Abstract
We improved the migration and survival of chimeric antigen receptor (CAR)-modified T cells in solid tumors by combining CAR-T cells with an armed oncolytic virus. Local delivery of the chemokine RANTES and the cytokine IL-15 by the oncolytic virus enhanced the trafficking and persistence of the CAR-T cells, resulting in improved antitumor effects.
Keywords: chimeric antigen receptor, GD2 antigen, IL-15, oncolytic virus, RANTES
Adoptive transfer of T cells modified to express chimeric antigen receptors (CARs) has had clinical success in B-lymphocyte derived malignancies.1 However, the clinical efficacy of CAR-T cells remains limited in solid tumors. This unfavorable outcome could be due to the insufficient migration of the infused T cells to the tumor site as well as to the immunosuppressive characteristics of the tumor environment that inhibits the effector function and proliferation of those few T cells that do reach the tumor.
CAR molecules have been further engineered to express co-stimulatory endodomains such as those derived from CD28 and tumor necrosis factor receptor superfamily member 9 (TNFRSF9, better known as 4–1BB) to promote T-cell proliferation and persistence upon encountering tumor cells. CD28 has proven effective in inducing CAR-T cell expansion in B-cell malignancies.2,3 The incorporation of 4–1BB seems, however, to sustain more robust engraftment of CD19-specific CAR-T cells.1 The incorporation of these co-stimulatory molecules in CARs targeting antigens expressed by solid tumors is anticipated to play a similarly crucial role.
CAR-T cells targeting CD19 seem to traffic physiologically to the bone marrow and lymph nodes, the primary sites of hematologic malignancies.1,3 In addition, they can encounter both normal B lymphocytes and leukemic cells directly in the circulation. By contrast, T-cell migration remains a relevant problem for solid tumors. For instance, while tumor-infiltrating T lymphocytes (TILs) isolated from tumor biopsies and expanded ex vivo show dramatic migration to melanoma lesions, polyclonal T lymphocytes isolated from the peripheral blood and engineered with a T-cell receptor seem less effective. This disparity suggests that TILs and peripheral blood T cells may have a different pattern of homing molecules.4 If the current CAR-T cell studies in solid tumors such as neuroblastoma, prostate cancer, pancreatic cancer, and mesothelioma reveal suboptimal migration, countermeasures to increase T-cell migration should be applied. Radiation or chemotherapy before the infusion of CAR-T cells in patients with solid tumors may favorably alter the pattern of T-cell migration. However, several preclinical models have already demonstrated that engineering CAR-T cells to express chemokine receptors that pair with chemokines produced by tumor cells is a strategy that can overcome the trafficking issue.5
When CAR-T cells reach the tumor bed in a sufficient number, tumor-associated inhibitory mechanisms are there to shutdown effective immune responses. In the treatment of small tumors, infusion of CAR-T cells after chemo or radiotherapy may partially reduce the impact of these inhibitory mechanisms. In addition, the recent introduction of antibodies that block T-cell inhibitory mechanisms, such as those abrogating the immune checkpoint CTLA-4 and PD1-PDL-1 pathways, shows great potential. Combinations of CAR-T cell therapies with these antibodies are anticipated to make a substantial difference in the near future. Similarly to the genetic modification of CAR-T cells aimed to express specific chemokine receptors, a plethora of genetic modifications has been proposed to increase the fitness of CAR-T cells within the tumor environment.5 Some of the proposed modifications are currently under clinical investigation. Although each single modification seems to have specific beneficial effects, multiple mechanisms of resistance can be developed by tumors. T cells must accomplish simultaneously optimal trafficking and persistence, while also retaining an acceptable safety profile. In our recent study,6 we developed an engineering strategy where an armed oncolytic virus (OV), another single biological agent, creates a favorable tumor environment for CAR-T cells.
