Abstract
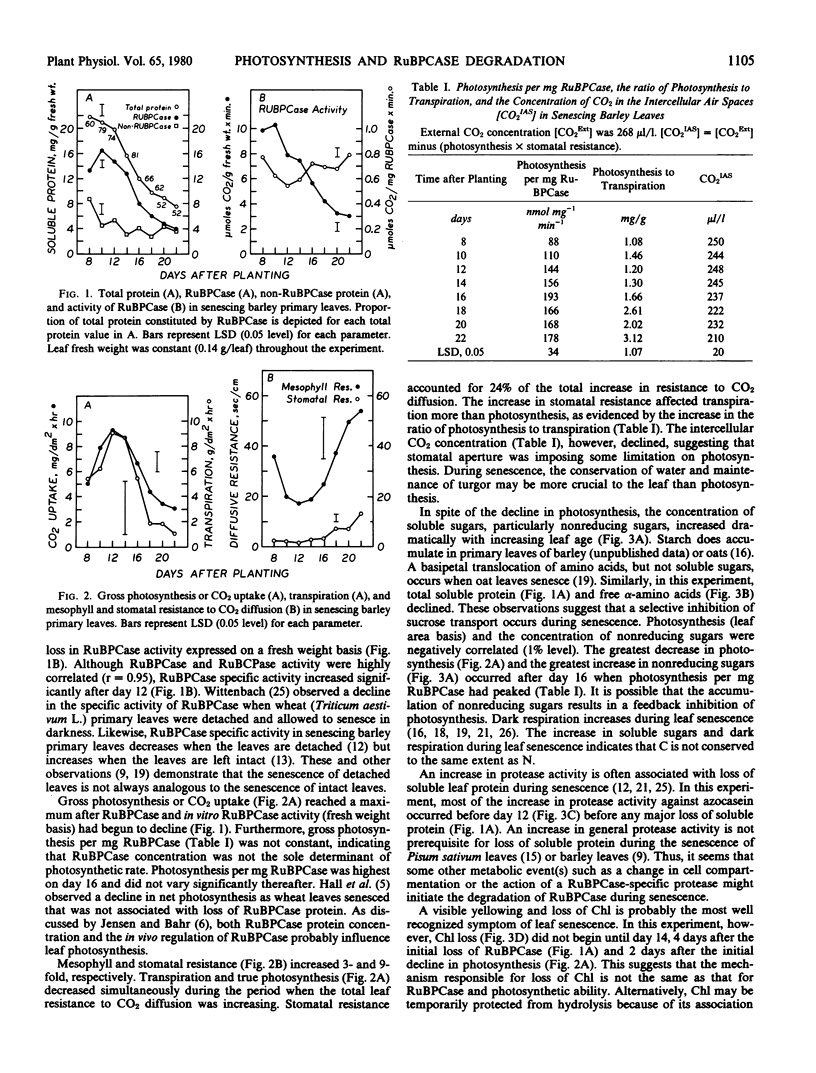
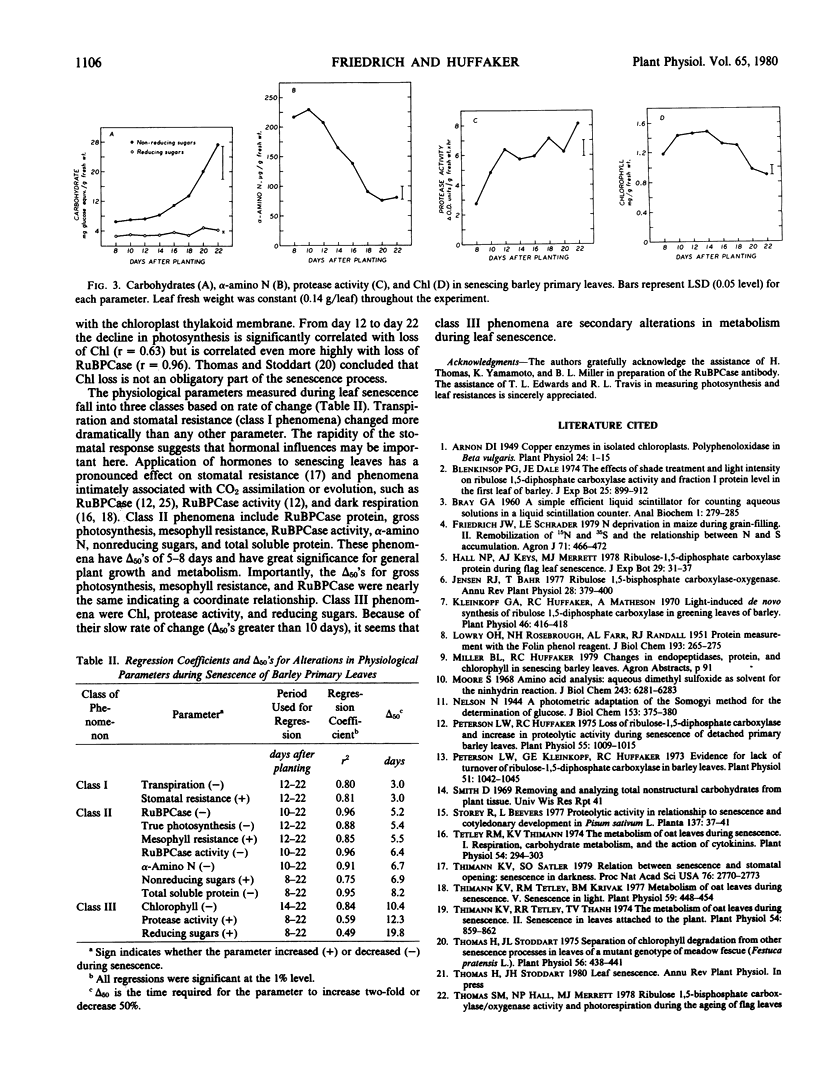
The relationship between loss of ribulose-1,5-bisphosphate carboxylase (RuBPCase) and the decline in photosynthesis during the senescence of barley primary leaves was assessed. Loss of RuBPCase accounted for about 85% of the decrease in soluble protein. RuBPCase was highly correlated with in vitro RuBPCase activity (r = 0.95) and gross photosynthesis (r = 0.96). However, the rate of photosynthesis per milligram RuBPCase increased during the early stages of leaf senescence. The concentration of nonreducing sugars was negatively correlated (1% level) with photosynthesis. Free α-amino N, in contrast to nonreducing sugars, declined markedly during senescence. A decrease in chlorophyll and an increase in in vitro protease activity was observed, but these changes did not appear to be closely related to the decline in photosynthesis and RuBPCase. Mesophyll resistance increased at the same rate that photosynthesis and RuBPCase declined. Stomatal resistance increased more rapidly than mesophyll resistance and accounted for about 24% of the total increase in resistance to CO2 diffusion. The concentration of CO2 in the intercellular air spaces decreased during the last stage of senescence. Although loss of RuBPCase probably is the primary event responsible for the decline in photosynthesis during leaf senescence, other factors such as in vivo regulation and stomatal aperture must also be considered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinkopf G. E., Huffaker R. C., Matheson A. Light-induced de Novo Synthesis of Ribulose 1,5-Diphosphate Carboxylase in Greening Leaves of Barley. Plant Physiol. 1970 Sep;46(3):416–418. doi: 10.1104/pp.46.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968 Dec 10;243(23):6281–6283. [PubMed] [Google Scholar]
