confirmed the functional activity of IDO and was linked with increased PD-L1+ cytotoxic T-cells (p = 0.009), relative lymphopenia (p = 0.036), and a higher mDC/pDC ratio (p = 0.002). High levels of circulating PD-L1+ cytotoxic T-cells were associated with increased CTLA-4 expression by Tregs (p = 0.005) and MDSC levels (p = 0.033). This illustrates that counter-regulatory immune mechanisms in melanoma should be considered as one interrelated signaling network. Moreover, both increased PD-L1+ T-cells and CTLA-4 expression in Tregs conferred a negative prognosis, indicating their in vivo relevance. Remarkably, circulating CTLA-4, IDO, and pDC levels were altered according to prior invasion of the sentinel lymph node and IDO expression in the sentinel was associated with more IDO+ PBMCs. We conclude that the expression of IDO, PD-L1, and CTLA-4 in the peripheral blood of melanoma patients is strongly interconnected, associated with advanced disease and negative outcome, independent of disease stage. Combination treatments targeting several of these markers are therefore likely to exert a synergistic response.
Keywords: cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), indoleamine 2-3-dioxygenase (IDO), melanoma, negative feedback mechanism, prognosis, programmed-death ligand 1 (PD-L1), regulatory T-cells
List of Abbreviations: AJCC; American Joint Committee on Cancer system; CC, correlation coefficientCTLA-4; Cytotoxic T Lymphocyte-Associated Antigen 4; DC, dendritic cells; HR, hazard ratio; IDO, indoleamine 2, 3-dioxygenase; IFNγ, interferon-gamma; IQR, interquartile range; Kyn, kynurenine; mDC, myeloid DC; MDSC, myeloid-derived suppressor cells; MFI, mean fluorescence intensity; mMDSC, monocytic MDSC; OS, overall survival; PBMC, peripheral blood mononuclear cells; pDC, plasmacytoid DC; PD-1, programmed cell death protein 1; PD-L1, Programmed-Death Ligand 1; pmnMDSC, polymorphonuclear MDSC; Treg, regulatory T-cell; Tryp, tryptophan; UPLC, ultra-performance liquid chromatography
Introduction
Malignant melanoma has become the prototype of an immunogenic tumor that can be successfully treated with immune checkpoint inhibitors such as anti-CTLA-4 and anti-PD1/PD-L1 antibodies.1,2 Clinical responses are more frequent in the subgroup with T-cell inflamed tumors,3 but still less than half of this patient subset is estimated to respond. This suggests that counter-regulatory immune mechanisms also play a role. Indeed, evidence emerges that antitumor immune responses are often accompanied by multiple immunosuppressive mechanisms, probably acting as negative feedback mechanisms. In this context, Spranger et al. were able to show that in metastatic melanoma tissue, the presence of IDO, PD-L1, and Forkhead box P3 (FoxP3+) Tregs coincides and is dependent on preceding IFN-gamma (IFNγ) secretion by CD8+ lymphocytes.4
IDO is an immunosuppressive intracellular enzyme that initiates the catabolism of the essential amino acid tryptophan to kynurenine and its derivatives.5 IDO expression has been described in a variety of immune and stromal cells, but is best characterized in dendritic cells.6 The function of IDO is the regulation of adaptive immune responses, consequently contributing to tumor-protective immune suppression.7 We were previously able to show that IDO expression in the primary tumor or sentinel lymph node has an independent negative prognostic effect on overall and relapse-free survival in melanoma.8,9
Cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) is a target gene of FoxP3 and is constitutively expressed by Tregs. It has a major physiological role in enhancing Treg activity and downmodulating T helper cell activity, by competing with CD28 for binding with CD80.10 CTLA-4 was the first immune-checkpoint to be clinically targeted, and CTLA-4 inhibition was the first treatment to induce a significant improvement of overall survival (OS) in stage IV melanoma patients.11 This success has paved the way for other checkpoints inhibitors, such as those targeting the PD1/PD-L1 axis. Engagement of PD1 by PD-L1 limits T-cell activity in peripheral tissues at the time of an inflammatory response to infection, leading to immune resistance in the tumor micro-environment.10
These immunosuppressive mechanisms are probably more complex than is currently understood. It has for example been shown that PD-L1 can also be a ligand for CD80 in vitro, and that this interaction inhibits T-cell proliferation and cytokine production.12 Furthermore, CTLA-4 can induce IDO activity in pDCs via reverse signaling with CD80. Adding more complexity to this issue, several different cell types have been reported to be implicated in these feedback loops, the most important being Tregs and myeloid-derived suppressor cells (MDSC).13
Even though IDO, CTLA-4 and PD-L1 are promising targets for immunotherapy, data on their expression by immune cells in the peripheral blood of melanoma patients are lacking. We previously demonstrated an increased mean-fluorescence intensity (MFI) for IDO in peripheral blood mononuclear cells (PBMCs) of melanoma patients with advanced disease after stimulation with CTLA-4.8 In the present study, we investigated the in vivo expression of IDO, PD-L1 and CTLA-4 by immune cells of the lymphoid and myeloid lineage in the peripheral blood of stage I–IV melanoma patients. To ascertain the functional relevance of the detected IDO, tryptophan metabolism was assessed by ultra-performance liquid chromatography (UPLC).