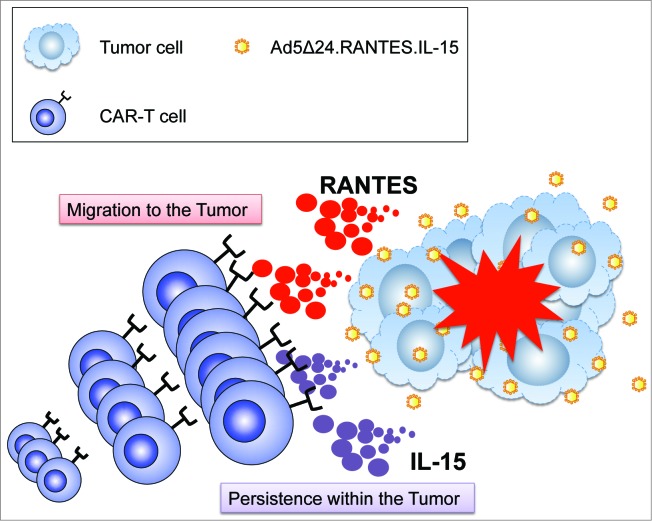
OVs selectively infect, lyse and replicate in malignant cells without affecting non-malignant cells.7 In addition, OVs have sufficient cargo capacity to insert multiple ectopic genes that can be beneficial for CAR-T cells. To investigate the effect of combining armed OV and CAR-T cells, we choose the neuroblastoma model since this tumor model has been previously targeted with CAR-T cells specific for the GD2 antigen.8 We also selected an oncolytic adenovirus (Ad5Δ24) that has been extensively used in the clinic. We exploited the tropism of the OV for the tumor cells and engineered the virus to express both a chemokine and a growth factor for the T cells in order to achieve optimal trafficking of the engineered T cells to the tumor site and to generate a cytokine milieu that sustains T-cell growth and survival. Therefore, we armed Ad5Δ24 with chemokine (C-C motif) ligand 5 (CCL5, better known as RANTES) and interleukin (IL)-15 (Fig. 1), 2 immunomodulatory molecules selected on the basis of both clinical and preclinical data. RANTES is also a very potent chemokine and its receptors are maintained in T cells expanded ex vivo. Using this strategy, we anticipated that tumor cells, regardless of their tissue origin, could be forced to ectopically express RANTES after infection with the armed OV. We hypothesized that this strategy would promote efficient migration of the infused CAR-T cells without the need to select a chemokine/chemokine receptor pathway specific for each single tumor type. The cytokine IL-15 was selected for its multiple beneficial effects on T cells, and its overall ability to increase T-cell antitumor functions. In our experimental design, we demonstrated that neuroblastoma cells infected with Ad5Δ24.RANTES.IL-15 produce functional levels of RANTES and IL-15 both in vitro and in vivo, while the cytopathic effect of the virus is conserved. In a xenogenic mouse model, combined therapy with Ad5Δ24.RANTES.IL-15 and GD2.CAR-T cells significantly enhanced the survival of mice as compared with either of the monotherapies. Furthermore, both RANTES and IL-15 released by the armed OV were predominantly detected at the tumor site, rather than in the serum, indicating a preferential local expression of both factors. This strategy thereby circumvented the toxicities associated with systemic administration of cytokines.6
Figure 1.
Combined therapy with CAR-T cells and Ad5Δ24 armed with RANTES and IL-15. Oncolytic viruses (OVs) selectively infect and lyse tumor cells, and then spread to neighboring tumor cells. Virus-infected tumor cells release both proteins and undergo apoptosis. The chemokine RANTES attracts circulating chimeric antigen receptor (CAR) modified T cells (CAR-T) to the virus infected tumor site and migrated CAR-T cells persist by virtue of interleukin 15 (IL-15) and specific antigen stimulation.
In conclusion, our preclinical study demonstrates that optimal trafficking and survival of CAR-T cells can be obtained in solid tumors by engineering an OV without compromising its cytopathic effect. In principle, similar genetic modifications can be applied to OVs such as vaccinia viruses and measles virus that have been administered intravenously into patients to treat metastatic tumors already.9,10 Arming these viruses using this proposed strategy will not only favor the rapid recruitment of tumor-specific T cells to the primary lesions so infected but will also enhance the spread of the virus to other tumor cells, thus amplifying the therapeutic effect of the viruses. In addition, considering the cargo capacity of these viruses, other relevant genes that may further overcome inhibitory mechanisms can be accommodated.
Acknowledgment
The authors would like to thank Catherine Gillespie for editing the invited auto commentary.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported in part by R01 CA142636 National Institute of Health-NCI and W81XWH-10–10425 Department of Defense and Technology/Therapeutic Development Award.
References
- 1. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. . Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371:1507-17; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1407222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, Kamble RT, Bollard CM, Gee AP, Mei Z, et al. . CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011; 121:1822-6; PMID:; http://dx.doi.org/ 10.1172/JCI46110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al. . CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013; 5:177ra38; PMID:; http://dx.doi.org/ 10.1126/scitranslmed.3005930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, Kammula US, Royal RE, Sherry RM, Wunderlich JR, et al. . Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 2009; 114:535-46; PMID:; http://dx.doi.org/ 10.1182/blood-2009-03-211714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev 2014; 257:107-26; PMID:; http://dx.doi.org/ 10.1111/imr.12131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nishio N, Diaconu I, Liu H, Cerullo V, Caruana I, Hoyos V, Bouchier-Hayes L, Savoldo B, Dotti G. Armed oncolytic virus enhances immune functions of chimeric antigen receptor-modified T cells in solid tumors. Cancer Res 2014; 74:5195-205; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer 2005; 5:965-76; PMID:; http://dx.doi.org/ 10.1038/nrc1750 [DOI] [PubMed] [Google Scholar]
- 8. Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al. . Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008; 14:1264-70; PMID:; http://dx.doi.org/ 10.1038/nm.1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, Cho M, Lim HY, Chung HC, Kim CW, et al. . Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 2013; 19:329-36; PMID:; http://dx.doi.org/ 10.1038/nm.3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Russell SJ, Federspiel MJ, Peng KW, Tong C, Dingli D, Morice WG, Lowe V, O'Connor MK, Kyle RA, Leung N, et al. . Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc 2014; 89:926-33; PMID:; http://dx.doi.org/ 10.1016/j.mayocp.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]