Abstract
As BRAFV600E-inhibitors become standard treatment for many metastatic melanoma patients, research has begun to elucidate their impact on the tumor immune landscape. Here, we highlight our recent studies demonstrating the ability of melanoma cell-intrinsic BRAFV600E-inhibition to selectively reduce intratumoral immunosuppressive cell populations and enhance antitumor CD8+ T-cell immunity.
Keywords: BRAF, CD8 T cells, melanoma, myeloid-derived suppressor cells, regulatory T cells
Abbreviations: MDSC, myeloid-derived suppressor cell; TME, tumor microenvironment; Treg, regulatory T cell
Author's View
As BRAFV600E inhibitors have become a new standard-of-care therapy for metastatic melanoma patients,1,2 the effect of BRAF-inhibition on host antitumor immunity has become a crucial field of study. Recent studies have shown that vemurafenib, which can induce rapid regression of BRAFV600E-mutant melanoma, also improves the effector T-cell composition within the tumor microenvironment (TME).3 Preclinical studies have thus begun to address the effects of BRAF-inhibition on tumor immune suppression, which is mediated primarily by 2 cell types: regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs). Studies in mouse models have recently demonstrated the ability of BRAF-inhibitors to decrease the proportion of Tregs in the TME, although there has been substantial disagreement regarding whether BRAF-inhibition also reduces numbers of MDSCs and whether reductions are specific to immunosuppressive cells.4-7 It also remains unclear if such immunological changes are secondary effects of inhibitor action on BRAFV600E in melanoma cells themselves (i.e., on-target). Our studies, highlighted herein, recently addressed the downstream consequences of BRAF-inhibition on Tregs and MDSC populations within tumors, and the resulting implications for T cell-mediated tumor control.8
To answer these questions we selected the Tyr-CreERT BrafCA Ptenlox/lox (Braf/Pten) model of BRAFV600E-driven melanoma,9 which we bred onto a C57BL/6 background.8 Tumors in these mice contained large populations of both MDSCs and Tregs, and had a dearth of CD8+ T cells, as has been previously described for human melanomas.3 Importantly, these tumors underwent stable growth arrest upon treatment with the BRAF-inhibitor PLX4720, a research analog of vemurafenib. This is in contrast to the weak drug sensitivity previously reported for transplantable BRAF-mutant melanoma models.4,5 Thus, this autochthonous melanoma may model a subset of aggressive and poorly-immunogenic tumors in patients receiving BRAF-inhibitors.
Our phenotypic analyses of these treated tumors showed that BRAF-inhibition induced a selective loss of immunosuppressive cells from the TME. The most prominent change was a loss of MDSCs, as evinced by decreases in both the proportion and total number of CD11b+Gr-1+ cells. Accordingly, CD11b+ cells isolated from PLX4720-treated tumors were less capable of suppressing T-cell function on a per-cell basis in vitro. In addition to myeloid cells, BRAF-inhibition also significantly decreased the proportion and absolute number of FoxP3+ Tregs in tumors. Analysis of untreated size-matched tumors demonstrated that reductions in Tregs and MDSCs were not due to reduced tumor size, but rather that BRAF-inhibition induced a quantitative loss of pre-existent cells from the TME. These changes were not observed in tumor-draining lymph nodes, nor in hosts bearing BRAF-wild-type melanoma, indicating that Treg and MDSC loss were downstream of melanoma cell-intrinsic BRAFV600E-inhibition.
In contrast to the effects of BRAF-inhibition on MDSCs and Tregs, the numbers of CD4+ and CD8+ T cell numbers were unchanged by treatment. Thus BRAF-inhibition reduced immunosuppressive cell populations selectively, which is contrary to what has been previously reported for this model.7 This failure to increase numbers of effector T cells was unexpected in light of clinical observations showing increased CD8+ and CD4+ T cells counts in tumor biopsies upon treatment with vemurafenib.3 Our adoptive transfer studies with naïve melanoma antigen-specific CD8+ T cells also demonstrated no new T cell priming as a result of PLX4720 treatment. Thus, our data in the Braf/Pten model is consistent with the idea that treatment increases the relative representation of pre-existing CD8+ T cell populations within tumors by selectively eliminating immunosuppressive cells— both MDSCs and Tregs (Fig. 1).
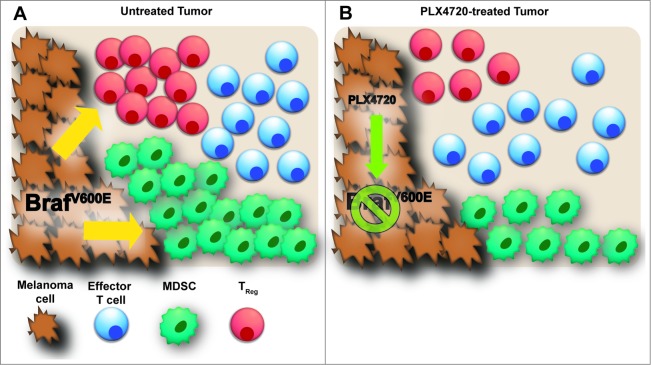
Figure 1.