Results
Flow cytometry was performed on PBMC samples of 72 American Joint Committee on Cancer (AJCC) stage I–IV melanoma patients who were not under active therapy for their disease at the time of sample procurement. Detailed patient characteristics can be found in Table 1.
Table 1.
Patient characteristics
| Number of patients, n | 72 |
| Follow-up time, months (median, IQR) | 16 (21–104.5) |
| Age at diagnosis, years (median, IQR) | 53 (40.5–65) |
| Female sex, % (n) | 52.9 (36) |
| Stage at inclusion, % (n) | |
| Local (stage I and II) | 50 (36) |
| Regional (stage III) | 34.7 (25) |
| Systemic (IV) | 15.3 (11) |
| Active disease at inclusion | 19.4 (14) |
| Stage IIIc | 4 (5.6) |
| Stage IV | 10 (13.9) |
| Melanoma characteristics | |
| Breslow (median, IQR) | 1.81 (1.10–2.80) |
| Ulceration, % (n) | 36.1 (22) |
| Sentinel invasion, % (n) | 22.2 (14) |
| Location of primary melanoma | |
| Head and neck, % (n) | 13 (9) |
| Trunk, % (n) | 42 (29) |
| Extremities, % (n) | 44.9 (31) |
| Unknown primary | 3 |
Indoleamine 2,3-dioxygenase
IDO expression by PBMCs was 3.8-fold upregulated after stimulation with IFNγ overnight. We compared the clinical relevance of IDO expression with and without stimulation with IFNγ, and found no correlation between stimulated IDO expression and any of the clinical variables. All further experiments are therefore based on spontaneous, unstimulated IDO expression values.
IDO expression was predominantly detected in pDCs and mMDSCs. A lower degree of IDO expression was also detected in Tregs, myeloid dendritic cells (mDCs) and polymorphonuclear MDSCs (pmnMDSC). There was no IDO expression in cytotoxic T-cells. For a complete overview of IDO expression in these cell types, we refer to Table 2. IDO expression by pDCs and mMDSCs was strongly correlated (p < 0.001, CC 0.621).
Table 2.
IDO and PD-L1 expression by different circulating cell types in AJCC stage I–IV melanoma patients
| Cell type | Observed cell frequency (mean % – SD)* | Cell frequency with IDO expression (mean % – SD) | Cell frequency with PD-L1 expression (mean % – SD) |
|---|---|---|---|
| All PBMCs | NA | 0.55 (0.58) | 1.22 (0.97) |
| Dendritic cells (DC) | 1.54 (0.54) | ||
| Plasmacytoid DCs | 0.34 (0.18) | 1.66 (2.27) | 0.71 (0.37) |
| Myeloid DCs | 0.96 (0.40) | 0.015 (0.028) | 0.91 (0.38) |
| Myeloid-derived suppressor cells (MDSC) | 4.00 (2.29) | ||
| Monocytic MDSC | 2.94 (2.81) | 0.96 (1.95) | 0.23 (0.61) |
| Polymorphonuclear MDSC | 1.04 (0.64) | 0.13 (0.26) | 1.10 (1.94) |
| CD3+ cells | 43.60 (9.32) | ||
| Cytotoxic T-cell | 14.79 (6.06) | 0.0030 (0.0046) | 0.17 (0.11) |
| Regulatory T-cell | 4.77 (1.35) | 0.083 (0.19) | 0.57 (0.24) |
*Percentage of live cells, except for Tregs (percentage of CD4+ cells).
To check the clinical relevance of the cell types with the strongest IDO-positivity, their relation to disease stage/activity and prognosis was assessed. IDO expression by MDSCs did not correlate with disease activity. In patients with stage IV disease, higher frequencies of IDO+ mMDSCs (p = 0.027) and IDO+ pmnMDSCs (p = 0.043) were found compared to patients in stage I–III. However, this had no impact on overall- or progression-free survival.
The percentage of circulating pDCs was dichotomized by ROC analysis into “low” (< 0.2515%) and “high” (> 0.2515%). Patients with high levels of pDCs had a positive prognosis on OS, independent of disease stage (p = 0.021, HR 4.16, CI 1.24–13.94 – Table 3, part 1). The frequency of IDO+ pDCs had no impact on prognosis in a model that corrects for disease stage and the percentage of circulating pDCs (Table 3, part 2).
Table 3.