- Peterson L. W., Huffaker R. C. Loss of Ribulose 1,5-Diphosphate Carboxylase and Increase in Proteolytic Activity during Senescence of Detached Primary Barley Leaves. Plant Physiol. 1975 Jun;55(6):1009–1015. doi: 10.1104/pp.55.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. W., Kleinkopf G. E., Huffaker R. C. Evidence for lack of turnover of ribulose 1,5-diphosphate carboxylase in barley leaves. Plant Physiol. 1973 Jun;51(6):1042–1045. doi: 10.1104/pp.51.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley R. M., Thimann K. V. The Metabolism of Oat Leaves during Senescence: I. Respiration, Carbohydrate Metabolism, and the Action of Cytokinins. Plant Physiol. 1974 Sep;54(3):294–303. doi: 10.1104/pp.54.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Satler S. Relation between senescence and stomatal opening: Senescence in darkness. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2770–2773. doi: 10.1073/pnas.76.6.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Tetley R. M., Krivak B. M. Metabolism of Oat Leaves during Senescence: V. Senescence in Light. Plant Physiol. 1977 Mar;59(3):448–454. doi: 10.1104/pp.59.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Tetley R. R., Van Thanh T. The Metabolism of Oat Leaves during Senescence: II. Senescence in Leaves Attached to the Plant. Plant Physiol. 1974 Dec;54(6):859–862. doi: 10.1104/pp.54.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H., Stoddart J. L. Separation of Chlorophyll Degradation from Other Senescence Processes in Leaves of a Mutant Genotype of Meadow Fescue (Festuca pratensis L.). Plant Physiol. 1975 Sep;56(3):438–441. doi: 10.1104/pp.56.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting I. P., Hanscom Z. Induction of Acid Metabolism in Portulacaria afra. Plant Physiol. 1977 Mar;59(3):511–514. doi: 10.1104/pp.59.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. A. Breakdown of Ribulose Bisphosphate Carboxylase and Change in Proteolytic Activity during Dark-induced Senescence of Wheat Seedlings. Plant Physiol. 1978 Oct;62(4):604–608. doi: 10.1104/pp.62.4.604. [DOI] [PMC free article] [PubMed] [Google Scholar]