Inhibition of oncogenic BRAFV600E in melanoma cells leads to a selective reduction in immunosuppressive Tregs and MDSCs within the tumor microenvironment. (A) Immune cell composition in Braf/Pten mouse model of BRAFV600E-driven melanomas. Myeloid-derived suppressor cells (MDSCs) represent the dominant immune cell population; regulatory T cells (Tregs), CD8+ T cells and CD4+ effector T cells are also present. (B) BRAFV600E-inhibition with the small molecule PLX4720 impairs the ability of melanoma cells to maintain Treg and MDSC populations, while leaving effector T-cell populations intact.
Studies in other models have suggested that either CD8+ T cells 4,5 or CD4+ T cells 6 are absolutely required for BRAF-inhibitor efficacy. In contrast, our own studies utilizing CD8 and CD4 depleting antibodies, or alternatively RAG1-/- mice, have demonstrated that αβ T cells are not essential for BRAF-inhibitor efficacy against autochthonous Braf/Pten tumors.8 However, during the period following BRAF-inhibitor treatment, we observed sustained depression of MDSC populations and delayed tumor progression dependent on CD8+ T cells. This implies that CD8+ T cells may provide a redundant mechanism of tumor control in PLX4720-treated mice. It has been suggested that an intermittent vemurafenib dosing schedule may limit the development of drug resistance.10 Our findings demonstrate that during these “drug holidays,” the patient's immune response may directly contribute to ongoing disease stability.
The mechanisms by which BRAF-mutant melanoma cells support immunosuppressive cells in the TME remain to be fully defined. Our findings are consistent with the idea that BRAF-inhibition selectively impairs melanoma cell production of Treg and MDSC survival factors. Accordingly, we showed that Tregs undergo apoptosis at an increased rate following BRAF-inhibition, although MDSC apoptosis was unchanged.8 BRAF-inhibition may also block the ability of tumor cells to secrete Treg and MDSC chemoattractants. It has been shown that the C-C chemokine ligand 2 (CCL2), a chemokine associated with recruitment of both TRegs and MDSCs, is decreased in the TME following BRAF-inhibition.4 Thus immunosuppressive cells may be lost through a combination of factors that modulate recruitment to, and fitness within, the TME.
Work with murine models is beginning to elucidate how dominant oncogenic signaling pathways shape the tumor immune landscape. Routine characterization of molecularly-targeted therapeutics for their impact on the tumor microenvironment may yield surprising information regarding the immunological basis of drug efficacy. These insights will provide a more solid foundation on which to build thoughtful combinatorial therapies that better address patient variability and the heterogeneous and dynamic nature of tumors.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Sosman JA, Kim KB, Schuchter L, Gonzalez R, Pavlick AC, Weber JS, McArthur GA, Hutson TE, Moschos SJ, Flaherty KT, et al. . Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012; 366:707-14; PMID:; http://dx.doi.org/ 10.1056/NEJMoa1112302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Jr., Kaempgen E, et al. . Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012; 380:358-65; PMID:; http://dx.doi.org/ 10.1016/S0140-6736(12)60868-X [DOI] [PubMed] [Google Scholar]
- 3. Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 2012; 18:1386-94; PMID:; http://dx.doi.org/ 10.1158/1078-0432.CCR-11-2479 [DOI] [PubMed] [Google Scholar]
- 4. Knight DA, Ngiow SF, Li M, Parmenter T, Mok S, Cass A, Haynes NM, Kinross K, Yagita H, Koya RC, et al. . Host immunity contributes to the anti-melanoma activity of BRAF inhibitors. J Clin Invest 2013; 123:1371-81; PMID:; http://dx.doi.org/ 10.1172/JCI66236 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5. Cooper ZA, Juneja VR, Sage PT, Frederick DT, Piris A, Mitra D, Lo JA, Hodi FS, Freeman GJ, Bosenberg MW, et al. . Response to BRAF inhibition in melanoma is enhanced when combined with immune checkpoint blockade. Cancer Immunol Res 2014; 2:643-54; PMID:; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ho PC, Meeth KM, Tsui YC, Srivastava B, Bosenberg MW, Kaech SM. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNgamma. Cancer Res 2014; 74:3205-17; PMID:; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hooijkaas A, Gadiot J, Morrow M, Stewart R, Schumacher T, Blank CU. Selective BRAF inhibition decreases tumor-resident lymphocyte frequencies in a mouse model of human melanoma. Oncoimmunology 2012; 1:609-17; PMID:; http://dx.doi.org/ 10.4161/onci.20226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steinberg SM, Zhang P, Malik BT, Boni A, Shabaneh TB, Byrne KT, Mullins DW, Brinckerhoff CE, Ernstoff MS, Bosenberg MW, et al. . BRAF Inhibition Alleviates Immune Suppression in Murine Autochthonous Melanoma. Cancer Immunol Res 2014; 2:1044-50; PMID:; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE, Jr., You MJ, DePinho RA, McMahon M, Bosenberg M. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 2009; 41:544-52; PMID:; http://dx.doi.org/ 10.1038/ng.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Das Thakur M, Salangsang F, Landman AS, Sellers WR, Pryer NK, Levesque MP, Dummer R, McMahon M, Stuart DD. Modelling vemurafenib resistance in melanoma reveals a strategy to forestall drug resistance. Nature 2013; 494:251-5; PMID:; http://dx.doi.org/ 10.1038/nature11814 [DOI] [PMC free article] [PubMed] [Google Scholar]