Cox regression models used to assess the prognostic impact of circulating immune cells on overall survival
| 95% Confidence Interval for HR | |||||
|---|---|---|---|---|---|
| Overall survival | Coefficient | p-value | HR | Lower | Upper |
| 1. Plasmacytoid dendritic cells (pDCs) | |||||
| Stage at inclusion | 1.97 | < 0.001 | 7.16 | 2.38 | 21.51 |
| pDC freq. (high vs. low) | 1.43 | 0.021 | 4.16 | 1.24 | 13.94 |
| 2. IDO+ pDCs | |||||
| Stage at inclusion | 1.96 | < 0.001 | 7.10 | 2.47 | 20.39 |
| pDC freq. (high vs. low) | 1.61 | 0.015 | 5.01 | 1.38 | 18.28 |
| IDO+ pDCs (%) | 0.26 | 0.104 | 1.29 | 0.95 | 1.77 |
| 3. PD-L1+ cytotoxic T-cells | |||||
| Stage at inclusion | 1.75 | 0.001 | 5.75 | 2.03 | 16.35 |
| PD-L1+ cytotoxic T-cell freq. (high vs. low) | 1.77 | 0.032 | 5.87 | 1.17 | 29.53 |
| 4. CTLA-4 expression by Tregs | |||||
| Stage at inclusion | 1.98 | <0.001 | 7.27 | 2.51 | 21.06 |
| CTLA-4 expression by Tregs (high vs. low) | 1.35 | 0.035 | 3.85 | 1.10 | 13.48 |
CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; freq., frequency; HR, hazard ratio; IDO, indoleamine 2,3-dioxygenase; OS, overall survival; pDC, plasmacytoid dendritic cell; PD-L1, programmed death ligand 1.
IDO and tryptophan metabolism
In order to confirm that the measurement of IDO by flow cytometry has functional relevance, we assessed tryptophan metabolism by UPLC-MS/MS. A higher MFI for IDO in PBMCs was correlated with a higher Kyn/Tryp ratio (p = 0.008, CC 0.389) and with lower tryptophan levels (p = 0.014, CC −0.364). This pattern matches tryptophan consumption, confirming metabolic activity of the IDO expression measured by flow cytometry.
There was no impact on prognosis of tryptophan levels or any of the IDO catabolites. However, patients with active disease at the time of diagnosis had lower serum tryptophan levels (p = 0.041). In parallel, higher serum anthralinic acid levels were found in stage IV patients compared to stage I–III (p = 0.012). For the other metabolites, trends were observed in the same direction. These results confirm that IDO activity is more prominent in patients with higher grade or active disease.
Next, we evaluated whether tryptophan catabolism in the serum influences circulating cell frequencies. Mean serum concentrations for tryptophan and its catabolites are summarized in Table 4. Patients with a high serum Kyn/Tryp ratio had lower levels of circulating CD3+ cells (p = 0.036, CC −0.267), increased PD-L1+ cytotoxic T-cells (p = 0.009, CC −0.328) and a higher MFI for IDO in Tregs (p = 0.009, CC 0.371). Moreover, in patients with elevated serum kynurenine levels a shift in DC subset frequencies was observed toward a higher mDC/pDC ratio (p = 0.002, CC 0.373).
Table 4.
Mean serum concentrations (nM) of tryptophan and tryptophan catabolites
| Molecule | Mean (SD) concentration (nM) |
|---|---|
| Tryptophan (Tryp) | 49586.11 (14926.746) |
| Kynurenine (Kyn) | 2095.94 (764.201) |
| Kyn/Tryp ratio | 4.43 (1.516) |
| Kynurenic acid | 179.67 (104.775) |
| 3-OH-kynurenine | 36.47 (40.656) |
| 5-OH-tryptophan | 64.63 (18.823) |
| 5-HT (serotonin) | 841.92 (550.497) |
PD-L1
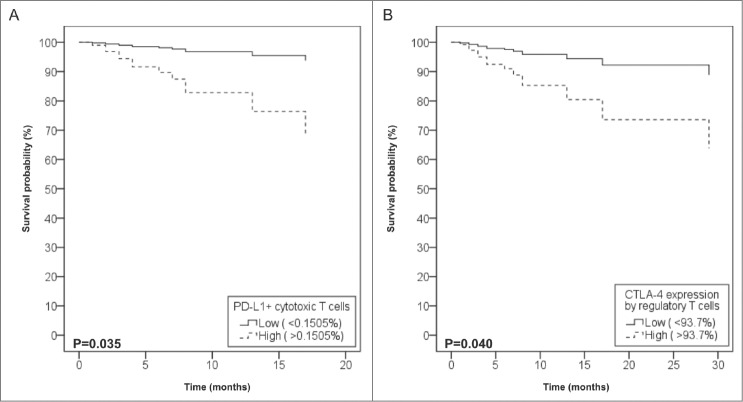
PD-L1 expression was most prominent in pmnMDSCs, but could also be found in other circulating cell types (Table 2). Despite its low frequency, PD-L1 expression by cytotoxic T-cells was clinically most relevant. Patients who died of melanoma had higher levels of PD-L1+ cytotoxic T-cells at the time of inclusion (p = 0.004). To further assess the impact of PD-L1+ cytotoxic T-cells on prognosis, the frequency of PD-L1+ cytotoxic T-cells was dichotomized by ROC analysis into “low” (>0.1505%). High PD-L1+ cytotoxic T-cell levels were present in 42.4%, these patients had a negative prognosis on OS (Log Rank test, p = 0.032, HR 5.87, CI 1.17–29.53) (Table 3, part 3). Moreover, in patients who were disease-free at inclusion but had disease progression in the following two y, a rise in PD-L1+ cytotoxic T-cell frequency could already be seen. The closer to relapse, the higher PD-L1+ cytotoxic T-cell frequencies were (p = 0.021, CC 0.829), suggesting that PD-L1+ cytotoxic T-cells could be relevant to detect impending or subclinical relapse.
Next, the relation of PD-L1 to IDO and Tregs was assessed. A high MFI for IDO correlated with a high MFI for PD-L1 in unstimulated, cultured cells (p < 0.001, CC 0.506), indicating that both immunosuppressive mechanisms occur together. PD-L1+ cytotoxic T-cells were also correlated with the percentage of Tregs (p = 0.048, CC 0.247), the level of CTLA-4 expression by Tregs (p = 0.005, CC 0.346) and with MDSCs (p = 0.033, CC 0.263). Furthermore, levels of PD-L1+ cytotoxic T-cells were inversely correlated with pDC levels (p = 0.044, CC −0.249). These results indicate that PD-L1 expression by cytotoxic T-cells is part of an immunosuppressive environment in the peripheral blood.
CTLA-4 expression by Tregs
CTLA-4 expression was only assessed in Tregs, which were all to some extent, CTLA-4-positive. The level of CTLA-4 expression by Tregs was dichotomized by ROC analysis into “low” (> 93.7%). The mean percentage of patients with high CTLA-4 expression was 37.3%. Patients with high levels of CTLA-4 expression by Tregs had a negative prognosis on OS (Log Rank test, p = 0.003), independent of disease stage (p = 0.035, HR 3.85, CI 1.10–13.48) (Table 3, part 4).
The link between Tregs, IDO, and PD-L1 was described in previous paragraphs. We also observed that the subgroup of patients with head and neck melanoma had increased frequencies of Tregs with high CTLA-4 expression (p = 0.026).
Link between the sentinel lymph node and circulating immune cells
As clinically relevant immune changes can even occur in tumor-free sentinel lymph nodes, we also evaluated whether invasion of the sentinel lymph node had an impact on circulating immune cells. The mean interval between surgical removal of the sentinel and venepuncture for PBMC isolation was 21 mo (IQR 6.5–79.5 mo). Patients with an invaded sentinel lymph node had increased frequencies of circulating highly CTLA-4+ Tregs (p = 0.045) and a higher MFI for CTLA-4 (p = 0.036). Invasion of the sentinel lymph node was also associated with higher frequencies of circulating pDCs (p = 0.034) (Table 5, upper part).
Table 5.
Systemic immune changes according to sentinel lymph node invasion (upper part) and sentinel IDO-positivity (lower part)
| Sentinel invaded | Sentinel non-invaded | ||||||
|---|---|---|---|---|---|---|---|
| Median | IQR | Min–max | Median | IQR | Min–max | p-value* | |
| CTLA-4 expression by Tregs (%) | 93.2 | 90.8–96.3 | 88.7–99.0 | 91.6 | 88.8–93.9 | 83.2–99.7 | 0.045 |
| MFI for CTLA-4 in Tregs | 1931 | 1720–2126 | 1261–2630 | 1695 | 1449–1955 | 1179–2985 | 0.036 |
| pDCs (% of live PBMCs) | 0.38 | 0.29–0.52 | 0.16–1.01 | 0.28 | 0.22–0.44 | 0.13–0.75 | 0.034 |
| Sentinel IDO-positive | Sentinel IDO-negative | ||||||
| Median | IQR | Min–max | Median | IQR | Min–max | p-value** | |
| IDO+ PBMCs (%) | 0.64 | 0.24–1.22 | 0.11–2.57 | 0.39 | 0.14–0.53 | 0.015–1.86 | 0.016 |
| IDO+ mMDSCs (%) | 0.60 | 0.16–1.29 | 0.00–14.10 | 0.095 | 0.035–0.60 | 0.00–1.52 | 0.018 |
CTLA-4, cytotoxic T-lymphocyte-associated antigen 4; IDO, indoleamine 2,3-dioxygenase; IQR, interquartile range; PBMC, peripheral blood mononuclear cell; pDC, plasmacytoid dendritic cell; Treg, regulatory T-cell. * p-values indicate significance of the difference between patients with invaded and non-invaded sentinel lymph nodes (Mann–Whitney-U Test). ** p-values indicate the significance of the difference between patients with IDO-positive and IDO-negative lymph nodes (Mann–Whitney-U Test).
We previously demonstrated that IDO expression in the sentinel is an early marker of immune suppression, irrespective of sentinel invasion. Therefore, the sentinel lymph nodes of the included patients were immunohistochemically stained for IDO and assessed as previously reported.8 Remarkably, patients with an IDO-positive sentinel had more circulating IDO-positive PBMCs (cultured, unstimulated cells, p = 0.016). This could be attributed to higher levels of IDO+ mMDSCs (Table 5, lower part), which were found to be associated with IDO status of the sentinel (p = 0.018). IDO-positivity of the sentinel was also associated with higher frequencies of circulating dendritic cells (p = 0.037, data not shown).
Discussion
The presence of a cytotoxic, inflammatory tumor micro-environment has been demonstrated to cause a concomitant upregulation of negative feedback mechanisms such as IDO, PD-L1, and FoxP3+ Tregs.4 Even though these immunosuppressive mediators are targets for current immunotherapies in melanoma, there is insufficient data on their expression by the different immune cells in the peripheral blood. In this paper we demonstrated that IDO, PD-L1, and CTLA-4 expression in the peripheral blood of melanoma patients is not only interconnected but also associated with advanced disease and a negative prognosis, independent of disease stage.
With regard to IDO, we demonstrated that spontaneous expression can be detected in the peripheral blood of melanoma patients, predominantly in pDCs and mMDSCs. IDO expression in mMDSCs is higher in patients with advanced disease. Up till now, systemic IDO expression was unreported in melanoma patients, but it had already been detected in other conditions. During pregnancy, circulating DCs express more IDO, contributing to fetal tolerance.14 IDO+ pDCs have been proposed to be a regulatory DC subset.6 In breast cancer patients, IDO expression by MDSCs in the tumor stroma was recently demonstrated to suppress immune responses.15 Circulating IDO+ cells could not be detected, but this might be due to insufficient numbers of recorded events during flow cytometry. As can be deduced from Table 2, absolute numbers of IDO+ cells in the peripheral blood are low, so analyzing a large sample (500,000 cells) is necessary for detection. The IDO expression we detected is metabolically active, as it is associated with tryptophan consumption in the serum. Tryptophan degradation by IDO was more prominent in patients with high grade or active disease, as previously reported.16 Tryptophan degradation was also associated with relative lymphopenia, a phenomenon that has also been observed in patients with myelodysplastic syndromes.17 Low tryptophan levels were associated with increased PD-L1+ cytotoxic T-cells and a higher mDC/pDC ratio. Taken together, our data suggest that IDO expression in the peripheral blood of melanoma patients in clinically relevant and is associated with tryptophan catabolism that seems to affect immune cell maturation and function.
Regarding PD-L1, we demonstrated that this marker is expressed by multiple circulating immune cells. The current paradigm on the PD1/PD-L1 axis states that PD-L1 expression by malignant cells will cause anergy by binding with PD-1 on T-cells.10 We demonstrated that clinically relevant PD-L1 expression also occurs in circulating cytotoxic T-cells, conferring a worse prognosis on OS, independent of tumor stage. To the best of our knowledge, these data have not been reported so far. Moreover, PD-L1+ cytotoxic T-cell counts were higher in disease-free patients that relapsed within a year after sample procurement, suggesting that PD-L1+ cytotoxic T-cells could already increase before relapse is clinically detected.
In our study, the levels of circulating Tregs were not related to disease stage or prognosis, but high CTLA-4 expression by Tregs did have a negative effect on OS independent of disease stage. Tregs have been reported to be overrepresented in the peripheral blood of melanoma patients, but contrary to their increased presence in the tumor micro-environment, the impact of circulating Tregs on prognosis had not been established yet.18
In this study we demonstrated that IDO, PD-L1, and CTLA-4 positivity of circulating immune cells are all individually of clinical importance. However, the expression of these three markers is also significantly intertwined. There was a strong correlation between the expression of IDO and PD-L1 in PBMCs. Furthermore the level of PD-L1+ cytotoxic T-cells was associated with increased CTLA-4 expression by Tregs. The combined expression of these three immunosuppressive markers was previously reported in the metastatic melanoma microenvironment.4 Both the interactions between PD-L1 and PD-1 and between CTLA-4 and CD80/CD86 have been abundantly described and provide the rationale for current melanoma immunotherapies.10 Nevertheless, our results support previous studies demonstrating that these markers have a much more complex, network-like interplay. In vitro experiments showed that when CTLA-4 on Tregs binds CD80/CD86 on dendritic cells, an IFNγ dependent induction of IDO is elicited with subsequent tryptophan catabolism.19 However, we could not withhold a correlation between CTLA-4 expression by Tregs and IDO in pDCs, suggesting that this might not be a dominant mechanism of IDO induction in the peripheral blood. Tregs have also been reported to functionally crosstalk with MDSCs through the PD-L1 pathway during melanoma development in mice.20 We did find a correlation between Treg and MDSC frequencies and PD-L1 expression in our patients, justifying further research on this interaction in the peripheral blood of melanoma patients.
Our results also illustrate that important immune changes can occur in melanoma patients with invaded and even with tumor-free sentinel lymph nodes. This is of importance, as about half of all melanoma-related deaths occur in those patients who had local stage disease (AJCC stage I and II) at the time of diagnosis.21 Sentinel node invasion was associated with systemic immune changes with a median delay of 19 mo (Fig. 1). Nevertheless we observed that irrespective of invasion, IDO expression in the sentinel also conferred systemic immunological changes. In patients with an IDO+ sentinel, a higher frequency of circulating DCs was seen (implying immune activation) but higher frequencies of IDO+ mMDSCs were also observed (suggesting concomitant immunosuppression). This clustering of IDO expression in the sentinel and in PBMCs is remarkable, as we previously showed that IDO expression is also consistent in the primary, sentinel, and even metastatic tissue of melanoma patients.8 Our results indicate that IDO expression by host cells is important in the pathogenesis of melanoma, but the question remains how this IDO expression is induced. Soluble tumor-derived factors could be responsible, but alternatively this clustering could also indicate a certain patient-related predisposition. Gene polymorphisms of both the IDO and IFNγ genes have been demonstrated to be associated with an upregulation of IDO expression and tryptophan metabolism in healthy individuals.22,23 If this would also be the case in melanoma patients, it could help explain a patient-dependent IDO expression pattern.
Figure 1.
Impact of PD-L1+ cytotoxic T-cells and CTLA-4+ Tregs on overall survival. Cox regression analysis of overall survival according to the levels of circulating PD-L1+ cytotoxic T-cells (A) and CTLA-4 expression level of regulatory T-cells (B), after adjustment for disease stage. Data are presented as percentages of live peripheral blood mononuclear cells (PBMCs).
There is a solid basis for investigating the effect of IDO inhibitors as part of combination strategies with other immunotherapies in melanoma patients. If IDO expression is associated with the spontaneous host antitumor immune response, it could also be induced by immunotherapy, hence limiting the effectiveness of the treatment.4 In a B16 mouse melanoma model, Holmgaard et al. have demonstrated that host IDO expression has an inhibitory role in both anti-CTLA-4 and anti-PD1/PD-L1 therapy and that pharmacological inhibition of IDO combined with CTLA-4 blockade gives superior effects.24 IDO expression analysis could also be useful for predictive immunoprofiling, as immunohistochemical IDO-positivity in the tumor micro-environment has been shown to predict response to anti-CTLA-4 therapy in patients with metastatic melanoma.25 Others have also demonstrated that lower levels of circulating mMDSCs before initiation of anti-CTLA-4 therapy are associated with increased response.26,27 The relevance of the IDO+ mMDSCs we observed should therefore be evaluated in melanoma patients, as it could not only provide predictive information, but also help elucidate the counter-regulatory mechanisms that hamper the efficacy of current immunological checkpoint inhibitors.
Methods
Patients
Seventy-two melanoma patients were enrolled in this study, with a median follow-up time of 16 mo after inclusion (i.e. the time of venepuncture for blood collection) and of 41.5 mo after diagnosis. For 69 patients both PBMCs and a serum sample were available, in an additional three patients only a serum sample was present. Only patients who were without treatment for their melanoma at the time of venepuncture were included, to avoid any possible influence on marker expression. Of all included patients (n = 72), 14 had active disease at the time of venepuncture. Disease activity was defined as the presence of metastatic melanoma; 4 patients had stage IIIc disease and 10 patients stage IV disease. The sentinel lymph node could be retrieved for additional immunohistochemistry in 51 patients, 27.7% of these lymph nodes was invaded and 72.3% was tumor-free. Detailed patient characteristics can be found in Table 2. The local medical ethical committee approved this study; all included patients signed written informed consent.
PBMC isolation
PBMCs were isolated from heparinized venous blood by centrifugation on a Ficoll-Paque gradient (GE Healthcare, Uppsala, Sweden) within 4 h of venepuncture. The PBMCs were cryopreserved in liquid nitrogen in heat-inactivated foetal bovine serum (FBS) supplemented with 10% dimethyl sulphoxide (DMSO) until analysis. Cells were thawed by submersion at 37° for 1–2 min and resuspended in a medium containing Iscove's Modified Dulbecco's Medium (IMDM) supplemented with 20% FBS and 1% glutamine.
Flow cytometry
MDSCs were characterized by the HLA-DR- lineage- (CD3, CD19, and CD56) CD33+ CD11b+ phenotype, mMDSCs are CD14+, pmnMDSCs are CD14–. Dendritic cells were characterized by the HLA-DR+ lineage– (CD3, CD14, CD16, CD19, CD20, and CD56) phenotype, pDCs are CD123+ CD11c– and mDCs are CD123– CD11c+. Tregs were defined as CD3+ CD4+ CD25+ FoxP3+ and cytotoxic T-cells as CD3+ CD8+ cells. All antibodies used in this study were fluorescently conjugated mouse anti-human monoclonal antibodies. The following antibodies were purchased from BD Biosciences; CD3 BV421 (563797), CD4 APC-Cy7 (561839), CD25 FITC (560990), CD33 BV421 (562854), CD11b APC-Cy7 (560914), CD123 BV421 (562517). The following antibodies were purchased from eBioscience; B7-H1 PE-Cy7 (25-5983-42), CD8 APC (9017-0087-025), CD3 FITC (11-0038-41), CD19 FITC (11-0199-41), CD56 FITC (11-0569-41), CD14 APC (17-0149-41), CD11c APC (17-0116-41), HLA-DR PerCP-Cy5.5 (45-9956-42). For intracellular stainings, after surface staining PBMCs were fixed and permeabilized with fixation/permeabilization solution (BD Biosciences), and then stained with antihuman IDO PE (R&D Systems, IC6030P), CTLA-4 APC (BD Biosciences, 560938) and FoxP3 PerCP-Cy5.5 (eBioscience, 45-4776-42) antibodies. Live/dead staining was performed using Live/dead® fixable aqua dead cell stain (Life Technologies Europe). Patient samples with less than 75% living cells were excluded (n = 4). Cells were analyzed on a FACSCanto™ II flow cytometer (BD Bioscience, Erembodegem, Belgium) using FlowJo software (Tree Star Inc., Ashland, OR, USA). For setting the gates, isotype and fluorescence-minus-one (FMO) controls were used. To provide a representative sample a median amount of 500,000 cells was analyzed per cell type (min 261000 – max 569750). Samples with less than 100,000 cells were excluded (n = 1). The reported frequencies of circulating cell types are percentages of live PBMCs, except for Treg frequencies which are percentages of CD4+ cells.
PBMC culture and stimulation
PBMCs were cultured overnight in a medium containing IMDM supplemented with 20% FBS and 1% glutamine. When sufficient cells were available, PBMCs were also stimulated to induce IDO expression by adding 1000 U/mL human recombinant interferon-gamma (Imukin) to the same medium for 12 h at 37°C in 10% CO2. After incubation, adherent and non-adherent cells were collected for flow cytometry.
Immunohistochemistry for IDO
All sentinel lymph nodes (n = 51) were formalin-fixed paraffin-embedded (FFPE) archival tissues. Immunohistochemical staining of 4–5 μm sections was performed according to standard avidin-biotin-peroxidase protocols (Envision Flex+, Dako, Denmark), a mouse linker was added to the protocol (Dako). The signal was visualized by incubation with 3-amino-9-ethylcarbazole (AEC) substrate (Dako). Sections were counterstained with hematoxylin. For antigen retrieval, slides were boiled (97°C) for 20 min (PT Link, Dako). Sections were incubated with a monoclonal anti-IDO antibody (clone 10.1, 1/200, Millipore) for 1 h.
Ultra-performance liquid chromatography (UPLC)
Tryptophan, kynurenine and its other downstream metabolites in patient sera were quantified by UPLC-MS/MS (Waters Acquity TQD), according to previously published methods, with slight modifications.28,29 An overview of the mean detected serum concentrations for the different molecules can be found in Table 4.
Statistical analysis
Median values between two groups were compared by the Mann–Whitney U-test, between >2 groups with the Kruskal–Wallis testing. To compare proportions of categorical variables, the Pearson's Chi-square test or Fisher's Exact test was used. To evaluate correlations, Spearman correlation coefficients (CC) were calculated. All statistical analyses were performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA), a p-value less than 0.05 was considered statistically significant (double-sided).
Acknowledgements
We would like to thank all the included patients for participating in this study. We also warmly thank Martine De Mil, Marie-Chantal Herteleer, Gabi Holtappels, and Els Van Maelsaeke for their technical assistance, and An Bosschaert and Nele Maes for their help in recruiting the patients.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This research was supported by a research grant to I. Chevolet from the Scientific Research Foundation Flanders (FWO PhD fellowship).
References
- 1. Eggermont A, Robert C, Soria JC, Zitvogel L. Harnessing the immune system to provide long-term survival in patients with melanoma and other solid tumors. Oncoimmunology 2014; 3:e27560; PMID:; http://dx.doi.org/ 10.4161/onci.27560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Speeckaert R, Van Geel N, Lambert J, Boone B, Chevolet I, Van Gele M, Speeckaert MM, Brochez L. Immune mediated mechanisms of melanocyte destruction: paving the way for efficient immunotherapeutic strategies against melanoma. Oncoimmunology 2012; 1:526-8; PMID:; http://dx.doi.org/ 10.4161/onci.19403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, et al. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012; 61:1019-31; PMID:; http://dx.doi.org/ 10.1007/s00262-011-1172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5:200ra116; PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J 1991; 5:2516-22; PMID: [PubMed] [Google Scholar]
- 6. Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science 2002; 297:1867-70; PMID:; http://dx.doi.org/ 10.1126/science.1073514 [DOI] [PubMed] [Google Scholar]
- 7. Johnson TS, Munn DH. Host indoleamine 2,3-dioxygenase: contribution to systemic acquired tumor tolerance. Immunol Invest 2012; 41:765-97; PMID:; http://dx.doi.org/ 10.3109/08820139.2012.689405 [DOI] [PubMed] [Google Scholar]
- 8. Chevolet I, Speeckaert R, Haspeslagh M, Neyns B, Kruse V, Schreuer M, Van Gele M, Van Geel N, Brochez L. Peri-tumoral indoleamine 2,3-dioxygenase expression in melanoma: an early marker of resistance to immune control? Br J Dermatol 2014; 171:987-95; PMID:; http://dx.doi.org/ 10.1111/bjd.13100 [DOI] [PubMed] [Google Scholar]
- 9. Speeckaert R, Vermaelen K, van Geel N, Autier P, Lambert J, Haspeslagh M, van Gele M, Thielemans K, Neyns B, Roche N, et al. Indoleamine 2,3-dioxygenase, a new prognostic marker in sentinel lymph nodes of melanoma patients. Eur J Cancer 2012; 48:2004-11; PMID:; http://dx.doi.org/ 10.1016/j.ejca.2011.09.007 [DOI] [PubMed] [Google Scholar]
- 10. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:; http://dx.doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363:711-23; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007; 27:111-22; PMID:; http://dx.doi.org/ 10.1016/j.immuni.2007.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khaled YS, Ammori BJ, Elkord E. Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol 2013; 91:493-502; PMID:; http://dx.doi.org/ 10.1038/icb.2013.29 [DOI] [PubMed] [Google Scholar]
- 14. Miwa N, Hayakawa S, Miyazaki S, Myojo S, Sasaki Y, Sakai M, Takikawa O, Saito S. IDO expression on decidual and peripheral blood dendritic cells and monocytes/macrophages after treatment with CTLA-4 or interferon-gamma increase in normal pregnancy but decrease in spontaneous abortion. Mol Hum Reprod 2005; 11:865-70; PMID:; http://dx.doi.org/ 10.1093/molehr/gah246 [DOI] [PubMed] [Google Scholar]
- 15. Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol 2013; 190:3783-97; PMID:; http://dx.doi.org/ 10.4049/jimmunol.1201449 [DOI] [PubMed] [Google Scholar]
- 16. Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology 2007; 214:8-14; PMID:; http://dx.doi.org/ 10.1159/000096906 [DOI] [PubMed] [Google Scholar]
- 17. Berthon C, Fontenay M, Corm S, Briche I, Allorge D, Hennart B, Lhermitte M, Quesnel B. Metabolites of tryptophan catabolism are elevated in sera of patients with myelodysplastic syndromes and inhibit hematopoietic progenitor amplification. Leuk Res 2013; 37:573-9; PMID:; http://dx.doi.org/ 10.1016/j.leukres.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 18. Jacobs JF, Nierkens S, Figdor CG, de Vries IJ, Adema GJ. Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy? Lancet Oncol 2012; 13:e32-42; PMID:; http://dx.doi.org/ 10.1016/S1470-2045(11)70155-3 [DOI] [PubMed] [Google Scholar]
- 19. Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, Candeloro P, Belladonna ML, Bianchi R, Fioretti MC, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol 2002; 3:1097-101; PMID:; http://dx.doi.org/ 10.1038/ni846 [DOI] [PubMed] [Google Scholar]
- 20. Fujimura T, Kambayashi Y, Aiba S. Crosstalk between regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs) during melanoma growth. Oncoimmunology 2012; 1:1433-4; PMID:; http://dx.doi.org/ 10.4161/onci.21176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Jr, Kirkwood JM, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol 2001; 19:3635-48; PMID: [DOI] [PubMed] [Google Scholar]
- 22. Soichot M, Hennart B, Al Saabi A, Leloire A, Froguel P, Levy-Marchal C, Poulain-Godefroy O, Allorge D. Identification of a variable number of tandem repeats polymorphism and characterization of LEF-1 response elements in the promoter of the IDO1 gene. PLoS One 2011; 6:e25470; PMID:; http://dx.doi.org/ 10.1371/journal.pone.0025470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raitala A, Pertovaara M, Karjalainen J, Oja SS, Hurme M. Association of interferon-gamma +874(T/A) single nucleotide polymorphism with the rate of tryptophan catabolism in healthy individuals. Scand J Immunol 2005; 61:387-90; PMID:; http://dx.doi.org/ 10.1111/j.1365-3083.2005.01586.x [DOI] [PubMed] [Google Scholar]
- 24. Holmgaard RB, Zamarin D, Munn DH, Wolchok JD, Allison JP. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J Exp Med 2013; 210:1389-402; PMID:; http://dx.doi.org/ 10.1084/jem.20130066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, Hyams DM, Gomez H, Bastholt L, et al. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med 2011; 9:204; PMID:; http://dx.doi.org/ 10.1186/1479-5876-9-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, Speiser DE. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother 2014; 63:247-57; PMID:; http://dx.doi.org/ 10.1007/s00262-013-1508-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kitano S, Postow MA, Cortez C, Rasalan T, Gallardo HF, Panageas K, Yuan JD, Wolchok JD, Lesokhin AM. Myeloid-derived suppressor cell quantity prior to treatment with ipilimumab at 10 mg/kg to predict for overall survival in patients with metastatic melanoma. J Clin Oncol 2012; 30:2518. [Google Scholar]
- 28. Schefold JC, Zeden JP, Fotopoulou C, von Haehling S, Pschowski R, Hasper D, Volk HD, Schuett C, Reinke P. Increased indoleamine 2,3-dioxygenase (IDO) activity and elevated serum levels of tryptophan catabolites in patients with chronic kidney disease: a possible link between chronic inflammation and uraemic symptoms. Nephrol Dial Transplant 2009; 24:1901-8; PMID:; http://dx.doi.org/ 10.1093/ndt/gfn739 [DOI] [PubMed] [Google Scholar]
- 29. Yamada K, Miyazaki T, Shibata T, Hara N, Tsuchiya M. Simultaneous measurement of tryptophan and related compounds by liquid chromatography/electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 867:57-61; PMID:; http://dx.doi.org/ 10.1016/j.jchromb.2008.03.010 [DOI] [PubMed] [Google Scholar]